Human β-Defensins in Colonic Mucosa of Patients with Irritable Bowel Syndrome, Inflammatory Bowel Disease and Healthy Controls – A Pilot Study
Received Date: December 17, 2022 Accepted Date: January 17, 2023 Published Date: January 21, 2023
doi: 10.17303/aglp.2021.3.101
Citation: Ghazaleh Mohammadian, Aldona Dlugosz, Greger Lindberg (2023) Human β-Defensins in Colonic Mucosa of Patients with Irritable Bowel Syndrome, Inflammatory Bowel Disease and Healthy Controls – A Pilot Study. J Ann Gastroenterol Liver Pancreatic Dis 3: 1-9
Abstract
Defensins are antimicrobial peptides involved in the innate immunity. They reside in the epithelial linings of many organs.In the gastrointestinal tract, both human beta- defensin type 1 and 2 (HBD1 and HBD2) have roles in the pathogenesis of inflammation and HBD2 mRNA expression is increased in patients with inflammatory bowel disease (IBD). For patients with irritable bowel syndrome (IBS) low grade inflammation has been suggested as a pathogenetic mechanism. Previously increase of fecal HBD2 has been described in patients with IBS but the peptide level in the epithelial mucosa has not been described. The aim of this pilot study was to identify and compare HBD2 in colonic mucosa of healthy volunteers and patients with IBS, using IBD patients as positive controls.
Methods: Endoscopic sigmoid biopsies from patients with IBS (n=13), IBD (n=15) and healthy controls (n=14) were formalin fixated and paraffin embedded. Sections were stained for HBD2 with immunohistochemistry. HBD2 positivity in the surface epithelium and crypt was calculated as percent of surface area with the NIS elements Image Analysis program.
Results and conclusion: HBD2 expression in the colonic mucosa of patients with IBS and IBD was significantly lower compared to healthy volunteers. The results are interesting since HBD2 expression has not been analyzed in this way before and mRNA HBD2 expression in previous studies have been increased in patients with inflammation. These results may indicate that even HBD2 may be involved in the innate immunity even without inflammation, but maybe not activated until an inflammation occurs, hence not detected mRNA. Further studies will be needed for confirmation.
Keywords: Irritable Bowel Syndrome; Human Defensins; Inflammatory Bowel Disease; Innate Immunity
Introduction
The pathogenesis of irritable bowel syndrome is still unknown and probably multifactorial. One of the factors that could be affected in IBS is the family of defensins [1]. Defensins are small antimicrobial peptides involved in the innate immunity [2] and divided in two families, alfa and beta, based on the position of the cystein residues and the disulfide bond-pairing pattern. Defensins are believed to form pores in the membranes of microbes, leading to cell lysis. ([3] and reviewed in [4, 5]). Two of six alfa-defensins are enteric in human (HD-5, -6) and expressed in small intestinal Paneth cells. There are four types of human betadefensins (HBD) where type 1 and 2 can be found through the whole GI-tract [6, 7]. HBD2 is produced in the epithelial cell lining for instance in skin or colon. HBD2 has been described to have a role in mucosal healing and have mucosal protecting properties [8]. HBD2 and HBD3 has also been described to be involved mainly in inflammation, in particular in patients with inflammatory bowel disease (IBD) [9-11]. It has been described that HBD2 expression is induced via toll like receptors (TLR) after stimulation by bacterial lipopolysaccharide or fungal infections, hence working as a barrier to entry of pathogens [12].
Different mechanisms have been suggested for the pathogenesis of IBD. Mutations in the nucleotide-binding oligomerization domain 2 (NOD2), a pattern recognition receptor, reduce production of HD5 and 6 [13] and have been suggested to have a role in the upcoming of Crohns disease (CD) [14,15]. For ulcerative colitis (UC), upregulation of defensins, including HBD2, have been studied, suggesting that HBD2 has a role in the inflammatory process in colon [16]. In gastric mucosa increase levels of HBD2 have been seen in gastritis due to Helicobacter pylori [17].
The knowledge of the HBD’s role in gastrointestinal disease is limited, and even less is known in IBS. Two studies on IBS and HBD have suggested an increase of the mRNA levels of patients with IBS and increased fecal levels of HBD2 [1]. However, the knowledge about the peptide expression in the gut mucosa is still unknown. The aim of this pilot study was to identify and compare HBD2 in colonic mucosa of healthy volunteers and patients with IBS. Sigmoid biopsies from IBD patients were taken as positive controls. Our hypothesis was that HBD2 expression in the colonic mucosa would be increased in patients with IBS compared to healthy controls and that IBD patients would have a higher expression compared to both IBS and healthy controls.
Materials and Methods
Participants
Thirteen patients (11 women) with IBS according to Rome-III (5 with IBS-C, 7 with IBS-D, 1 with IBS-M) were consecutively included for analysis. Median age at biopsy was 35 (range 19-65) years. The participants were included year 2011, hence why Rome-III was used for the IBS diagnosis.
Fifteen patients with active IBD, meaning having a light to moderate flair, (8 with UC, 7 with CD) were included as positive controls. Median age at biopsy for CD (4 women) was 42 (range 22-58) years. Median age at biopsy for UC (4 women) was 44 (range 26-60) years. 6 out of 8 patients with UC and all patients with CD had macroscopic and microscopic light inflammation. Three of these patients had no active treatment but three patients with ulcerative colitis had corticosteroids, one had azathioprine and one had treatment with 5-aminosalicylic acid. Among the CD patients one had budesonide, two were treated with infliximab, two with 5-aminosalicylic acid, one with metronidazole, and one with azathioprine.
Fourteen healthy volunteers (11 women; median age 30 (range 22-60) years) without any gastrointestinal symptoms were included as negative controls.
Exclusion criteria of patients were pregnancy.
Mucosal biopsies
Immunohistochemistry
The biopsies were sectioned into 4 μm sections in the Department of Pathology. The slides were rehydrated and antigen retrieval was done with citrate buffer pH 6.0 and blocked for peroxidase with Avidin and Biotin. Primary antibody was rabbit polyclonal antibody against beta-2–defensin (Abcam, Cambridge, UK.), incubated for 1.5 hours in room temperature. Secondary antibody was goat anti-rabbit biotin conjugated according to standard protocols. Hematoxylin counterstaining was done.
Analysis method
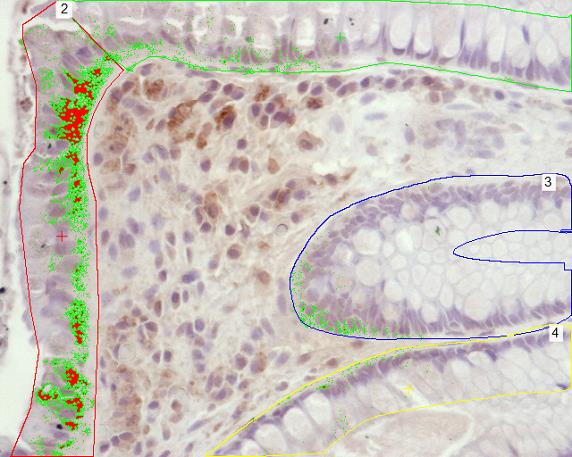
All samples were coded prior to staining and analysis. The slides were analyzed under light microscopy (Nikon Eclipse E1000, Tokyo, Japan) in a blinded fashion and the area of HBD2 was calculated with an image analysis program from NIS-elements (Nikon, Japan) by calculating fraction of the area where HBD2 was positive in surface epithelium and crypt epithelium. The area of the epithelia was calculated with the help of the image analysis program by marking it. The area of the positive staining was identified with the help of the image analysis program as seen in Figure 1.
The results are presented as medians and ranges since they did not follow a normal distribution. Mann- Whitney U-test was used for group comparisons. P-value was set at <0.05.
Ethical considerations
The Regional Ethical Review Board in Stockholm approved the study, 2007/1151-31/2. Investigation follows the principles outlined in the Declaration of Helsinki. All participants gave oral and written consent to participate in the study.
Results
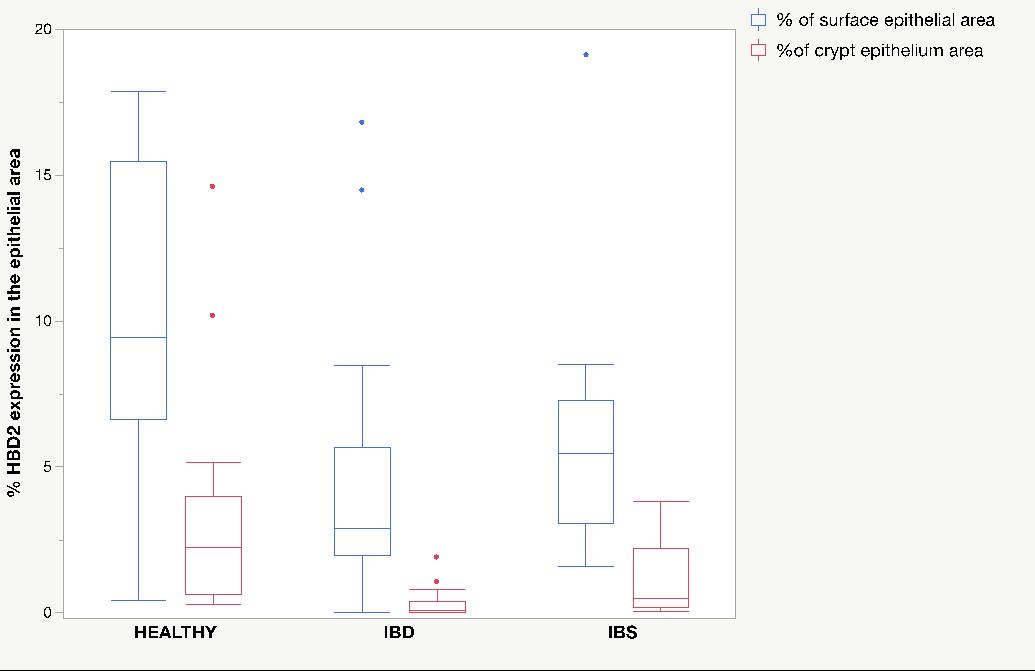
HBD2 expression was located mainly in the surface epithelium and some could be seen in the crypt epithelium. There was no expression of HBD2 in lamina propria of colon mucosa. The results are expressed as percent area covered in surface epithelium and in crypt epithelium (Figure 2).
There was a significantly lower expression of HBD2 in patients with IBS (Median 5.48% (Range 1.58-19.11) compared to healthy volunteers (Median 9.42% (0.43-17.84)) in the surface epithelium (p=0.04) and in the crypt epithelium (Median 0.48% (0.05-3.85) vs. 2.24% (0.43-17.89)) (p=0.03). Interestingly there was also a significantly lower expression of HBD2 in patients with IBD compared to healthy controls in surface epithelium (Median 2.91% (0.002-16.8) vs. Median 9.42% (0.43-17.84)) (p=0.01) and crypt epithelium (Median 0.11% (0-1.91) vs. 2.24% (0.43-17.89), p=0.0001), the group that was hypothesized to be the positive control. In the IBD group there was no difference in HBD2 expression per surface area or crypt respectively. There was no difference between patients with IBD and IBS in the surface epithelium (p=0.18) but there was significantly lower expression of HBD2 in the crypt epithelium of patients with IBD, (0.11%, 0-1.91) vs. 0.48% ,0.05-3.85) (p=0.01) (Figure 3).
Discussion
In this study, we found a lower expression of HBD2in sigmoid mucosa of patients with IBS compared to healthy volunteers. We also found a lower expression of HBD2 in sigmoid mucosa of patients with ulcerative colitis and Crohn’s disease compared to healthy controls. We could also see that there was no difference in the expression of HBD2 in surface epithelium between patients with IBD and IBS but patients with IBD had lower expression of HBD2 in the crypt epithelium compared to patients with IBS.
Human defensins have a role in the innate immunity and the non-specific host response to microorganisms in the gastrointestinal lumen. HBD1 mRNA is expressed throughout the whole GI-tract in normal epithelial mucosa and upon inflammation in the colon the mRNA expression has not been affected [7]. On the other hand, the mRNA expression of HBD2 has been shown to be increased in colonic mucosa of patients UC but not necessarily in the colon of patients with CD [8]. Crohn’s disease has previously been described as a “paneth cell disease”, where the expression of human defensin 5 has been shown to be decreased [18]. The signaling of HBD2’s antibacterial effect is thought to be through TLR signaling [2], and regulated with NFkB and G-protein coupled protease activated receptors after induction by lipopolysaccharide (LPS) [19, 20], peptidoglycans [19], IL-1 and TNF-[18]. NODs are required for NFkB activation [21, 22] and the dysfunction of TLR signaling leads to inappropriate activation of NF-kB [15], thus may disrupt the activation of HBD2.
For HBD2 it has been suggested that mRNA level is upregulated upon infection [4]. Another study also showed that there is a lower level of HBD2 in serum of patients with ulcerative colitis compared to healthy volunteers [23].
Reduced production of defensins was associated with less effective clearing of infection with E.coli in knockout mice [24]. This suggests that HBD2 might have a role in both IBD and IBS disease pathogenesis.
Our findings of a decrease of HBD2 expression in sigmoid epithelial cells in patients with IBS as well as those with IBD is somewhat surprising but very interesting. There can be many reasons for these findings. One, is reduced HBD2 production in patients with IBS and IBD but this seems unlikely given that previous studies have shown an increase of mRNA levels of HBD2 during intestinal inflammation. Increase of HBD2 mRNA has also been found in Helicobacter pylori-induced gastritis [25]. An increase of mRNA is however not necessarily associated with an increase at the protein level, since there may be a dysfunction in the transcription from mRNA to protein. An increase in the expression of DEFB4 gene that codes for HBD2, has in some studies been unable to show an increase at the protein levels in patients with IBD and also in patients with psoriasis which makes the mechanisms of protein expression less simple. [26] [27] [28].
Decreased levels of HBD2 mRNA has been found in children with CD [29] compared to UC and since some patients with CD have NOD2 mutation this was put forward as a mechanism for the lower mRNA level in CD. Since there are theories that dysbiosis in the gut of patients with IBS and IBD could have a role in the pathogenesis, a dysfunction of defensin production could lead to a defective barrier mechanism to micro-organisms in the lumen.
It could also be speculated that HBD2 production is downregulated by bacteria. For instance, Shigella can downregulate the expression of HBD1 and another defensin LL37, suggesting a mechanism that the micro-organisms themselves can affect host innate defenses [30].
Another possible reason for low levels of HBD2 in the epithelium of sigmoid colon in IBD could be that the HBD2 has been lost into the lumen. That could explain the high fecal levels of HBD2 seen previously in patients with IBD and IBS [1]. Measuring fecal HBD2 levels was not a part of our pilot study but future studies should examine this.
In this pilot study, we have looked at the epithelial expression both at the crypt level and at the surface epithelial level. When comparing active and non-active UC though, there was a significantly reduced expression of HBD2 in the crypts of active UC but not for the surface epithelium. For patients with CD, only 2 didn’t have active disease and there were no differences in the expression of the HBD2. However,caution should be taken in making any larger conclusion from this since the number of patients were low and further studies are needed for evaluating the colonic expression of HBD2 in IBD patients.
One could also argue that immunohistochemistry is not a good way to analyze the expression of HBD. However, immunohistochemistry allows us to locate where the protein is bound. By only measuring HBD2 in feces or mRNA expression it is unknown where the protein is located and where the level of HBD2 is altered. It is expected for HBD2 to exist in the epithelium since it is a part of the first line of defense against microorganisms and we expected it to be localized in the surface epithelium as we see in this study. We have seen a lower expression level HBD2 in crypts and this is expected since the exposure to bacteria is lower in the crypts. In infants with necrotising enterocolitis (NEC) lower levels of HBD2 both mRNA and at protein level via immunohistochemistry has been shown [31].
The findings of our study are interesting but limited by the size of our cohort. This was a pilot study with limited statistical power and in the future a larger cohort of IBS patients is needed to also include the different subtypes. For future analysis measuring fecal HBD2 and mRNA expression and also comparing with HBD1 can give us a clue about the involvement of HBD in IBS.
Acknowledgements
Thanks to Kristina Eckes for her support in the laboratory and to late professor Rolf Hultcrantz for access to the laboratory.
Conflicts of interests
The authors declare that they have no conflict of interests in this study.
- Langhorst J et al. (2009) Elevated human betadefensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am J Gastroenterol 104: 404-10.
- Pazgier M et al. (2006) Human β-defensins. Cellular and Molecular Life Sciences 63: 1294-313.
- Lehrer RI et al. (1989) Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest 84: 553-61.
- Cunliffe RN, YR Mahida (2004) Expression and regulation of antimicrobial peptides in the gastrointestinal tract. J Leukoc Biol 75: 49-58.
- White SH, WC Wimley, ME Selsted (1995) Structure, function, and membrane integration of defensins. Curr Opin Struct Biol 5: 521-7.
- Frye M et al. (2000) Differential expression of human alpha- and beta-defensins mRNA in gastrointestinal epithelia. Eur J Clin Invest 30: 695-701.
- Fahlgren A et al. (2003) Increased expression of antimicrobial peptides and lysozyme in colonic epithelial cells of patients with ulcerative colitis. Clin Exp Immunol 131: 90-101.
- Otte JM et al. (2008) “Human Beta Defensin 2 Promotes Intestinal WoundHealing In Vitro” Journal of Cellular Biochemistry 104: 2287-97.
- Wehkamp J et al. (2003) Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis 9: 215-23.
- Antoni L et al. (2013) Human colonic mucus is a reservoir for antimicrobial peptides. J Crohns Colitis 7: 652-64.
- Meisch JP et al. (2013) Human beta-defensin 3 peptide is increased and redistributed in Crohn's ileitis. Inflamm Bowel Dis 19: 942-53.
- Cieślik M et al. (2021) Human β-Defensin 2 and Its Postulated Role in Modulation of the Immune Response.Cells 10: 2991
- Tan G, B Zeng, FC Zhi (2015) Regulation of human enteric alpha-defensins by NOD2 in the Paneth cell lineage.Eur J Cell Biol 94: 60-6.
- Ogura Y et al. (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature 411: 603-6.
- Hugot JP et al. (2001) Association of NOD2 leucinerich repeat variants with susceptibility to Crohn's disease. Nature 411: 599-603.
- Wehkamp J et al. (2002) Human beta-defensin 2 but not beta-defensin 1 is expressed preferentially in colonic mucosa of inflammatory bowel disease. Eur J Gastroenterol Hepatol 14: 745-52.
- Donnarumma G et al. (2016) beta-Defensins: Work in Progress. Adv Exp Med Biol.
- Wehkamp J et al. (2005) Reduced Paneth cell alphadefensins in ileal Crohn's disease. Proc Natl Acad Sci U S A 102: 18129-34.
- Vora P et al. (2004) Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol 173: 5398-405.
- Schneider JJ et al. (2005) Human defensins. J Mol Med 83: 587-95.
- Ogura Y et al. (2001) Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NFkappaB.J Biol Chem 276: 4812-8.
- Inohara N et al. (2001) Human Nod1 confers responsiveness to bacterial lipopolysaccharides. J Biol Chem 276: 2551-4.
- Chang C et al. (2017) Human beta-Defensin 2 in Primary Sclerosing Cholangitis. Clin Transl Gastroenterol 8: 80.
- Wilson CL et al. (1999) Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science 286: 113-7.
- Hamanaka Y et al. (2001) Expression of human betadefensin 2 (hBD-2) in Helicobacter pylori induced gastritis: antibacterial effect of hBD-2 against Helicobacter pylori. Gut 49: 481-7.
- Bentley RW et al. (2010) Association of higher DEFB4 genomic copy number with Crohn's disease. Am J Gastroenterol 105: 354-9.
- Aldhous MC, CL Noble, J Satsangi (2009) Dysregulation of human beta-defensin-2 protein in inflammatory bowel disease. PLoS One 4: 6285.
- Jansen PA et al. (2009) Beta-defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PLoS One 4: 4725.
- Zilbauer M et al. (2010) Expression of human betadefensins in children with chronic inflammatory bowel disease. PLoS One 5: 15389.
- Islam D et al. (2001) Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med 7: 180-5.
- Jenke AC et al. (2012) Human beta-defensin 2 expression in ELBW infants with severe necrotizing enterocolitis. Pediatr Res 72: 513-20.

Figures at a glance