Utilization and Perceptions of a Novel Cervical Visualization Tool, The Callascope, For Home-Based Self-Cervical Examinations
Received Date: November 13, 2021 Accepted Date: December 13, 2021 Published Date: December 15, 2021
doi: 10.17303/jwhg.2021.8.306
Citation:Júlia S Agudogo (2021) Utilization and Perceptions of a Novel Cervical Visualization Tool, The Callascope, For Home-Based Self-Cervical Examinations. J Womens Health Gyn 1: 1-24.
Abstract
Background:Cervical visualization is critical for a wide array of reproductive health maintenance procedures. Yet, it has largely been limited to clinical providers, who utilize the speculum to access the cervix for diagnostic or treatment purposes. Self-cervical visualization, using the speculum and a mirror, has been attempted, but is often characterized as painful, awkward, and uncomfortable. We have developed a novel speculum-free device, the Callascope, that enables self-cervical visualization and image capture without clinician assistance. Self-cervical visualization has immense potential for increased confidence in knowing one’s body, seeking help from a provider, and feeling empowered to take charge of one’s own reproductive health. Additionally, self-cervical visualization could enable access to home-based basic reproductive health applications, such as intrauterine device placement monitoring, and eventually preliminary self-cervical cancer screening using contrast-agent application in combination with HPV self-sampling.
Methods:This mixed-methods study involved training healthy volunteers (n=12) to use the Callascope at home to assess ease-of-use and feasibility of imaging their cervix without clinician guidance. This involved (1) on-site training at the study site, followed by initial self-imaging of the cervix, (2) self-imaging at home, and (3) an optional audio reflection.
Results:The on-site training examinations resulted in 83% of participants (10 out of 12) visualizing their cervix, upon their first attempt. During the home examinations, 92% (11 out of 12) participants visualized their cervix. Overall, all participants captured at least one image of their cervix and would recommend the Callascope to others. Audio reflections showed high acceptability with participants described the Callascope experience as “comfortable”, “easy to use”, “empowering”, and “fascinating”.
Conclusion:We have shown high acceptability and feasibility of the Callascope for person-centered, home-based, comfortable, and cost-effective self-cervical visualization and image capture.
Keywords:Cervix Imaging; Speculum-Free; Home-Based; Cervical Cancer
Introduction
Cervical visualization is a critical component of reproductive health maintenance. Healthcare providers use the speculum for cervical visualization in a wide variety of clinical evaluations [1-5]. While the speculum is an important tool in obstetric and gynecologic care, it is associated with pain and discomfort leading to fear of pelvic examinations [6]. Fear of the speculum is an evident component of non-adherence to cervical cancer screening recommendations. Though cervical cancer is preventable with regular screening and the HPV vaccine, half a million women are diagnosed and a quarter million dies annually [7]. One of the main deterrents to screening is pain due to the speculum- based examination, which leads to a four-fold decrease in adherence to screening [8,9]. The barriers the speculum poses to health-seeking behavior creates a need for alternative approaches of viewing lower internal reproductive anatomy. Additionally, lack of awareness about reproductive anatomy significantly decreases women’s reproductive health- seeking behavior [10]. Several studies conducted in socioculturally diverse settings found that educating women on their cervix and cervical cancer increased screening rates more than two-fold [11-15]. These studies suggest that women are more likely to advocate for their reproductive healthcare when educated on the subject.
We developed the Callascope, a low-cost cervical visualization device, to comfortably view internal lower reproductive anatomy and potentially substitute for the speculum (Figure 1) [16,17]. In our previously published studies, we evaluated clinician-based cervical imaging, and self-cervical imaging using the Callascope under clinician guidance [16,17]. In this study, we investigated the utilization of the Callascope for self-cervical imaging outside of a clinical setting to assess the Callascope’s potential for future use in home-based reproductive health applications and reproductive health education programs.
Methods
Healthy volunteer studies
This study was approved by Duke University’s institutional review board (IRB) and was performed with an approved protocol, informed consent process, and data storage system (Pro00008173). Participants were healthy, between 21 to 65 years old, had a history of at least one pap smear, and were not pregnant or not in 2nd or 3rd trimester of pregnancy (n = 12). Volunteers were recruited from the general Durham community using flyers and online on the Craigslist platform (Supplemental Figure 1). During the first phase of the study, participants completed the training module, and independently performed an on-site training examination. In the second phase, which consisted of a home examination, participants were given the Callascope in their homes for one week (Figure 2). In the last phase, the participants had the option of doing an audio reflection using the Calla imaging phone application.
On-site training and initial cervix imaging
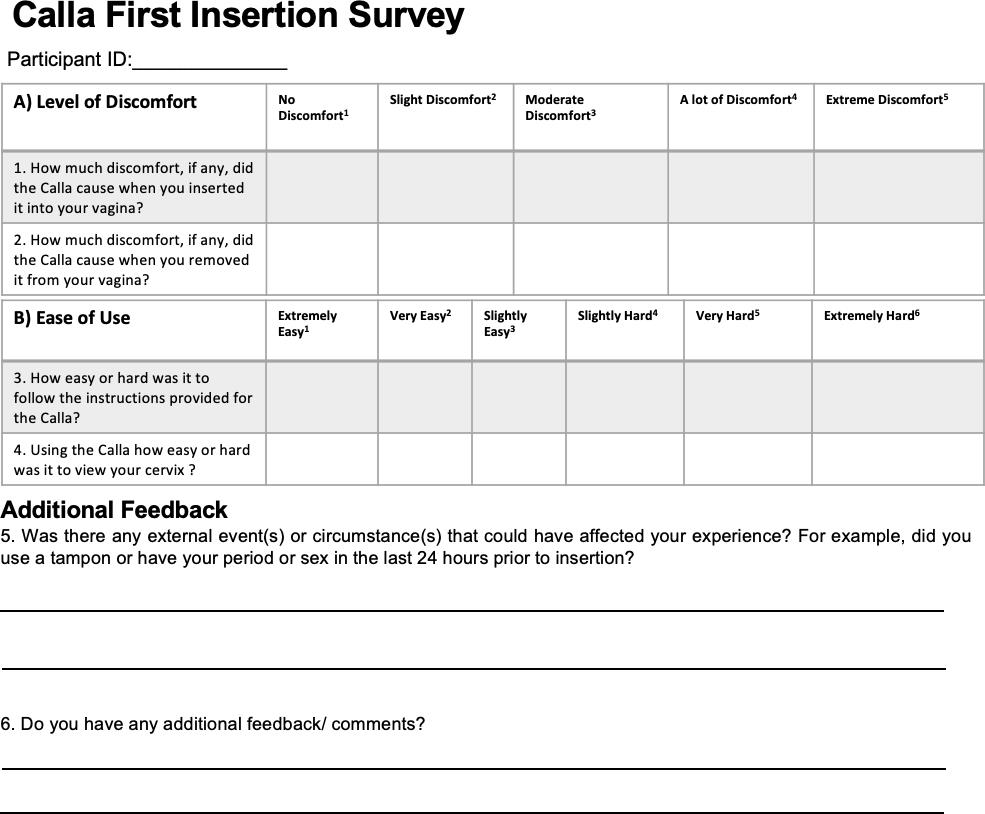
Participants were seen at an initial visit to the Duke University Medical Center (DUMC) research study site during which they provided written informed consent for the study. Following informed consent, participants completed a pre-insertion baseline survey to assess their demographics, prior experiences with the standard speculum, and initial impressions of the Callascope (Supplementary Figure 2.1-2). Participants were then given a user kit containing a Callascope, an android phone and phone charger, Sani wipes, vaginal wipes, lubricating jelly, a printed user guide (Supplementary Figure 3), and an audio reflection guide (Supplementary Figure 2.3). Participants watched a video tutorial (this is the video tutorial link) available on the custom Calla mobile application consisting of information on the assembly and use of the Callascope. After watching the tutorial, participants were allowed to ask the study coordinators, who were well-versed with the Callascope design and function, any questions they had related to the Callascope. Participants were then privately allowed to capture an image of their cervix with the Callascope in a reserved room at DUMC. A study nurse confirmed whether the images captured were that of the cervix. If the image was not that of the cervix, the volunteers were given additional opportunities to capture an image of their cervix before attempting the home-examination part in the second phase of the study. Upon completion of the on-site exam, participants were asked to complete a survey to assess the comfort and ease-of-use of the Callascope, when used for the first time, using a Likert scale.
Home self-examination
After completing the initial examination in DUMC, participants were provided with the Calla user kit to take home for a week. At home, participants were asked to perform a self-cervix visualization examination one to three times and to capture images of their cervix. At the end of the one week period, participants completed a final survey to assess the ease-of-use and comfort level after using the Callascope upon their last attempt at home. Participants also completed an optional audio reflection guide to share their thoughts on exploring their inner reproductive parts and to give feedback on the study. Before the distribution of the Callascope user kit to the participants, the Callascope, and the phone were disinfected using high-level disinfection procedures [18-19].
Quantitative analysis
Since this study was exploratory, no power analysis was performed. Quantitative data is represented as a mean with standard deviation indicated. Additionally, Likert scale responses of participants regarding discomfort and ease-of- use of the Callascope were analyzed quantitatively. The percentage of the unobstructed cervix area visualized was calculated using a circular grid that was superimposed on cervix images captured by participants (Supplemental Figure 3) to standardize comparison of cervix images [17]. The percent cervix visualization area (PVA) was calculated by dividing the total number of squares within the cervix area visualized by the total number of squares in the grid (equation 1).
Qualitative analysis
Audio reflections which were recorded on phones provided in the user kit were transcribed and then de-identified. The audio reflection guide (Supplemental Figure 1.3) was optional for all participants, it consisted of questions focusing on the acceptability and feasibility of self-imaging with the Callascope in a home-based setting. The recordings were analyzed using a validated qualitative data analysis platform, NVivo v11 (QSR International, London, UK) to organize, manage, and code the data. Three authors (JSA, MNA, MED) read all the transcripts, discussed the transcripts as a group and conducted content analysis20. Codes were developed deductively (a priori) from topics in the audio reflection guide, and inductively (emergent themes) from transcripts. Deductive codes were developed based on the feedback on the Callascope from volunteers in prior clinical studies under clinician guidance [16-17]. Deductive codes included comfort and ease-of-use of Callascope and change in awareness of reproductive anatomy experience. Inductive codes were obtained from the analysis of audio reflections recorded after home-based examination with the Callascope. Three researchers (JSA, MNA, MED) independently coded the transcripts and agreed on coding decisions that were then applied to all transcripts. Authors (JSA, MNA, MED) met to review and revise the codes, resolve discrepancies, and agree on the final organization of the thematic structure.
Results
Participant Demographics
Participant demographics are presented in Table 1. Participants represented different races and ages, vaginal birth experiences, and BMIs. Participant ages ranged from 22 to 56 years (median of 28.5 years). Most of the participants, 66.7% (8 out of 12) of participants, identified as non-Hispanic white, with 25.0% (3 out of 12) of participants identifying as Black, and 1 participant identifying as Asian. Slightly more than half of participants, 58.3% (7 out of 12) were in a normal BMI range (18.5 – 24.9). Additionally, 16.7% (2 out of 12) were underweight, and 25.0% (3 out of 12) of participants were either overweight or obese. The majority, 75% (9 out of 12) of participants, were both nulliparous and regular users of tampons/menstrual cups. Half of the participants had more than 6 prior speculum examinations.
Pre-Insertion Survey
Pre-insertion survey responses collected prior to cervix visualization are summarized in Table 2. Fifty percent of participants found the speculum to be a barrier to cervical cancer screening. Based on appearance only, more participants were willing to use the Callascope over the speculum. 25% (3 out of 12) reported being ‘extremely willing’ or ‘very willing’ to use the speculum based on appearance alone compared to 100% (12 out of 12) who reported being ‘extremely willing’ or ‘very willing’ to use the Callascope. 75% (9 out of 12) reported being ‘not willing’ or ‘slightly willing’ to use the speculum based on appearance alone compared to 0% (0 out of 12) who reported being ‘not willing’ or ‘slightly willing’ to use the Callascope.
Participants ranked comfort as one of the top three factors impacting their experience with cervical cancer screening (cost and adequate assessment of cancer risk ranked higher). Procedure and travel time were least important for participants in the self-examination group.
Cervical Visualization Examinations
During the on-site training (initial cervix imaging session), the study nurse confirmed that 10 out of 12 (83%) of the study participants could visualize their cervix upon their first attempt. 2 participants (17%) were not able to capture images of their cervix during their first attempt at the on-site training phase; they also were not able to capture images on their second attempt on-site. Of the two participants who were not able to capture images of their cervix on their first attempt, one had a BMI > 30, and the other had a normal BMI (18.5 – 24.9). The duration of the initial self- imaging sessions ranged from 4-15 minutes. Representative images of the cervix captured during the on-site training examination and the home-examination are shown in Figure 3a-c, and bar graphs of the cervix area visualized per participant is illustrated in Figure 3d. A representative video of the insertion of the Callascope, showing the vaginal wall and cervix manipulation, is shown at this link. The cervix percent area visualized was calculated via the number of cervix quadrants visualized as described in our previous work [17]. All participants were able to capture at least one image of their cervix either during the initial attempt (on-site training examination) or at home. Of the 12 participants, 11 (92%) were also able to visualize and capture an image of their cervix at home. Nine participants (75%) performed one self- examination, and two participants (17%) performed two self-examinations at home. Of the two participants who performed the examination twice, both captured images of their cervix on each attempt. One participant (8%) was unable to do so due to a self-reported device failure with the camera of the Callascope at home. Overall, the images did not show any significant differences in percent cervix area visualization between the on-site examination and home examination. The mean percent cervix visual area in the images captured during on-site and home examination were comparable at 81% +/- 9%, and 82% +/- 7% respectively. There was no association between percent visual area and BMI.
The results from the post-insertion questionnaire comparing the ease-of-use of the Callascope in terms of ease of understanding the instructions/tutorial materials (S3), and ease of use of the Callascope to visualize the cervix, are shown in Figure 4. Most of the participants found the instructions easy (extremely, very, or slightly easy) to use both during on-site (11 out of 12) and home use (11 out of 11) respectively. One participant did not complete this portion of the last insertion survey at home. Further, 67% (8 out of 12) of participants found it easy (extremely, very, or slightly easy) to visualize their cervix using the Callascope during their first attempt (on-site), compared to 33% (4 out of 12) who found it hard (extremely, very, or slightly hard). Of the participants who attempted to visualize their cervix twice on-site, due to inability to capture an image of their cervix on the first attempt, one participant found it extremely hard, and the other found it very hard. In terms of ease-of-use at home, 55% (6 out of 11) of participants found it easy, while 45% (5 out of 11) found it hard. There was some overlap between responses recorded during the on-site visit and home examinations. Specifically, 8% (1 out of 12) reported cervical visualization was extremely easy, 8% (1 out of 12) reported very easy, 33% (4 out of 12) reported slightly easy, 8% (1 out of 12) reported slightly hard, and 8% (1 out of 12) reported extremely hard for both first and last post-insertion surveys. 8% (1 out of 12) of participants changed from reporting very hard on-site to extremely hard at home, 8% (1 out of 12) of participants changed from reporting very hard on-site to slightly hard at home, 8% (1 out of 12) of participants changed from reporting slightly easy on-site to slightly hard at home, and finally, 8% (1 out of 12) of participants did not complete this portion of the last insertion survey.
The results from the post-insertion questionnaire comparing discomfort associated with insertion and removal of the Callascope during on-site versus home examination use are shown in Figure 5. 50% (6 out of 12) of the women had slight to no discomfort inserting the Callascope during the initial use, 42% (5 out of 12) found insertion moderately uncomfortable, and only 8% (1 out of 12) had a lot of discomfort during insertion. In the case of final use, the same participant continued to report a lot of insertion discomfort (9% or 1 out of 11). Additionally, 73% (8 out of 11) reported slight to no discomfort, and 18% (2 out of 11) reported moderate discomfort. The majority of participants reported the same rating for discomfort with insertion for both on-site and home-based settings; 33% (4 out of 12) reported no discomfort, 17% (2 out of 12) reported slight discomfort, 17% (2 out of 12) reported moderate discomfort, and 8% (1 out of 12) reported a lot of discomfort respectively for both sessions. 8% (1 out of 12) of participants changed from reporting moderate discomfort on-site to no discomfort at home, 8% (1 out of 12) of participants changed from reporting moderate discomfort on-site to slight discomfort at home, and 8% (1 out of 12) of participants did not complete this portion of the last insertion survey. All participants found that removal of the Callascope posed no discomfort or only slight discomfort both in the on-site training and home-based settings; 75% (9 out of 12) reported no discomfort and 17% (2 out of 12) reported slight discomfort respectively for both settings; 8% (1 out of 12) did not complete this portion of the last insertion survey. In the last post-insertion survey administered to participants, 100% of participants (12 out of 12) indicated that they would recommend the Callascope to others.
Audio reflections and optional comments provided more in-depth reviews by participants
Participants from the home study provided optional comments in their final surveys as well as audio reflections of their experience with the Callascope. The audio recordings ranged in duration from 2 minutes to 26 minutes; the mean duration was 9 minutes, with a standard deviation of 7 minutes. Themes were developed based on both deductive and inductive methodologies. These themes were comfort and ease-of-use of Callascope, change in awareness of reproductive anatomy, excitement at future applications of the Callascope for gynecologic examinations, and feelings of empowerment associated with the use of the Callascope and visualization of the cervix. Keywords associated with the use of the Callascope include “comfortable/ easy to use”, “excitement”, “self-awareness”, and “empowering” (Table 3). Briefly, self-examination participants mentioned that the device was easy to use at home and expressed excitement over being able to view their cervix themselves. Participants also mentioned feeling empowered and having improved cervix awareness by being able to visualize their reproductive anatomy themselves, which they had not been able to do previously. Additionally, one participant mentioned uncertainty about whether her cervix looked healthy based on the image captured, and two participants stated that they would have preferred to have remote access to a healthcare provider to confirm whether their cervix, and its associated discharge, looked was normal. One participant did not complete an audio reflection, hence was excluded from the qualitative analysis of the audio reflections.
Discussion
The Callascope is a portable, low-cost imaging device that enables cervical visualization by either a clinician in a clinic/hospital or by individuals themselves in the comfort of their home or within their community. In our prior work, we showed that healthy volunteers could undergo cervical visualization with the assistance of a healthcare provider and/or in a clinical setting [16-17]. In this study, we built upon our prior work by testing the feasibility of healthy participants to independently visualize their cervix without clinician assistance in both an in-person and at-home setting. Our results are consistent with studies of acceptability and feasibility of HPV-self-sampling at home [21,22]. For example, a study of 818 participants in Ontario, reported that 89.7% of participants found self-HPV testing acceptable, and 88% would recommend it to a friend; comparable to 100% who would recommend the Callascope to a friend in this study [21]. Another study of 878 Appalachian women (a region with lower income levels than our study site, Durham) found that 99% of those who collected self-specimens were able to obtain adequate samples; this is comparable to 92% (11 out of 12) participants who were able to obtain cervical images at home, and 100% who obtained at least one image during the study using the Callascope. However, it should be noted that the study reported in this manuscript had a much smaller sample size [23-25]. Overall, our results are comparable to studies of self-HPV testing and support future work pairing self-cervical visualization with HPV sample collection.
The need for travel associated with multiple hospital visits poses a significant challenge to receiving gynecologic examinations due the financial cost of transportation, childcare, and taking time off work to visit a healthcare center [26]. Since many rural areas lack well-established cervical cancer screening centers, women from these areas bear the burden of long travel times and significant costs for obtaining care [27]. The travel to screening sites poses a significant challenge for cervical cancer prevention and this has only been exacerbated in the era of COVID-19, which has brought to light the emerging and critical need for novel, affordable, and effective technologies for remote patient care [28-29]. In addition to challenges regarding travel, ethnic/racial minority groups [6,30-31], sexual minority groups [32], adolescents [33], obese women [34], victims of sexual assault and other trauma [35], and women with disabilities [36] have also cited emotional distress and fear of pain as reasons for non-adherence to cervical cancer screening. Speculum examinations are also especially painful for women with certain medical conditions that reduce elasticity, cause spasms or pain with manipulation of the labia or the vestibule, such as atrophic vaginitis [36-37], vaginal stenosis, vulvodynia and vaginismus [38]. International studies corroborate that the speculum is a barrier to cervical cancer screening for many across the globe [39,40]. One of the most successful approaches to increasing the accessibility of cervical cancer screening is the provision of tests that individuals themselves can perform such as self-collected HPV sampling tests [41]. While self-collected HPV sampling tests have been shown to be highly sensitive, they unfortunately have low specificity; only 10% of patients with high-risk HPV develop persistent infections that progress to cervical cancer [42-45]. Thus, the low specificity of HPV tests can lead to the unnecessary overburdening of already resource-limited healthcare facilities [42-45]. There is potential for a significant clinical benefit of combining self-collection of HPV samples with self-cervical imaging with the Callascope to increase the specificity of preliminary cervical cancer screening. Additionally, the increased privacy, comfort, and empowerment associated with self-cervical imaging could increase the acceptability of preliminary cervical cancer screening among patients with a prior history of pain or emotional distress associated with screening.
Apart from the potential for routine gynecologic applications, the Callascope also offers immense potential as an educational tool for people with cervices to understand their internal reproductive anatomy and be empowered to explore their internal anatomy, often for the very first time. One strategy to empower individuals to advocate for their reproductive autonomy is the provision of self-exploration technology that facilitates visualization, understanding, and appreciation of their own bodies as expressed in audio reflections and comments from this study. There have been published studies concerning the utilization of self-examination as a learner-centered pedagogical methodology for health promotion. For example, a study in Brazil examined the use of self-eye examination as a method for health promotion reported improved awareness of eye care among 324 student participants46. In another study in Turkey, 174 men investigated the impact of testicular self-examination (TSE) on knowledge, performance, and health beliefs, in relation to testicular cancer and TSE, showing significantly improved positive beliefs towards TSE after performing a self-examination47. These studies of self-examination support future work to develop a reproductive health educational curriculum harnessing the pedagogical value of physical self-examination in the context of the cervix. To our knowledge, there have not yet been any published studies of self-examination as a learner-centered pedagogical tool for cervical health. However, there have been public health initiatives, such as The Beautiful Cervix Project that promote cervical health through displaying images of cervices, captured at home using a speculum, mirror and phone, via an online platform [48]. Future studies will investigate the use of the Callascope with an online basic reproductive health educational program to increase knowledge, awareness, and health-seeking behavior with a focus on cervical health. This educational program would consist of multimedia content on basic reproductive health including images detailing a variety of pathological conditions of the cervix to educate users on the need to adhere to screening recommendations and warning signs for which to seek medical attention immediately with the knowledge that early diagnosis is essential for a positive prognosis.
This study was limited to a small participants size (n = 12) in this explanatory phase to prove feasibility before launching a larger scale study. In future studies, in addition to accrual from a larger and more diverse population, the user guide will be upgraded with trouble-shooting modules for the rare incidences of difficulties with camera use. Additionally, 2 out of 12 (17%) participants who were not able to capture images of their cervix on their first attempt, one had a BMI > 30 which may have contributed to difficulties with image-capture. In our previous clinical studies, we observed a correlation between increased BMI and failure to capture an adequate image of the cervix with the Callascope [16-17]. Thus, in future studies we plan to provide a variety of Callascope form-factors optimized for women with larger body masses by increasing the length of the introducer and enlarging the opening of the Callascope introducer tip. Additionally, we received feedback requesting more detailed educational resources on vaginal discharge. Therefore, we will include an extensive multi-media tutorial on expected discharge throughout the menstrual cycle as well as other reproductive experiences, so that participants have an increased understanding of what they observe with the Callascope. Finally, artificial intelligence and machine learning (AI/ML) combined with the Callascope device and mobile application could democratize reproductive health by vastly increasing accessibility [49]. Various data-based scenarios could be explored such as fertility/cycle prediction, and assessment for infections or preliminary cervical cancer screening based on cervix images. We are currently exploring the use of AI/ML for automated risk assessment of cervix images and plan to apply this to the Callascope in future studies
Limitations
Due to the exploratory nature of the study, a small sample size was used. This study however provides a foundation upon which to conduct a larger study. One of the challenges was accrual of a more diverse population in the sites where we posted flyers in the Durham community and online on Craigslist platform. Another continuing challenge was the inability to accrue additional patients owing to the COVID-19 pandemic.
Conclusion
This pilot study demonstrates feasibility of two novel scenarios for self-imaging of the cervix; (1) in a clinic setting, as an alternative to speculum-based imaging, patients can be given the Callascope imaging kit, with the training guide and provided a private room to take images of the cervix, which can then be used for clinical decision making (this is performed for self-HPV testing when testing at home is challenging or not affordable), and (2) in the home setting where users could purchase the Callascope to perform basic imaging of their cervix either for education and awareness or for health care in their homes. We have shown that the Callascope has the potential to positively transform access to gynecologic examinations through enabling a person-centered, home-based, and comfortable approach to viewing internal female reproductive anatomy. In future studies, we will increase efforts to recruit a more diverse cohort through collaborations with community health centers and minority women’s health advocacy groups. In addition, these studies will also be conducted in low-resource settings to be more representative of populations most affected by cervical cancer. Finally, we will explore the use of the Callascope for more specific clinical and diagnostic applications beginning with IUD placement monitoring and eventually preliminary self-cervical cancer screening using contrast-agent application in combination with HPV self-sampling.
Acknowledgements
The authors thank Bonnie Thiele for her assistance with reviewing images captured by our participants. We also thank Bibi Gnagno and Tuania Wright (Women’s Center, Duke University) for their assistance with scheduling and conducting reproductive anatomy and health interviews with women in the Durham community. Last but not least, we would like to thank artist Courtney Reid-Eaton for helping to name the device by observing that the shape of the introducer tip resembled a Calla Lily.
Declaration of interests
MNA, MSK, and NR, have founded a company called Calla Health Foundation to commercialize the Callascope. They and JSA have developed technologies related to this work where the investigators or Duke may benefit financially if this system is sold commercially. For the concept of this system a patent has been awarded to JSA, MNA and NR with the title: “Colposcopes and mammoscopes having curved ends and flat ends, associated methods, and speculum-free imaging methods”. U.S. Patent Application No. 16/089,522. This does not alter the authors’ adherence to policies on sharing data and materials. Other authors declare no competing interests.
Author Contributions
Conceptualization: JSA MNA MK MH GS WH NR.
Data curation: JSA MNA MED MK.
Funding acquisition: JSA MNA MK NR.
Investigation: JSA MNA MK.
Methodology: JSA MNA WH NR.
Project administration: MK NR.
Resources: NR WH.
Supervision: NR WH MK.
Writing – original draft: JSA MNA NR.
Writing – review & editing: JSA MNA MK MH GS JWS DJ WH NR JSS.
Sources of Funding
This work was supported by the National Institute of Health Quick Trials (Grant: 1R01CA195500 https://projectreporter.nih.govproject_info_description.cfm?projectnumber=1R01CA195500-01) to NR and National Institute of Health Academic-Industrial Partnerships (Grant: 1R01CA193380 https://projectreporter.nih.gov/project_info_description.cfm?aid=8864360&icde=0) to NR. We also received funding from the Franklin Humanities Lab, Duke Intellectual Community Planning Grant, and Collaborative Art Proposal grant, all of which are Duke University Internal Grants. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We are not being paid to write this article.
Supplemental Materials

S1. Study Recruitment Flyer
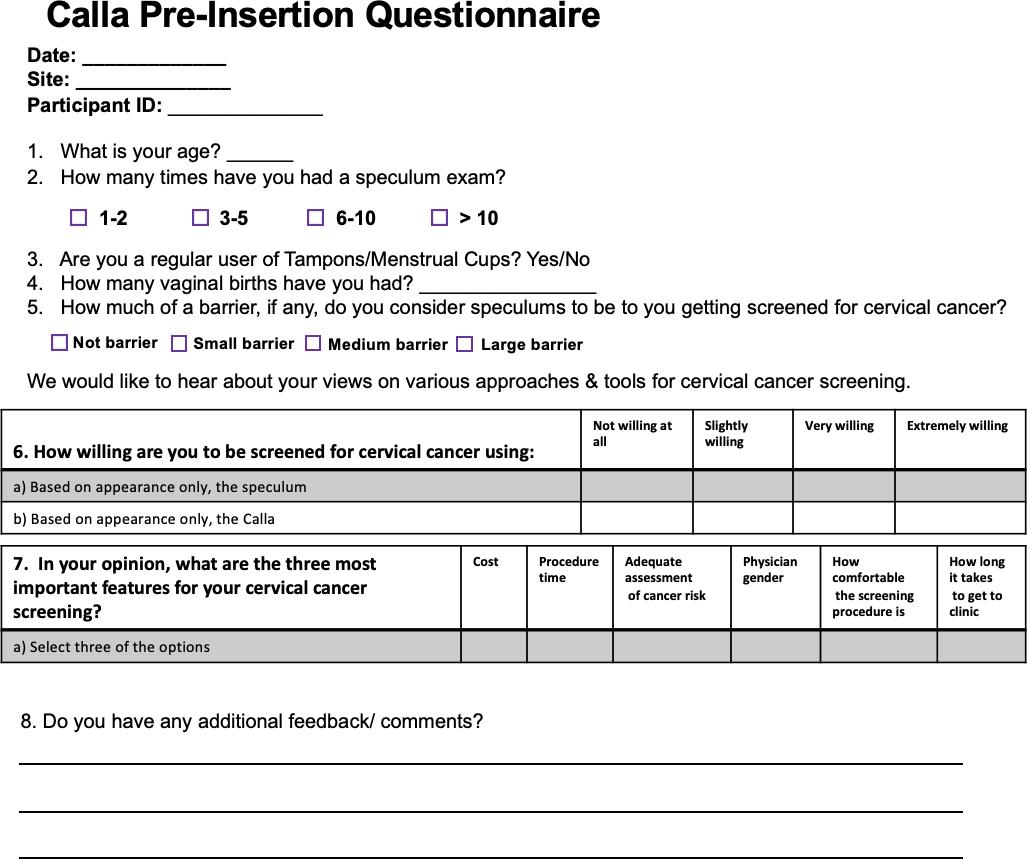
S2. Volunteer study questionnaires
S.2.1. Pre-insertion survey
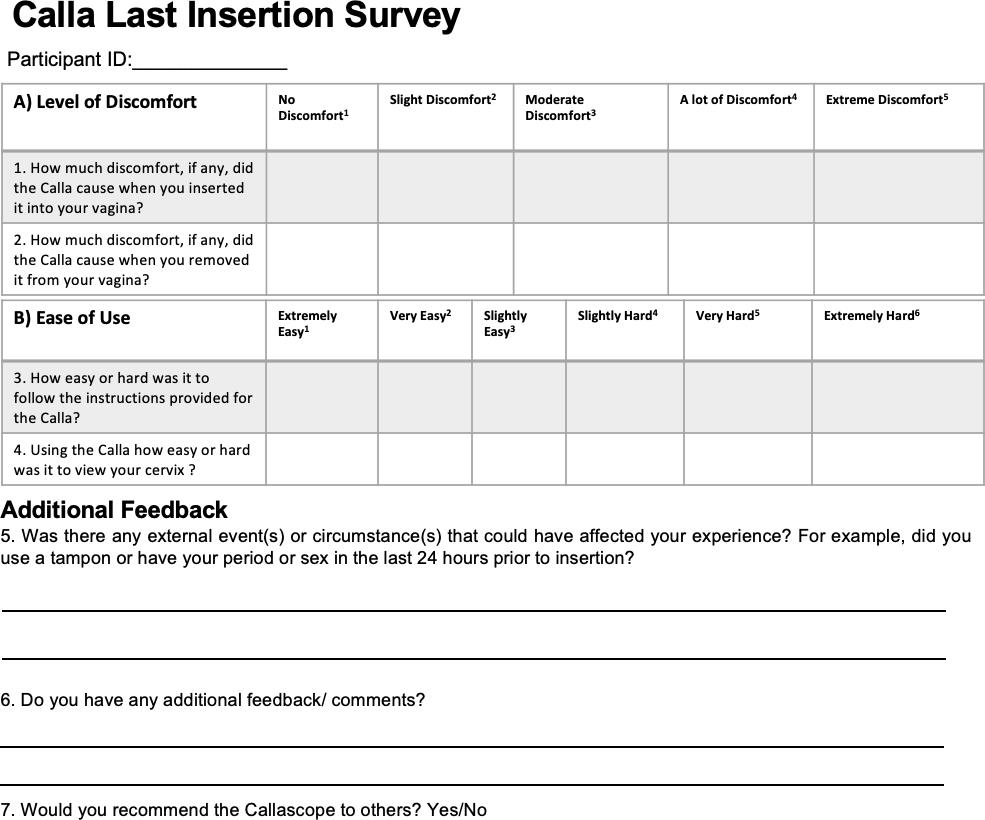
S.2.2. Post-insertion surveys (first and last attempt)
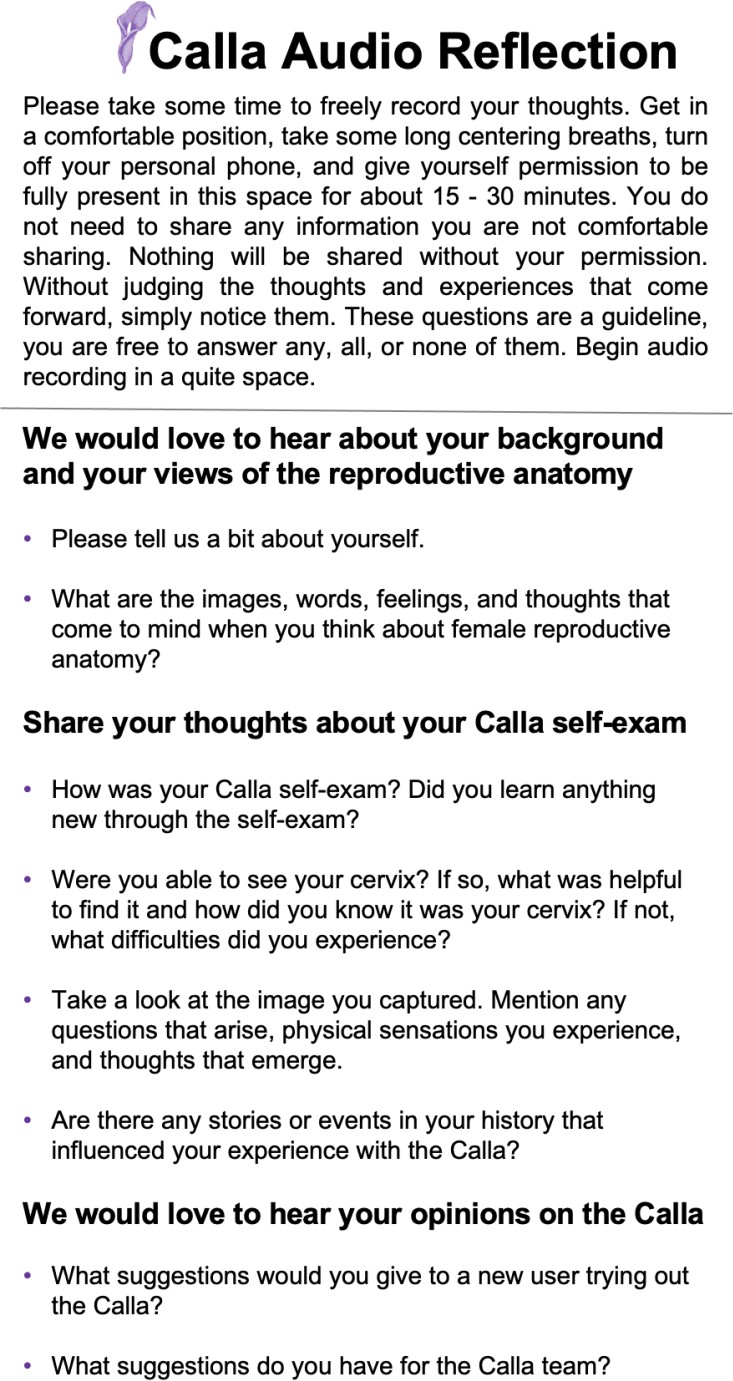
S.2.3. Audio reflection survey

S3. Volunteer study tutorial
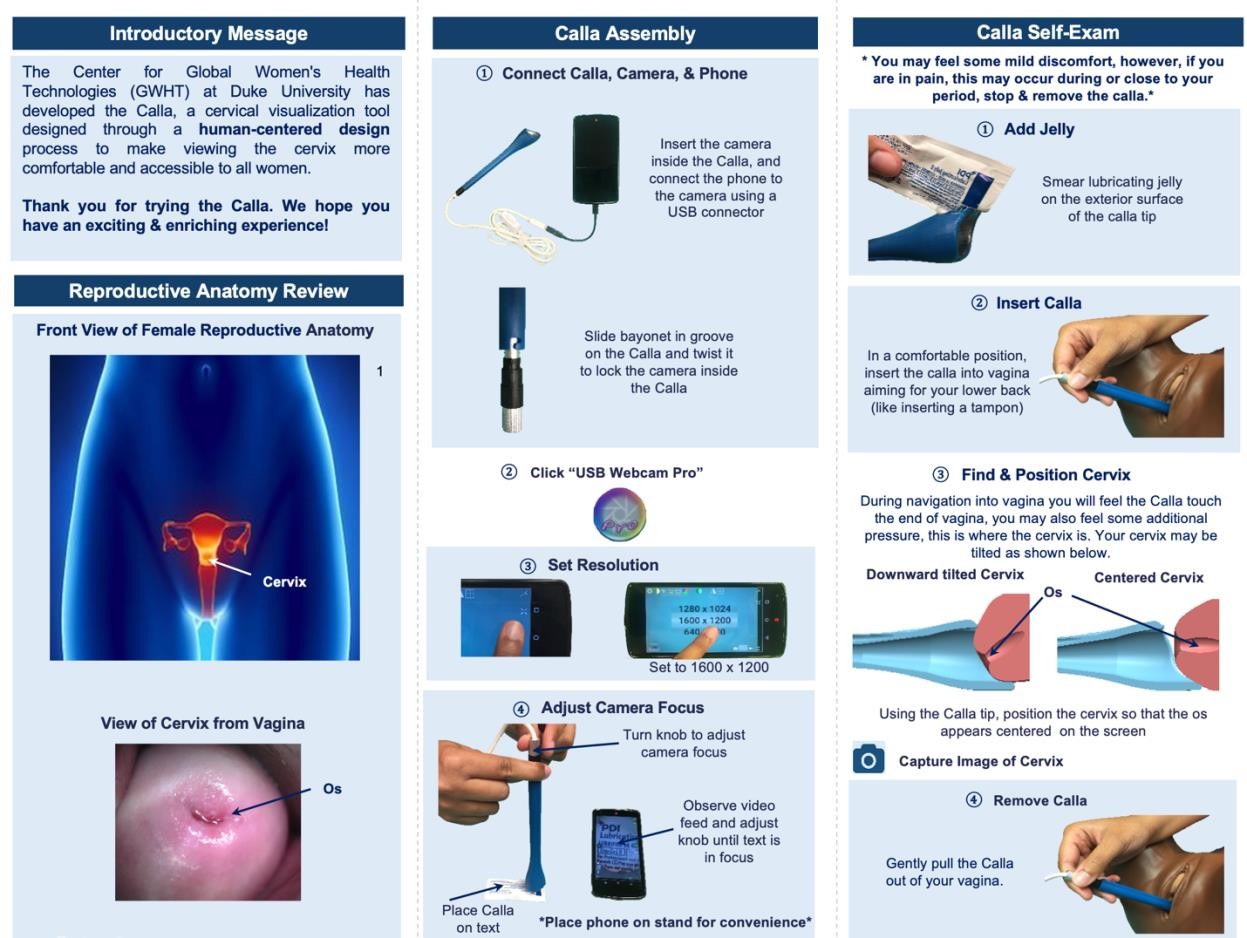
S.3.1. Paper tutorial (trifold – back and front)
- Moyer VA (2012) Screening for Cervical Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 156: 880.
- Singh RH, Erbelding EJ, Zenilman JM, Ghanem KG (2007) The role of speculum and bimanual examinations when evaluating attendees at a sexually transmitted diseases clinic. Sex Transm Infect 83: 206-10.
- Brown CL, Ludwiczak MH, Blanco JD, Hirsch CE (1993) Cervical dilation: accuracy of visual and digital examinations. Obstet Gynecol 81: 215-6.
- Insertion and Removal of Intrauterine Devices - American Family Physician. 2021.
- Sachs CJ, Ladd M, Chapman J (2021) Sexual Assault History and Physical. In: StatPearls. StatPearls.
- Hoyo C, Yarnall KSH, Skinner CS, Moorman PG, Sellers D, et al. (2005) Pain predicts non-adherence to pap smear screening among middle-aged African American women. Prev Med 41: 439-45.
- Small W, Bacon MA, Bajaj A (2017) Cervical cancer: A global health crisis. Cancer 123: 2404-12.
- Hoyo C, Yarnall KSH, Skinner CS, Moorman PG, Sellers D, et al. (2005) Pain predicts non-adherence to pap smear screening among middle-aged African American women. Prev Med 41: 439-45.
- Maguire RL, Vidal AC, Murphy SK, Hoyo C (2017) Chapter Six - Disparities in Cervical Cancer Incidence and Mortality: Can Epigenetics Contribute to Eliminating Disparities? In: Ford ME, Watson DK, eds. Advances in Cancer Research. Vol 133. Cancer Disparities. Academic Press 2017: 129-56.
- Musa J, Achenbach CJ, O’Dwyer LC (2017) Effect of cervical cancer education and provider recommendation for screening on screening rates: A systematic review and meta-analysis. PloS One 12: e0183924.
- Jayant K, Rao RS, Nene BM, Dale PS (1995) Improved stage at diagnosis of cervical cancer with increased cancer awareness in a rural Indian population. Int J Cancer 63: 161-3.
- Suarez L, Roche RA, Nichols D, Simpson DM (1997) Knowledge, behavior, and fears concerning breast and cervical cancer among older low-income Mexican-American women. Am J Prev Med 13: 137-42.
- Patra S, Upadhyay M, Chhabra P (2017) Awareness of cervical cancer and willingness to participate in screening program: Public health policy implications. J Cancer Res Ther 13: 318-23.
- Eze JN, Umeora OU, Obuna JA, Egwuatu VE, Ejikeme BN (2012) Cervical cancer awareness and cervical screening uptake at the Mater Misericordiae Hospital, Afikpo, Southeast Nigeria. Ann Afr Med 11: 238-43.
- Coronado Interis E, Anakwenze CP, Aung M, Jolly PE (2015) Increasing Cervical Cancer Awareness and Screening in Jamaica: Effectiveness of a Theory-Based Educational Intervention. Int J Environ Res Public Health 13: ijerph13010053.
- Asiedu MN, Agudogo J, Krieger MS (2017) Design and preliminary analysis of a vaginal inserter for speculum-free cervical cancer screening. PloS One 12: e0177782.
- Asiedu MN, Agudogo JS, Dotson ME (2020) A novel speculum-free imaging strategy for visualization of the internal female lower reproductive system. Sci Rep 10: 16570.
- Concepcion DB (2008) The environmental aspects of infection control in the dialysis clinic. Nephrol News Issues 22: 39-41.
- Health C for D and R. FDA-Cleared Sterilants and High Level Disinfectants with General Claims for Processing Reusable Medical and Dental Devices. FDA. Published online 2021.
- Schreier M (2014) Qualitative Content Analysis. In: The SAGE Handbook of Qualitative Data Analysis. SAGE Publications Ltd 2014: 170-83.
- Randomized Intervention of Self-Collected Sampling for Human Papillomavirus Testing in Under-Screened Rural Women: Uptake of Screening and Acceptability. J Women’s Health. 2021.
- Self-test device for cytology and HPV testing in rural Appalachian women: an evaluation - PubMed. Accessed June 15, 2021.
- Soisson AP, Reed E, Brown P (2008) Self-test device for cytology and HPV testing in rural Appalachian women: an evaluation. J Reprod Med 53: 441-8.
- Employment, Income, And Migration in Appalachia: A Spatial Simultaneous Equations Approach*-Gebremariam-2011- J Regional Sci 2021.
- Östensson E, Alder S, Elfström KM (2015) Barriers to and facilitators of compliance with clinic-based cervical cancer screening: population-based cohort study of women aged 23-60 years. PloS One 10: e0128270.
- Östensson E, Alder S, Elfström KM (2015) Barriers to and Facilitators of Compliance with Clinic-Based Cervical Cancer Screening: Population-Based Cohort Study of Women Aged 23-60 Years. PLOS ONE 10: e0128270.
- Miller MJ (2020) Impact of COVID-19 on Cervical Cancer Screening Rates Among Women Aged 21–65 Years in a Large Integrated Health Care System — Southern California, January 1–September 30, 2019, and January 1–September 30, 2020. MMWR Morb Mortal Wkly Rep 2021: 70.
- Paz-Zulueta M, Álvarez-Paredes L, Rodríguez Díaz JC (2018) Prevalence of high-risk HPV genotypes, categorised by their quadrivalent and nine-valent HPV vaccination coverage, and the genotype association with high-grade lesions. BMC Cancer 18: 112.
- Byrd TL, Chavez R, Wilson KM (2007) Barriers and facilitators of cervical cancer screening among Hispanic women. Ethn Dis 17: 129-34.
- Wang JH, Sheppard VB, Schwartz MD, Liang W, Mandelblatt JS (2008) Disparities in cervical cancer screening between Asian American and Non-Hispanic white women. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 17: 1968-73.
- Diamant AL, Wold C, Spritzer K, Gelberg L (2000) Health behaviors, health status, and access to and use of health care: a population-based study of lesbian, bisexual, and heterosexual women. Arch Fam Med 9: 1043-51.
- Millstein SG, Adler NE, Irwin CE (1984) Sources of anxiety about pelvic examinations among adolescent females. J Adolesc Health Care Off Publ Soc Adolesc Med 5: 105-11.
- Wee CC, McCarthy EP, Davis RB, Phillips RS (2000) Screening for cervical and breast cancer: is obesity an unrecognized barrier to preventive care? Ann Intern Med 132: 697-704.
- Farley M, Golding JM, Minkoff JR (2002) Is a history of trauma associated with a reduced likelihood of cervical cancer screening? J Fam Pract 51: 827-31.
- Iezzoni LI, McCarthy EP, Davis RB, Harris-David L, O’Day B (2001) Use of screening and preventive services among women with disabilities. Am J Med Qual Off J Am Coll Med Qual 16: 135-44.
- Bergmark K, Avall-Lundqvist E, Dickman PW, Henningsohn L, Steineck G (1999) Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med 340: 1383-9.
- Bachmann G, Lobo RA, Gut R, Nachtigall L, Notelovitz M (2008) Efficacy of low-dose estradiol vaginal tablets in the treatment of atrophic vaginitis: a randomized controlled trial. Obstet Gynecol 111: 67-76.
- Badura AS, Reiter RC, Altmaier EM, Rhomberg A, Elas D (1997) Dissociation, somatization, substance abuse, and coping in women with chronic pelvic pain. Obstet Gynecol 90: 405-10.
- Wright D, Fenwick J, Stephenson P, Monterosso L (2005) Speculum ‘self-insertion’: a pilot study. J Clin Nurs 14: 1098-111.
- Lyimo FS, Beran TN (2012) Demographic, knowledge, attitudinal, and accessibility factors associated with uptake of cervical cancer screening among women in a rural district of Tanzania: three public policy implications. BMC Public Health 12: 22.
- Prevalence of Genital Human Papillomavirus Among Females in the United States, the National Health and Nutrition Examination Survey, 2003–2006 | The J Infectious Diseases | Oxford Academic 2021.
- Ho GYF, Burk RD, Klein S (1995) Persistent Genital Human Papillomavirus Infection as a Risk Factor for Persistent Cervical Dysplasia. JNCI J Natl Cancer Inst 87: 1365-71.
- Molano M, Van den Brule A, Plummer M (2003) Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol 158: 486-94.
- Kuehn BM (2021) Dramatic Cervical Cancer Screening Decline During Pandemic. JAMA 325: 925-5.
- Ivanuš U, Jerman T, Oblak UG (2021) The impact of the COVID-19 pandemic on organised cervical cancer screening: The first results of the Slovenian cervical screening programme and registry. Lancet Reg Health – Eur 2021: 5.
- Lima MA de, Nascimento JC do, Silva ABR da, Barros LM, Pagliuca LMF, et al. (2018) Evaluation of the self- eye examination method for health promotion. Rev Esc Enferm USP 52: e03340.
- Asgar Pour H, Kunter D, Norouzzadeh R, Heidari MR (2018) The Effect of Testicular Self-Examination Education on Knowledge, Performance, and Health Beliefs of Turkish Men. J Cancer Educ 33: 398-403.
- Beautiful Cervix Project 2012.
- Asiedu, Mercy Nyamewaa, Anish Simhal, Usamah Chaudhary, Jenna L. et al. (2018) Development of algorithms for automated detection of cervical pre-cancers with a low-cost, point-of-care, pocket colposcope. IEEE Transactions on Biomedical Engineering 8: 2306-18.

Tables at a glance
Figures at a glance