Efficacy of a Dietary Supplement Containing Choline, Glutathione, Selenium, zinc, L. rhamnosus and B. lactis on Short-term Steatosis and Liver Function: A Pilot Study
Received Date: March 12, 2025 Accepted Date: April 12, 2025 Published Date: April 15, 2025
doi:10.17303/aglp.2025.2.101
Citation: Guerriero Ciro, De Luca Massimo (2025) Efficacy of a Dietary Supplement Containing Choline, Glutathione, Selenium, zinc, L. rhamnosus and B. lactis on Short-term Steatosis and Liver Function: A Pilot Study. Ann Gastroenterol Liver Pancreatic Dis 2: 1-12
Abstract
Background: The incidence of metabolic dysfunction-associated steatotic liver disease (MASLD) has steadily increased across the globe, affecting more than a third of adult population, driven by the raised rates of obesity and metabolic syndrome. Treatment options are limited; thus, our aim is to evaluate a valid and effective approach for the management of MASLD.
Methods: Fifty-two adults with MASLD were enrolled in this controlled pilot study. The participants were randomly assigned to the consumption of a food supplement containing choline, glutathione, selenium, zinc, and 2 probiotic strains once a day along with a balanced diet for 4 months, or to a balanced diet for 4 months. The reduction of serum alanine aminotransferase (ALT) was set as primary outcome, and aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), homeostatic model assessment for insulin resistance (HOMA-IR) and the controlled attenuation parameter (CAP) were set as secondary outcomes of the study.
Results: After 4 months of intervention, the food supplement group had significantly lower ALT (46.04 ± 14.51 U/L vs. 60.91 ± 17.67 U/L, p < 0.05), AST (35.58 ± 6.11 U/L vs 43.83 ± 12.62 U/L, p < 0.05), and CAP (239.88 ± 32.65 dB/m vs. 261.83 ± 33.79 dB/m, p < 0.05), compared to the control group. Furthermore, improvement in the liver and metabolic profile following the food supplement intervention was observed, including FIB 4, liver stiffness, body mass index (BMI), fasting blood glucose, insulinemic and lipids parameters in the MASLD patient.
Conclusion: The results of the present study indicated that consumption of a food supplement containing choline, glutathione, selenium, zinc, and 2 probiotic strains once a day along with a balanced diet for 4 months, is a valid and effective approach in the management of MASLD.
Keywords: MASLD; Fat Liver; Liver Enzymes; Probiotics; Choline
Introduction
Liver disease now accounts for two million deaths per year worldwide. These numbers are expected to rise as obesity and metabolic diseases are increasing worldwide. Globally, metabolic dysfunction-associated steatotic liver disease (MASLD) is the most common chronic liver disease to date, affecting over 30% of the adult population[1-3]. MASLD is recognized as the hepatic manifestation of metabolic disorders, such as obesity, metabolic syndrome (MetS), insulin resistance (IR), dyslipidemia and type 2 diabetes (T2D) [4,5]. Indeed, excess caloric consumption and/or physical inactivity, which induce hyperglycemia, hyperinsulinemia, and elevated levels of proinflammatory factors, leads to insulin resistance and subsequently fatty liver disease [5]. Furthermore, lipotoxicity, oxidative stress and chronic inflammation play a crucial role in the exacerbation of MASLD into metabolic dysfunction-associated steatohepatitis (MASH) and eventually into cirrhosis of the liver [6]. A growing body of evidence indicates that gut dysbiosis may be involved in the progression to MASLD/MASH [7-9]. Gut microbiota alteration is characterized by bacteria overgrowth that cause an increased intestinal barrier permeability leading to bacterial translocation and lipopolysaccharide (LPS) leakage, which can cause endotoxemia and long-term damage to liver cells [9]. The chronic low grade inflammation triggered by LPS is known as a lipogenic factor involved in hepatic steatosis progression [9,10]. Furthermore, an increased endogenous ethanol production by the gut microbiota and impaired gut barrier may lead to an aberrant translocation in the portal and, eventually, systemic circulation, exacerbating the fatty liver condition [9,11]. The recognition and diagnosis of MASLD in primary care is often poorly understood and applied [11]. Thus, in order to improve the diagnosis and prognostication of chronic liver diseases it is important to improve the accessibility of liver assessments and provide accurate healthcare support [12]. To date, no therapeutic interventions are available for MASLD prevention and management, except for some OFF-label drugs [13]. Since there are no specific treatments currently available for MASLD, it is essential to explore effective alternatives that can help patients with fatty liver resolve the issue in a timely manner. Treating MASLD in its early stages is far less costly than managing advanced liver disease, which often requires hospitalizations, expensive treatments, or even liver transplantation [14]. Diet and physical activity are first-line approaches, even though a poor compliance of patients is often reported [15,16]. The lack of specific, targeted treatments for this condition highlights a significant gap in medical research. Despite the increasing prevalence of MASLD, the current therapeutic strategies remain limited and mainly focused on managing risk factors such as obesity, diabetes, and dyslipidemia, rather than addressing the root cause of fatty liver disease. This underscores the critical need for innovative approaches to effectively treat MASLD, particularly through the use of adjunctive therapies, such as food supplements [17]. A number of studies have investigated the potential beneficial effect of dietary antioxidants, such as glutathione on the management of oxidative stress in patients with MASLD [18-20]. Also choline, an essential element that is metabolized in the liver, has been shown to influence the liver function and in case of its deprivation hepatosteatosis and liver cell death may occur [21]. Since fatty liver is correlated with an alteration of the intestinal microbiota, the intervention in the gut flora with probiotics has been subject of current research in patients with MASLD [22]. However, substantial gaps remain in our understanding of their long-term efficacy, as well as the need to provide a reliable basis for clinical application.
Thus, the study aims to evaluate the efficacy of a dietary supplementation based on choline, glutathione, selenium, zinc, Lactobacillus rhamnosus and Bifidobacterium lactis on short-term steatosis and liver function. This research is crucial as it addresses the lack of clear, evidence-based recommendations for managing MASLD outside of conventional pharmacological treatments. By exploring the role of food supplements, we aim to identify viable, cost-effective alternatives that could be easily integrated into patient care, particularly for those who do not respond well to current treatments or prefer non-pharmacological approaches.
Patients and Methods
A single-centre, controlled, parallel-group observational pilot clinical study was conducted between October 2023 and May 2024. Patients were divided into the intervention and control groups. Inclusion criteria were: subjects with age ≥18 years old, movement alanine transaminase (ALT >40 U/L) liver indices, otherwise healthy individuals with MASLD, ultrasound diagnosis of steatosis using the controlled attenuation parameter (CAP>230 dB/m), overweight/obese individuals, able to understand and sign informed consent. Subjects with the following criteria were excluded from the study: body mass index (BMI) ≥ 35 kg/m2, alcohol intake, intake of drugs or hepatolytic substances, chronic liver, biliary and pancreatic diseases, concomitant initiation of targeted therapies, pregnancy or breastfeeding, history of allergy to ingredients contained in the investigational treatment (dietary supplement), malabsorption; history of dependence or abuse of medication, drugs or alcohol, eating disorders, heart disease; neoplastic diseases, genetic-metabolic diseases, rheumatological disease, chronic haematological diseases, neuropsychiatric or neurological diseases, current or in the week prior to enrolment use of prescription or over-the-counter drugs that may influence inflammatory levels (NSAIDs and others). Patients in the intervention group were asked to take one capsule of the food supplement containing 92.3 mg choline, 200 mg glutathione, 27.5 mcg selenium, 5 mg zinc, 2 billion Bifidobacterium lactis and 2 billion Lactobacillus Rhamnosus (Deltha Pharma, Rome, Italy) once a day, in combination with a balanced diet for 16 weeks. The control group was asked to follow a balanced diet for 16 weeks. During the study period, a physician examined each patient in two determination points, at baseline (T0) and after 4 months (T1). As ALT is considered a more specific biomarker for the liver than aspartate aminotransferase (AST), the primary outcome of this study was resolution of ALT elevation. Secondary outcomes were set as the reduction of intrahepatic lipid deposit with examination through the elastography using the Fibroscan® (Echosens, Paris, France) and changes on serum levels of liver enzymes including AST and gamma-glutamyl transferase (GGT), on lipid profile including total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and on fasting blood glucose (FBG), as well as homeostatic model assessment for insulin resistance (HOMA-IR). Subjects were all screened at baseline to assess enrolment according to inclusion criteria and informed consent. After enrolment, at baseline and 4 months post-treatment, a peripheral blood sampling and the hepatic elastography were performed to obtain all parameters set in the primary and secondary objectives. The sample size calculation was set up considering the primary endpoint, the ALT transaminases. The calculation was obtained from previous literature (Honda et al. 2017) with the assumption of a 25% change of ALT in the food supplement group. The inputs required power of at least 80% with type I error = 5%. The required sample size for each group was 23. Allowing for a potential 10% dropout, the required sample size was 26 participants per group. The study protocol was in accordance with the Declaration of Helsinki, and all patients gave informed consent to participate in the study.
Statistical Analysis
Descriptive statistics are presented as mean ± standard deviation for continuous data. All statistical analyses were conducted using IBM SPSS Statistics for Windows (Version 26.0. Armonk, NY) and statistical significance was set at p < .05. For biomarkers including liver enzymes and lipids, there were gender-specific reference ranges, however because of the small numbers included in this pilot study these values were combined for statistical analysis.
Results
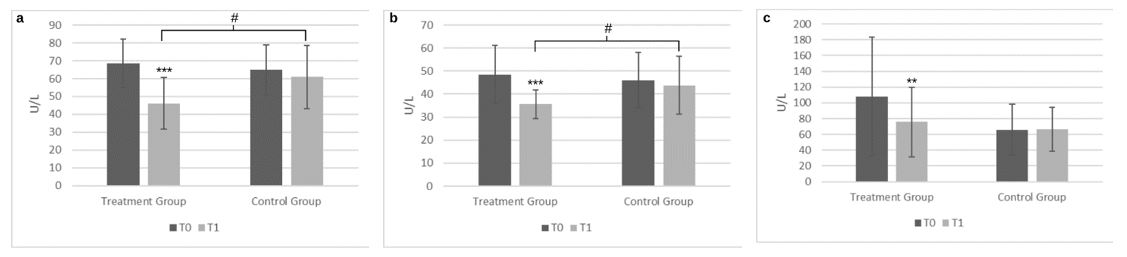
Fifty-two participants were included and 49 completed the study. The mean age was 54.18 ± 16.18 years old in the interventional group and 51.67 ± 12.27 years old in the control group. The baseline demographic, clinical and biochemistry characteristics are shown in Table 1. Following 16 weeks of intervention there was a statistically significant improvement of the ALT levels in the food supplement group representing approximately a 33% reduction, (68.54 ± 13.58 U/L to 46.04 ± 14.51 U/L, p < 0.0001). In comparison, there was a non-significant reduction in the diet group, (Figure 1a). There was a significant reduction (-28%) of AST levels following the food supplement intervention (48.38 ± 12.71 U/L to 35.58 ± 6.11 U/L, p < 0.0001) and a non-significant reduction in the diet group (46.00 ± 12.09 U/L to 43.83 ± 12.62 U/L), (Figure 1b). The differences at the end of the intervention were significant between-groups for ALT levels (p = 0.0022) and for AST levels (p = 0.0047). There was also a significant reduction (-30%) in GGT levels in the food supplement group (108.19 ± 74.98 U/L to 75.73 ± 44.00 U/L, p = 0.0002), and a non-significant reduction in the diet group (65.91 ± 32.23 U/L to 66.35 ± 27.97 U/L), (Figure 1c); however, the baseline values for GGT were notably higher in the food supplement group and the differences at the end of the intervention were not significant between-groups (p = 0.38). The CAP determined by Fibroscan was reduced significantly (-14%) in the food supplement group (278.96 ± 24.11 db/m to 239.88 ± 32.65 db/m, p < 0.0001), but not in the diet group (262.96 ± 33.85 db/m to 261.83 ± 33.79 db/m, p = 0.88), and the changes observed at T1 between-groups were significant (p= 0.02), (Figure 2). BMI was reduced significantly in both groups; 30.73 ± 3.76 Kg/m2 to 29.61 ± 3.61 Kg/m2, p = 0.0006, (-4%) in the food supplement group, and 28.67 ± 4.40 Kg/m2 to 28.07 ± 4.38 Kg/m2, p = 0.0044) (-2%) in the diet group, although there were no between-group differences. FIB 4 and stiffness were significantly reduced by 10% and 7%, respectively as shown in table 2. Fasting blood glucose and insulinemic changes were statistically significant following the food supplement intervention, however non-significant reductions were noted for HOMA-IR. Not significant changes were observed in the diet group for all 3 parameters (Table 2). The changes in the lipid’s parameters within both groups, from pre- to post-intervention are shown in table 2. In particular, low-density lipoprotein (LDL) levels were reduced significantly in the food supplement group (134.8 ± 25.8 mg/dL to 125.7 ± 24.0 mg/dL, p<0.05), while in the control group remained stable (126.20 ± 32.97 mg/dL to 126.89 ± 28.17 mg/dL). High-density lipoprotein (HDL) levels significantly increased in the food supplement group (51.54 ± 8.79 mg/dL to 56.27 ± 8.48 mg/dL, p<0.0001), and no changes were observed in the control group (54.43 ± 10.24 mg/dL to 54.65 ± 7.85 mg/dL). Regarding total cholesterol and triglyceride levels, no significant changes were observed in either group, as shown in Table 2.
Discussion
Obesity, and metabolic disorder rates are rising worldwide, accounting approximately 2 billion obese or overweight adults and over 400 million with diabetes; both of which are risk factors for MASLD and hepatocellular carcinoma [23,24]. Progression of the hepatic steatosis may lead to cirrhosis and hepatocellular carcinoma, and ultimately death [23]. The current study demonstrated that a food supplement based on choline, glutathione, selenium, zinc, and 2 probiotic strains reduces the liver fat content by 14% in obese subjects with MASLD. Furthermore, the food supplement achieved a reduction in transaminases levels of 33%, while the only diet had little impact on either transaminases and fat liver deposit. Our results are in line with previous animal and clinical studies where is shown that high intake of dietary choline may be correlated to a reduced risk of MASLD [25]. Indeed, it was shown that hepatosteatosis and liver cell death occur when humans are deprived of choline [21]. A study indicated that dietary choline had a lipid-lowering effect attenuating high fat diet-induced inflammation and alleviation of steatosis in an animal model, by modulating intrahepatic lipid metabolism, reducing lipid droplet accumulation and suppressing NFκB activation [26]. In previous clinical studies were found a correlation with choline deficiency and an increased fibrosis in postmenopausal women with MASLD [27], as well as in normal weight Chinese women was reported an inverse association between dietary choline intake and risk of MASLD [28]. According to these results, a number of studies suggested that a good intake of choline can attenuate the risk of visceral obesity-related hepatic steatosis [28,29]. Mitochondrial dysfunction not only promotes fat accumulation, but also leads to generation of reactive oxygen species (ROS) and lipid peroxidation, resulting in oxidative stress in hepatocytes [6]. Glutathione (GSH), is a tripeptide particularly concentrated in the liver, recognized as the most important thiol reducing agent involved in the modulation of redox processes. It represents one of the most commonly analysed redox-active molecules, as changes in its content contribute to the pathogenesis of many diseases. Oxidative stress contributes to the pathogenesis of many liver diseases, such as activation and fibrotic progression in MASLD and MASH; hence, supplementation of GSH demonstrated to be a valid approach for the management of varied hepatic conditions [19]. One multicentre pilot trial evaluated the efficacy of glutathione 300 mg/day for 4 months in patients presenting MASLD. A total of twenty-nine patients reported a significant reduction of ALT levels following treatment with glutathione for 4 months, as long as reduction of triglycerides, non-esterified fatty acids, and ferritin levels [18]. Selenium plays a role in protecting the body cells from damage, and as a cofactor for the production and the activity of glutathione, thus its precense augment glutathione antioxidant activity[30]. Oxidative stress is a pathophysiological hall-mark of metabolic liver disease, as is implicated in the course of inflammatory, metabolic and proliferative liver diseases [31]. High reactive oxygen species (ROS) levels are correlated with an impairment of intracellular GSH homeostasis, leading to a reduction in GSH levels, a diminution of its antioxidant hepato-protective activities [18,20,32-34]. Since GSH is involved in several functions, in primis the management of oxidative stress and maintenance of redox balance, cell cycle regulation, immune system modulation, and fibrogenesis [35], it might be useful to further explore its clinical use in MASLD [20,36]. Indeed, clinical evidences have shown the positive effect of GSH in reducing the transaminase levels in MASLD patients [18]. A number of studies have shown an inverse relationship between serum zinc level and FIB-4 index in MASLD [37-39]. Deficiency of this element, that is involved in the glucose, lipid, and protein metabolism, plays a critical role In the pathogenesis of MASLD [39].
Recent publications shown the correlation of an overgrowth of intestinal bacterial with an increased severity of steatosis [40,41]. The increased permeability appears to be caused by disruption of intercellular tight junctions in the intestine, and it may play an important role in the pathogenesis of hepatic fat deposition [41]. Furthermore, an intestinal bacterial overgrowth, elevated levels of endotoxins and inflammatory cytokines into the blood circulation were found in patients with MASH [42]. Many studies have shown the activity of probiotics in modulating the intestinal ecological disorders and the integrity of the intestinal mucosal barrier, thereby reducing the inflammatory response of the liver [43]. In a meta-analysis, a positive effect on reducing liver enzymes, lipid profiles, and inflammatory cytokines was observed in patients with MASLD, after consumptions of probiotics [44]. These results are mainly linked to an improvement in the intestinal bacteria homeostasis [45,46], permeability [47], inflammation [44], liver enzymes and lipid profile [44,48], thus promoting the gut-liver axis balance [49,50]. Selected probiotics have been studied and extensively analysed in their potential activity on the liver, such as Lactobacillus rhamnosus GG and Bifidobacterium animalis ssp. lactis. Lactobacillus rhamnosus GG (LGG) has shown to prevent alcoholic liver injury by decreasing the levels of lipopolysaccharide and tumour necrosis factor-α and prevent liver steatosis by suppressing triglyceride, free fatty acid, and malondialdehyde production in liver [51]. Bifidobacterium animalis ssp. lactis was found to improve fat deposition, fasting hyperinsulinemia, fatty acid synthase (FAS), sterol regulatory element-binding protein 1 (SREBP-1), carbohydrate-responsive element-binding protein (ChREBP) and inflammatory cytokine expression, thereby lowering the hepatic steatosis score [52].
Furthermore, supplementation with specific nutrients has demonstrated significant improvements in the metabolic profiles of patients with MASLD, aligning with our observed case. A randomized, double-blind, placebo-controlled trial assessed the impact of a micronutrient cocktail on liver parameters in adults with obesity and metabolic syndrome. The treatment group experienced notable reductions in both controlled attenuation parameter (CAP) and transient elastography (TE) scores, indicative of decreased liver fat content and fibrosis [53]. Similarly, a systematic review and meta-analysis evaluated vitamin E's effects on serum markers of liver inflammation and histology in MASLD patients. Vitamin E supplementation significantly reduced serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels, and improved histological features such as steatosis, lobular inflammation, and hepatocyte ballooning. However, it did not significantly impact liver fibrosis [54]. These studies collectively support the efficacy of targeted nutritional interventions in enhancing metabolic and hepatic health in MASLD patients. Our case report contributes to this growing body of evidence, highlighting the potential therapeutic benefits of dietary supplementation in managing MASLD.
All these scientific evidences encourage the use of nutraceuticals that are useful for the management of oxidant stress, lipid profile, insulinemic, and gut microbiota in MASLD subjects. Early diagnosis and intervention are the keys to manage this disease and preventing it from reaching advanced stages. Our study highlighted the efficacy of a food supplement based on choline, glutathione, selenium, zinc, Lactobacillus rhamnosus GG and Bifidobacterium animalis ssp. lactis in significantly reducing the transaminases and the intrahepatic lipid deposition in MASLD subjects. Furtheremore, ssupplementation with this dietary supplement led to an improvement in the metabolic profile of the patient affected by MASLD.
The study has some limitations, including the small sample size, despite it being powered on hepatic steatosis, the absence of a placebo group, and the short duration of the treatment. To address potential biases, efforts were made to ensure that patient selection was as representative as possible of the target population, and statistical adjustments were applied to minimize the impact of the small sample size. Furthermore, while the lack of a placebo group may limit the ability to draw definitive conclusions about treatment efficacy, the study design focused on providing preliminary evidence of the food supplement’s potential benefits. Future studies with larger sample sizes, a placebo-controlled design, and longer follow-up periods are needed to confirm these findings and reduce the risk of biases, particularly in relation to outcomes such as fat liver deposition.
Conclusion
In conclusion these findings show significant reductions of the liver enzymes levels, steatosis and metabolic parameters from baseline to post-intervention after 16 weeks of consumption of a food supplement based on choline, glutathione, selenium, zinc, Lactobacillus rhamnosus GG and Bifidobacterium animalis ssp. lactis.
In this case, dietary supplementation was associated with a marked improvement in the patient's metabolic profile. The MASLD patient showed favourable changes in key biochemical parameters following the introduction of the supplement. Improvements were observed in liver enzyme levels, intra-hepatic lipid deposit, insulin sensitivity, and lipid profile, suggesting a potential therapeutic benefit of the supplement in the management of MASLD. These findings support the growing body of evidence highlighting the role of targeted nutritional interventions in metabolic associated liver diseases.
- A Mantovani, E Scorletti, A Mosca, A Alisi, CD Byrne, G. Targher (2020) Complications, morbidity and mortality of nonalcoholic fatty liver disease, Metabolism, 111: 154170.
- T Marjot, A Moolla, JF Cobbold, L Hodson, JW Tomlinson (2020) Nonalcoholic Fatty Liver Disease in Adults: Current Concepts in Etiology, Outcomes, and Management, Endocr. Rev., vol. 41, fasc. 1: 66-117.
- L Miao, G Targher, CD Byrne, YY Cao, MH Zheng (2024) Current status and future trends of the global burden of MASLD, Trends Endocrinol. Metab., 35: 697-707.
- AF Godoy-Matos, WS Silva Júnior, CM Valerio (2020) NAFLD as a continuum: from obesity to metabolic syndrome and diabetes, Diabetol. Metab. Syndr. 12: 60.
- E Buzzetti, M Pinzani, EA Tsochatzis (2016) The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD)», Metabolism, 65: 1038-48.
- L Xu, N Nagata, T Ota (2019) Impact of glucoraphanin-mediated activation of Nrf2 on non-alcoholic fatty liver disease with a focus on mitochondrial dysfunction, Int. J. Mol. Sci., 20: 5920.
- L Zhu, RD Baker, SS Baker (2015) Gut microbiome and nonalcoholic fatty liver diseases, Pediatr. Res., 77: 245–51.
- Y Ji, Y Yin, Z Li, W Zhang (2019) Gut microbiota-derived components and metabolites in the progression of non-alcoholic fatty liver disease (NAFLD), Nutrients, 11: 1712.
- H Hu, et al. (2020) Intestinal microbiome and NAFLD: Molecular insights and therapeutic perspectives, J. Gastroenterol., 55: 142–58.
- Q Li, et al. (2024) DNMT3B alleviates liver steatosis induced by chronic low-grade LPS via inhibiting CIDEA expression, Cell. Mol. Gastroenterol. Hepatol., 17: 59–77.
- C Gofton, Y Upendran, MH Zheng, J George (2023) MAFLD: How is it different from NAFLD?, Clin. Mol. Hepatol., 29: S17–31.
- H Wazir, et al. (2023) Diagnosis and treatment of liver disease: Current trends and future directions, Cureus.
- AISF, SID, SIO (2021) Steatosi epatica non alcolica 2021. Linee guida per la pratica clinica dell’Associazione Italiana per lo Studio del Fegato (AISF), della Società Italiana di Diabetologia (SID) e della Società Italiana dell’Obesità (SIO).
- W Eskridge, et al. (2023) Metabolic dysfunction-associated steatotic liver disease and metabolic dysfunction-associated steatohepatitis: The patient and physician perspective, J. Clin. Med., 12: 6216.
- ZM Younossi, S Zelber-Sagi, L Henry, LH Gerber (2023) Lifestyle interventions in nonalcoholic fatty liver disease, Nat. Rev. Gastroenterol. Hepatol., 20: 708–22.
- ZM Younossi, KE Corey, JK Lim (2021) AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: Expert review, Gastroenterology, 160: 912–8.
- L Wang, P Zhang, H Yan (2023) Functional foods and dietary supplements in the management of non-alcoholic fatty liver disease: A systematic review and meta-analysis, Front. Nutr., 10: 1014010.
- Y Honda, et al. (2017) Efficacy of glutathione for the treatment of nonalcoholic fatty liver disease: An open-label, single-arm, multicenter, pilot study, BMC Gastroenterol., 17: 96.
- M Vairetti, LG Di Pasqua, M Cagna, P Richelmi, A Ferrigno, C Berardo (2021) Changes in glutathione content in liver diseases: An update, Antioxidants, 10: 364.
- M Irie, T Sohda, A Anan, A Fukunaga, K Takata, T Tanaka (2016) Reduced glutathione suppresses oxidative stress in nonalcoholic fatty liver disease, Euroasian J. Hepato-Gastroenterol., 6: 13–8.
- Corbin KD, Zeisel SH (2012) Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr Opin Gastroenterol. 28: 159–65.
- Loman BR, Hernández-Saavedra D, An R, Rector RS (2018) Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutr Rev. 76: 822–39.
- Asrani SK, Devarbhavi H, Eaton J, Kamath PS (2019) Burden of liver diseases in the world. J Hepatol. 70: 151–71.
- Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS (2023) Global burden of liver disease: 2023 update. J Hepatol. 79: 516–37.
- Chai C, Chen L, Deng M-G, Liang Y, Liu F, Nie JQ (2023) Dietary choline intake and non-alcoholic fatty liver disease (NAFLD) in U.S. adults: National Health and Nutrition Examination Survey (NHANES) 2017–2018. Eur J Clin Nutr. 77: 1160–6.
- Jin M, Li X, Li J, Xue M, Liang H, Wu R, et al. (2019) Dietary choline supplementation attenuated high-fat diet-induced inflammation through regulation of lipid metabolism and suppression of NFκB activation in juvenile black seabream (Acanthopagrus schlegelii). J Nutr Sci. 8: e38.
- Guerrerio AL, Colvin RM, Schwartz AK, Molleston JP, Murray KF, Torbenson MS, et al. (2012) Choline intake in a large cohort of patients with nonalcoholic fatty liver disease. Am J Clin Nutr. 95: 892–900.
- Yu D, Yin L, Zhang J, Lyu Z, Gao X, Li Y, et al. (2014) Higher dietary choline intake is associated with lower risk of nonalcoholic fatty liver in normal-weight Chinese women. J Nutr. 144: 2034–40.
- Gao X, Randell E, Zhou H, Sun G (2016) Higher dietary choline and betaine intakes are associated with better body composition in the adult population of Newfoundland, Canada. PLoS One. 11: e0155403.
- Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, et al. (2018) Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 172: 409–22.e21.
- Cichoż-Lach H (2014) Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol. 20: 8082–91.
- Bresci G, Piccinocchi M, Banti S (1991) The use of reduced glutathione in alcoholic hepatopathy. Minerva Med. 82: 753–5.
- Scotto G, Tantimonaco G, Dentico P, Fazio V, Buongiorno R (1996) Ultrasound monitoring of alcoholic liver steatosis during glutathione (GSH) treatment. Minerva Gastroenterol Dietol. 42: 195–200.
- Sacco R, Eggenhoffner R, Giacomelli L (2016) Glutathione in the treatment of liver diseases: insights from clinical practice. Minerva Gastroenterol Dietol. 62: 316–24.
- Pizzorno J (2014) Glutathione Integr Med (Encinitas). 13: 8–12.
- Santacroce G, Gentile A, Soriano S, Novelli A, Lenti MV, Di Sabatino A (2023) Glutathione: pharmacological aspects and implications for clinical use in non-alcoholic fatty liver disease. Front Med. 10: 1124275.
- Kim MC, Lee HJ, Lee YJ, Park HS, Shin CM, Kim N (2020) Serum zinc level and hepatic fibrosis in patients with nonalcoholic fatty liver disease. PLoS One. 15: e0240195.
- Chen SD, Chen CC, Tsai NW, Lin TK, Chang CC, Huang CR, et al. (2022) J-shaped relationship between serum zinc levels and the severity of hepatic necro-inflammation in patients with MAFLD. Nutr Metab Cardiovasc Dis. 32: 1259–65.
- Barbara M, Mindikoglu AL (2021) The role of zinc in the prevention and treatment of nonalcoholic fatty liver disease. Metab Open. 11: 100105.
- Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, et al. (2016) The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 63: 764–75.
- Miele L, Valenza V, La Torre G, Montalto M, Cammarota G, Ricci R, et al. (2009) Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 49: 1877–87.
- Wigg AJ, Roberts-Thomson IC, Dymock RB, McCarthy PJ, Grose RH, Cummins AG (2001) The role of small intestinal bacterial overgrowth, intestinal permeability, endotoxaemia, and tumour necrosis factor alpha in the pathogenesis of non-alcoholic steatohepatitis. Gut. 48: 206–11.
- Cao C, Shi M, Wang X, Yao Y, Zeng R (2023) Effects of probiotics on non-alcoholic fatty liver disease: a review of human clinical trials. Front Nutr. 10: 1155306.
- Pan Y, Yang Y, Wu J, Zhou H, Yang C (2024) Efficacy of probiotics, prebiotics, and synbiotics on liver enzymes, lipid profiles, and inflammation in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. BMC Gastroenterol. 24: 283.
- Van Baarlen P, Wells JM, Kleerebezem M (2013) Regulation of intestinal homeostasis and immunity with probiotic lactobacilli. Trends Immunol. 34: 208-15.
- Chandrasekaran P, Weiskirchen S, Weiskirchen R (2024) Effects of probiotics on gut microbiota: an overview. Int J Mol Sci. 25: 6022.
- Kim M-J, Lee YJ, Hussain Z, Park H (2024) Effect of probiotics on improving intestinal mucosal permeability and inflammation after surgery. Gut Liver.
- Behrouz V, Aryaeian N, Zahedi MJ, Jazayeri S (2020) Effects of probiotic and prebiotic supplementation on metabolic parameters, liver aminotransferases, and systemic inflammation in nonalcoholic fatty liver disease: a randomized clinical trial. J Food Sci. 85: 3611–17.
- Paolella G (2014) Gut-liver axis and probiotics: their role in non-alcoholic fatty liver disease. World J Gastroenterol. 20: 15518.
- C Mandato, AP Delli Bovi, P Vajro (2021) The gut-liver axis as a target of liver disease management, Hepatobiliary Surg. Nutr., 10: 100–2.
- Z Gu et al. (2020) Lactobacillus rhamnosus Granules Dose-Dependently Balance Intestinal Microbiome Disorders and Ameliorate Chronic Alcohol-Induced Liver Injury, J. Med. Food, 23: 114–24.
- MH Do, MJ Oh, HB Lee, CH Kang, G Yoo, HY Park (1965) Bifidobacterium animalis ssp. lactis MG741 Reduces Body Weight and Ameliorates Nonalcoholic Fatty Liver Disease via Improving the Gut Permeability and Amelioration of Inflammatory Cytokines, Nutrients, 14: 9.
- I Perva et al. (2024) Use of a Micronutrient Cocktail to Improve Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD) in Adults with Obesity: A Randomized, Double-Blinded Pilot Clinical Trial, Medicina (Mex.), 60: 1366.
- NM Chee, RP Sinnanaidu, W Chan (2024) Vitamin E improves serum markers and histology in adults with metabolic dysfunction‐associated steatotic liver disease: Systematic review and meta‐analysis, J. Gastroenterol. Hepatol., 39: 2545-54.

Tables at a glance
Figures at a glance