Molecular Detection and Characterization of Aneuploid CD31- Ctcs and CD31+Ctecs Expressing Epcam or Ki-67 in the Comprehensive Diagnosis of a Rare Case of Merkel Cell Carcinoma
Received Date: September 25, 2023 Accepted Date: October 25, 2023 Published Date: October 28, 2023
doi: 10.17303/aicb.2023.1.106
Citation: Sirui Li, Sujun Luo, Na Wei, Jiahao Xie, Rongyi Chen et al. (2023) Molecular Detection and Characterization of Aneuploid CD31- Ctcs and CD31+ Ctecs Expressing Epcam or Ki-67 in the Comprehensive Diagnosis of a Rare Case of Merkel Cell Carcinoma. Ann Immunol Cell Biol 1: 1-10
Abstract
Merkel cell carcinoma (MCC) is a rare, aggressive malignancy of the skin, which is an epithelial origin of cutaneous neuroendocrine cancer, with a poor prognosis. MCC frequently manifests in locations regularly exposed to the sun in older patients. Due to the absence of symbolic cutaneous symptoms, diagnosis of MCC is often overlooked at the time ofpresentation. The current study describes a very rare case of an 89-year-old woman, who presented with a 4 × 3 cm red nodule on the right side of her face, which was consistent with pathological diagnosis of MCC performed with histopathological and immunohistochemical examinations. In addition, the patient had a total of 35 aneuploid circulating rare cells detected, including CD31- aneuploid circulating tumor cells(CTCs) and CD31+ aneuploid circulating tumor endothelial cells (CTECs), with some of them expressing EpCAM or Ki-67. Recent clinical progress with respect to MCC is thoroughly discussed, which may assist diagnosis and treatment of this unique type of carcinoma.
Keywords: Merkel Cell Carcinoma (MCC); Head and Neck Tumor; Face Tumor; Aneuploid Ctcs; Ctecs
Introduction
Merkel cell carcinoma (MCC), a rare epithelial origin of cutaneous neuroendocrine carcinoma [1] manifests most frequently on skin exposed to the sun. Toker originally referred to it as "trabecular cancer" in 1972 [2].
According to Olsen et al ’s findings from 2021, MCC incidence increased from 1997 to 2016 at a rate of about 2-4% every year. Age-standardized incidence of MCC rose from 0.55 per 100,000 men in 1997 to 1.03 per 100,000 men in 2016, and from 0.28 to 0.45 per 100,000 women in the United States, [3] but in Brazil, the equivalent increases from 2000 to 2015 were from 0.31 to 1.21 for men and from 0.50 to 0.55 for women [4]. Additionally, men typically have a higher incidence of MCC than women.
MCC is quite common in older people and skin regions frequently exposed to the sun, such as the head and neck. The analyses of Kieran et al. show that the median age of diagnosis in Vitoria, Australia, between 1986 and 2016 was 79.5 years old [5]. The results by Olsen et al. further reveal that the age-specific incidence was highest for individuals who were 80 years old, that the increase in incidence over time was mostly confined to this age group, and that MCC of the head and neck experienced the largest increase in absolute numbers. Merkel cell carcinoma is manifested by asymptomatic erythematous/violaceous nodules or plaques, which grow rapidly and solitary. The clinical characteristics of MCC have been encapsulated by the abbreviation AEIOU,where AE stands for asymptomatic, E for quickly expanding, I for immune suppression, O for older than 50 years, and U for UV-exposed [6,7]. Here, a comprehensive cellular and molecular diagnosis of a rare case of Merkel cell carcinoma is reported. Recent progress regarding clinical manifestations, pathological features, and treatment of MCC is also discussed.
Case report
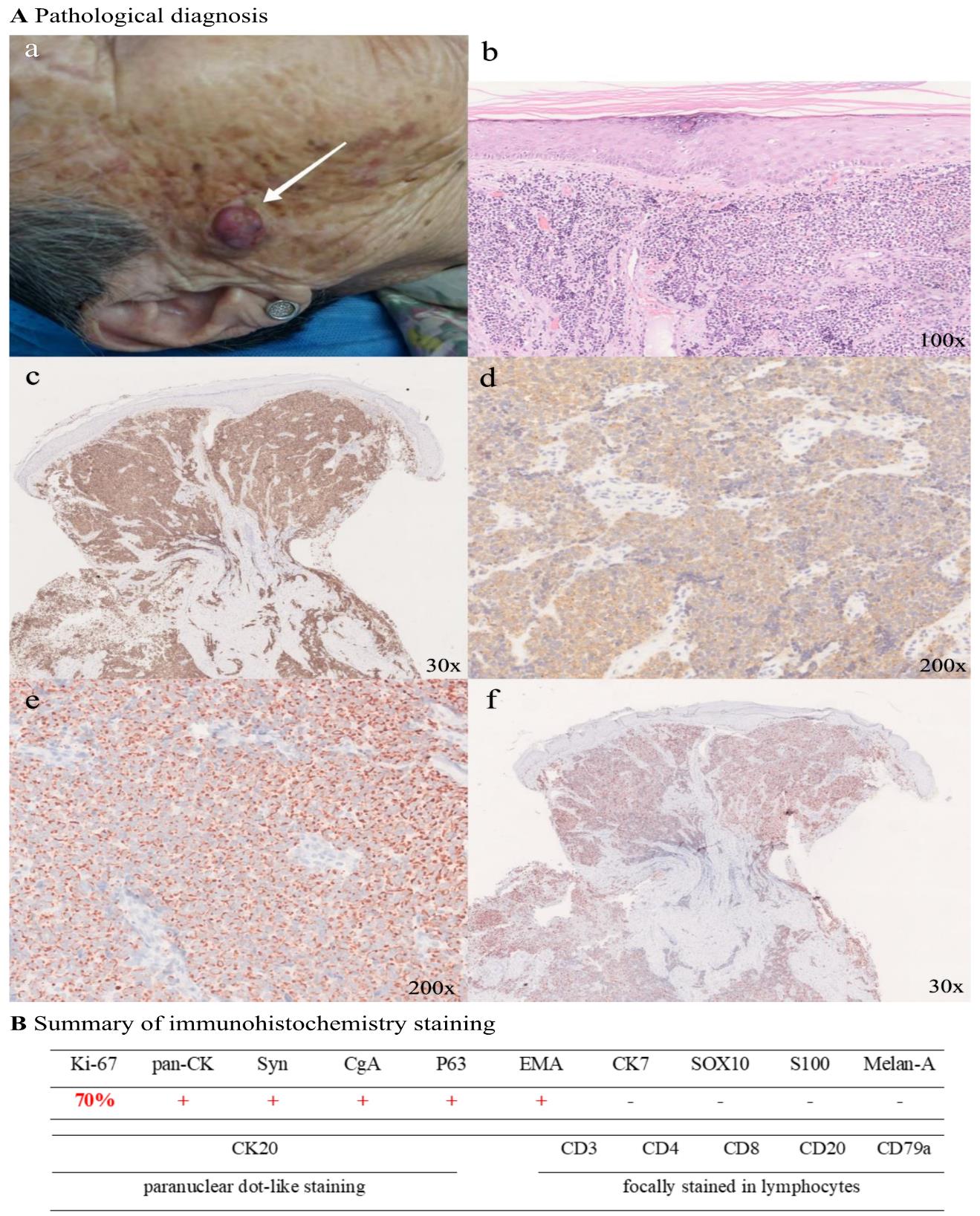
An 89-year-old woman presented with a 4-month history of a violaceous painless nodule on the right side of her face, gradually increasing. The nodule was wellcircumscribed, hemispherical in shape, violaceous in color, and measured 4 × 3 cm. The mobility was acceptable, and there was no erosion, ulcer, exudation, scale, or tenderness (Fig.1A-a). Pathological diagnosis revealed that nuclear mitosis was evident, the epiderm was absent, and little round cell clusters may be detected in the deep dermis (Figure.1Ab). As shown in Figure. 1A-c, d, and e, abundant expression of Syn, CgA, and Ki-67 in the specimen was observed. In addition, immunohistochemical staining of CK20 demonstrated paranuclear dot-like staining (Figure. 1A-f). Results of additional comprehensive pathological immunohistochemistry staining are summarized in Figure. 1B, indicating pan-CK+, Syn+, CgA+, P63+, EMA+, CK7-, SOX10-, S100-, Melan-A-, and focal staining of CD3, CD20, CD8, CD4, and CD79a in lymphocytes. Upon histological and immunohistochemical analysis, the tumor was consistent with the diagnosis of MCC.
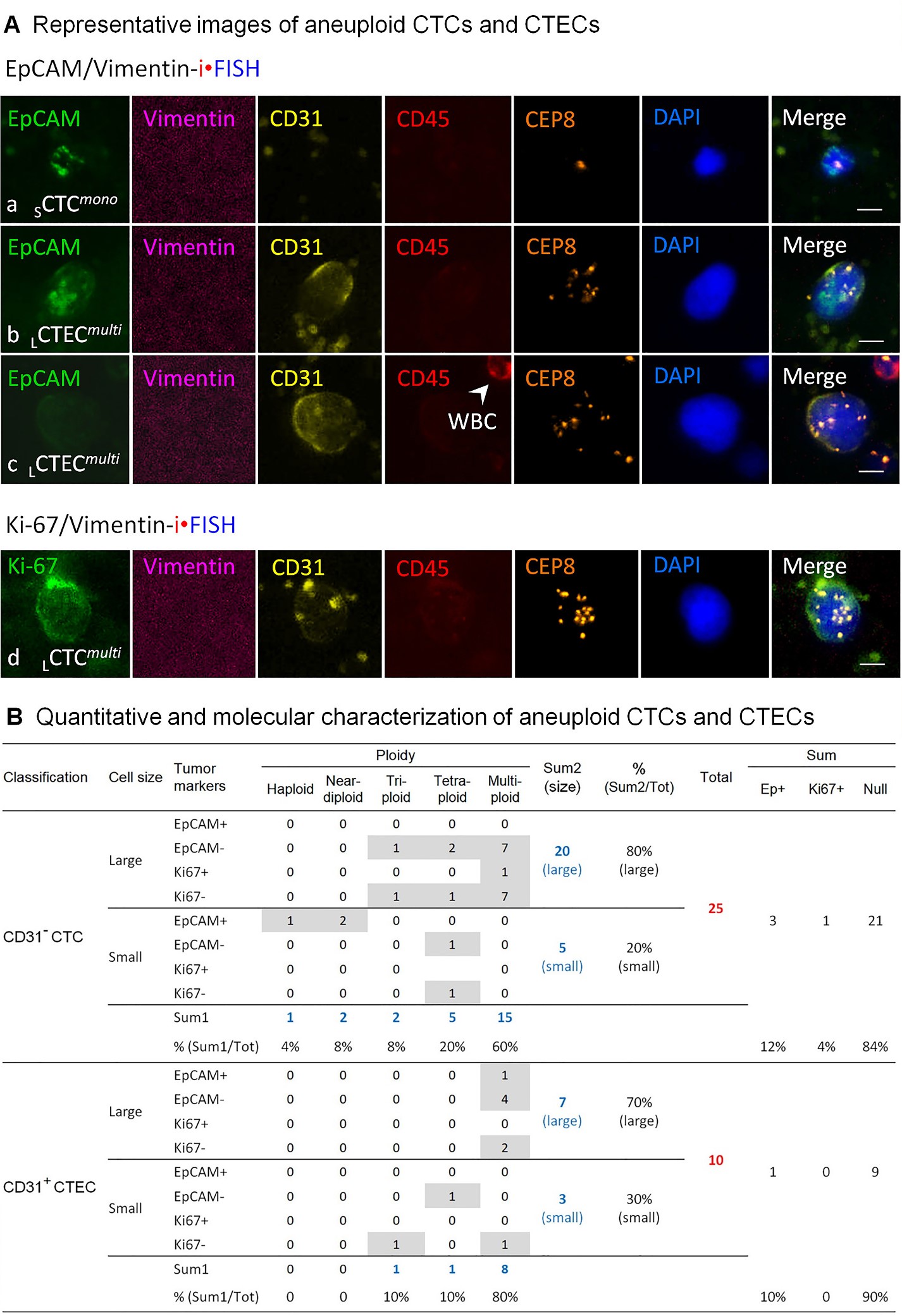
Aneuploid CD31- circulating tumor cells (CTCs) and CD31+ circulating tumor endothelial cells (CTECs) in this MCC patient were detected by the subtraction enrichment integrated with immunostaining-fluorescence in situ hybridization (SE-iFISH) [8]. Briefly, six ml of peripheral blood was collected from the subject, followed by subtraction enrichment processing. Enriched circulating rare cells were split, and each half specimen was subjected to 6-channel EpCAM/Vimentin-iFISH and Ki-67/Vimentin-iFISH, respectively. As illustrated in Figure 2, 25 CTCs and 10 CTECs were detected in a total of six ml of blood, including three EpCAM+ CTCs, one Ki67+ CTC, and one EpCAM+ CTEC. Most of the detected aneuploid CTCs and CTECs were EpCAM-/Ki-67- null cells. Among 25 CD31- CTCs, 60% of them (15 out of 25) were multiploid (≥ pentasomy 8), and 80% of the detected CTCs (20 out of 25) were large cells (>5 mm). Similarly, 70% of CD31+ CTECs were large cells (7 out of 10) with 80% of the detected CTECs exhibiting multiploid Chr8 (8 out of 10). Expression of Vimentin on CTCs or CTECs was not observed in this MCC patient.
The family of the patient stopped seeking treatment after being informed of her condition and understanding the basics of MCC. The patient is currently still alive and not in any specific discomfort.
Discussion and conclusions
Merkel cell carcinoma (MCC) is a rare and aggressive malignant tumor of the skin, which is a kind of epithelial neuroendocrine carcinoma of the skin. Its clinical manifestation is nonspecific and its prognosis is poor.
The mechanism of carcinogenesis in MCC may be related to Merkel cell polyomavirus (MCPyV) infection and UV radiation. Feng et al. showed that MCPyV infection may have a role in the pathogenesis of MCC since they found MCV sequences in 80% of MCC tumors and viral DNA clonally integrated into the genome of MCC cells in 75% of MCPyV+ tumors. The preservation of the oncogenic phenotype is significantly associated with the expression of LT, which causes a certain DNA damage response.ST is more important in tumor transformation and induces tumor formation [9]. ST plays a bigger role in the transformation of tumors since it promotes tumor development, impairment, proliferation, and apoptosis and activates the DNA damage response. However, MCPyV is widespread in the population, with approximately 60-80% of the population infected with this virus. Therefore, despite the strong association of MCPyV with MCC, the exact mechanism of its carcinogenesis remains unclear [10]. A high correlation between MCC and UV radiation has been reported in the literature [11]. UV radiation can induce mutations in the viral genome, leading to DNA damage, the effects of which are more pronounced in MCPyV-tumors. In MCPyV- tumors, cells cannot effectively repair the damage caused by UV radiation and proliferate as mutations accumulate [12].In contrast, in MCPyV+ tumors, UV radiation causes tumor development by inducing immunosuppression: UV radiation induces altered expression and function of inflammatory mediators in antigen-presenting dendritic cells, which modulates immune sensitivity and causes immunosuppression. MCC is commonly seen in patients with leukemia, lymphoma, HIV infection, and immunosuppression due to organ transplantation [13]. The pathogenic mechanism of MCC due to UV radiation may explain this phenomenon: MCC patients often have other skin tumors associated with sun exposure, such as basal cell carcinoma, squamous cell carcinoma, and melanoma.
The tumor typically presents as a rapidly growing flesh-colored or violaceous nodule or plaque. MCC frequently occurs in sun-damaged skin. The most commonly affected sites, in order of frequency of involvement, are the head, neck, extremities, and trunk [14]. Because of the lack of specificity of cutaneous presentation, clinical features rarely assist with diagnosis, therefore histopathology and immunohistochemistry play a crucial role in the diagnosis.
Hematoxylin and eosin stains of MCC demonstrate an infiltrate of small round blue cells, arranged mainly in sheets and nests. And they exhibit a high nuclear: cytoplasmic ratio with a “salt and pepper” nuclear chromatin pattern and indistinct nucleoli [15,16] MCC can be differentiated from other diseases by pathological manifestations. In contrast to basal cell carcinoma, MCC lacks peripheral palisading. In contrast to melanoma, MCC usually spares the epidermis and is free of pigment [17] MCC cells are positive for several types I or type II cytoskeletal keratins, particularly CK20 with a distinctive perinuclear dot staining pattern. The tumor also expresses neurofilament (NF) and neuroendocrine markers such as chromogranin A and synaptophysin. The co-expression of cytokeratins and neurofilament is characteristic of MCC. Recently, insulinoma-associated protein 1 (INSM1) is a sensitive nuclear marker of MCC [13,17].
MCC has a poor prognosis, a low survival rate, and is highly aggressive. Harms et al. analyzed data related to 9387 cases of MCC and came to the following conclusions: Five-year OS estimates for c patients with localized MCC were 51%, while with the nodal disease was 35%, and for patients presenting with distant metastatic disease was 14% [18]. The analysis by Maloney et al. showed that 42.3% of cases with a head/neck primary site of MCC had liver metastases, and 39.6% of cases with a trunk primary site of MCC had bone metastases. Increasing age, and liver and brain metastases were independent prognosticators of poorer prognosis [19].
In this case, it is the first time SE-iFISH was employed to detect the CTCs and CTECs in the MCC patient. Circulating tumor cells (CTCs) are considered the real-time liquid biopsy for patients with cancer [20]. The majority of endothelial cells in tumor vasculatures are tumorderived endothelial cells (TECs). TECs contribute to morphological abnormal tumor vasculature, leading to increased vascular permeability and transendothelial intravasation as well as extravasation during tumor metastasis. CTECs are TECs shed into the peripheral blood [21]. CTCs and CTECs constitute a pair of circulating tumor biomarkers in cancer patients [22]. Detection and molecular characterization of CTCs and CTECs may offer real-time insights into the course, prognosis, and effectiveness of cancer treatment [20].
The occurrence, development, and outcome of human diseases could be considered a spatiotemporal ecological process [23]. The tumor microenvironment, which is a complex, dynamic entity, has been proven to actively assist the process of tumor development [24]. The direct interaction of CTCs with TMEs is essential for tissue invasion and tumor metastasis. The association between neutrophils and CTCs promotes cell cycle development in circulation and expands the metastatic potential [25]. Also, the association between CTCs and tumor-associated macrophages (TAMs) promotes advanced tumor metastasis. Chen et al. found that the JAK2/ STAT3/ miR-506-3p/ FoxQ1 axis is regulated by TAMs to enhance colorectal cancer migration, invasion, and CTC-mediated metastasis [26]. According to the research, a significant number of CTCs are detected in many solid metastatic tumors, and several malignancies of epithelial origin, including pancreatic, colorectal, and hepatocellular carcinomas, also exhibit a significant number of EpCAM+ CTC.[25] As the study by Allen et al. shows, 6 of 11 colorectal cancer patients with liver metastases had positively significant numbers of apoptotic CTCs in peripheral blood as measured by Fischer's exact test [27]. So it is clear that CTCs have the potential to cause distant cancer metastases and that CTCs can be used as a dynamic tool to assess the metastatic tendency of tumors and to evaluate prognosis.
SE-iFISH enables to effectively enrich nonhematologic circulating rare cells regardless of cell sizes and cell surface molecule (such as EpCAM) expression, followed by in situ karyotypic and phenotypic co-examination of chromosomal aneuploidy as well as protein expression of various tumor markers [8,22]
The epithelial molecule EpCAM participates in epithelial-to-mesenchymal transition (EMT) and cancer metastasis [28,29]. As previously reported, the detection of EpCAM+ aneuploid CTCs could be utilized to evaluate surgical efficacy and to predict poor prognosis and tumor recurrence in malignancies, such as hepatocellular carcinoma and breast cancer [30,31] Besides, abundant expression of Ki-67 was reported to be highly associated with cancer cell proliferation, growth, metastasis, and the tumor’s clinical stage [32,33]. Moreover, the degree of aneuploidy is relevant to tumor grades as well as cancer cell proliferation, showing the higher degree of chromosomal ploidy, the higher grade of the tumor [34]. In regards to MCC CTC, Boyer et al.’s research showed that CTCs were detected mainly in patients with stage III/IV MCC, but also in some patients with earlystage MCC [20]. Riethdorf et al.’s research showed that up to 55% of patients with MCC had one or more CTCs/7.5 ml detected, and among those MCC patients, CTCs were detected in 70% of subjects with lymph node metastases and in all patients with distant metastases [35].
In the current presented case, 25 CD31- CTCs and 10 CD31+ CTECs were detected, with some of them expressing EpCAM or Ki-67. The majority of detected CTCs and CTECs were large multiploid cells. Obtained results suggested that the patient might be undergoing an active distant tumor metastasis and might have a poor prognosis. Longitudinal monitoring of CD31- CTCs and CD31+ CTECs along the following treatment will provide a feasible and applicable tumor liquid biopsy approach which enables a more effective assessment of subsequent therapeutic efficacy in this patient, ultimately illustrating the clinical significance of the detected MCC CTCs and CTECs, particularly those expressing EpCAM or Ki-67.
The first-line treatment for patients with primary Merkel cell cancer is surgery. Advocate adjuvant radiotherapy (RT) after full excision with clinical safety margins of 1 cm as the optimal course of action [36]. Mohsmicrographic surgery (MMS) or wide local excision (WLE) is the latest surgical excision technique [15]. We assessed the results of primary MCC patients receiving MMS as monotherapy at a single institution, there was no local recurrence. Stage I malignancies had a 5-year MCC-specific survival rate of 91.2% (historical controls ranged from 81% to 87%). Stage IIA cancers had a 68.6% 5-year MCC-specific survival rate (historical controls ranged from 63% to 67%). A survival rate that is at least as excellent as WLE+RT may be available with Mohs surgery alone [37].
The 5-year local relapse-free rate following treatment with radiotherapy alone is as high as 90% in MCC, a highly radiosensitive tumor. Radiation therapy is therefore an effective alternative therapy for patients with advanced MCC, MCC patients with primary lesions that are difficult to perform surgery on, and inoperable patients [38]. Sentinel lymph node biopsy can clinically identify lymph node metastases and indicate lymph node dissection. According to the study by Harounian et al., 29% of the clinically N0 MCC patient group who underwent SLN examination had pathologic proof of metastases. The existence of involvement changed the tumor stage and the size of the adjuvant radiation field in comparison to tumor-free SLN [39].
Nowadays, Anti-PD-1/PD-L1 agents have achieved significant efficacy in treating metastatic MCC (mMCC). Avelumab, an anti–PD-L1 monoclonal antibody, became the first treatment for patients with mMCC to receive approval in the United States, the European Union, and Japan. An analysis of a clinical trial showed that avelumab had antitumor activity and a manageable safety profile in patients with mMCC [40]. Nivolumab can mediate substantial tumor regression. Data from Topalian et al. showed that 47.2% of patients treated with Nivolumab for 4 weeks followed by surgery achieved a pathologic complete response (pCR, which was defined as the absence of residual viable invasive cancer on completely resected tumor specimens including all sampled lymph nodes). 87.9% of patients show radiographic tumor reduction [41].
Ethics statement
The study was conducted according to the Declaration of Helsinki Principles. An informed consent form, approved by the Ethics Review Committees (ERC) of the Dermatology Hospital of Southern Medical University, Guangzhou, China, was signed and obtained from the patient.
Author contributions
SRL, SUL, NW, JX, JR, and RC participated in patient treatment and analysis of results. SRL and AYL contributed to writing the original draft. AYL and DDW contributed methodology and validation. RC and PPL contributed to the conceptualization, reviewing, and editing of the manuscript. All authors approved the submitted version of the manuscript.
Funding
This research was supported by Natural Science Foundation of Guangdong Province of China 2016A030313682 and Natural Science Foundation of Guangdong Province of China 2020A1515010281.
Acknowledgments
The authors sincerely thank the staffs in the Dermatology Hospital, Southern Medical University, Cytointelligen (China Medical City, Taizhou, Jiangsu, China) and Cytelligen (San Diego, CA, USA) for providing support to this study.
Conflict of Interest
i•FISH® is the registered trademarks of Cytelligen. PL is the president at Cytelligen. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- Thibault K (2022) Evidence of an epithelial origin of Merkel cell carcinoma. Mod Pathol 35: 446-8.
- Toker C (1972) Trabecular carcinoma of the skin. Arch Dermatol105: 107-10.
- Olsen CM, Pandeya N, Whiteman DC (2021) International Increases in Merkel Cell Carcinoma Incidence Rates between 1997 and 2016. J Invest Dermatol 141: 2596-601.
- de Melo AC, Santos Thuler LC (2021) Trends in the incidence and morbidity of Merkel cell carcinoma in Brazil.Future Oncol 17: 2857-65.
- Garbutcheon Singh KB, Curchin DJ, McCormack CJ, Smith SD (2020) Trends in the incidence of Merkel cell carcinoma in Victoria, Australia, between 1986 and 2016. Australas J Dermatol 61: e34-e8.
- Becker JC, Stang A, DeCaprio JA, Cerroni L, Lebbé C et al. (2017) Merkel cell carcinoma. Nat Rev Dis Primers 3:17077.
- Coggshall K, Tello TL, North JP, Yu SS (2018) Merkel cell carcinoma: An update and review: Pathogenesis,diagnosis, and staging. J Am Acad Dermatol 78: 433-42.
- Lin PY, Hsieh CW, Hsieh S (2017) Rapid and Sensitive SERS Detection of Bisphenol a Using Selfassembled Graphitic Substrates. Sci Rep 7: 16698.
- Feng H, Shuda M, Chang Y, Moore PS (2008) Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319: 1096-100.
- Verhaegen ME, Mangelberger D, Harms PW,Vozheiko TD, Weick JW et al. (2015) Merkel cell polyomavirus small T antigen is oncogenic in transgenic mice. J Invest Dermatol 135: 1415-24.
- Miller RW, Rabkin CS (1999) Merkel cell carcinoma and melanoma: etiological similarities and differences.Cancer Epidemiol Biomarkers Prev. 1999 Feb;8(2):153-8. Erratum in: Cancer Epidemiol Biomarkers Prev 8: 485.
- Coggshall K, Tello TL, North JP, Yu SS (2018) Merkel cell carcinoma: An update and review: Pathogenesis,diagnosis, and staging. J Am Acad Dermatol 78: 433-42.
- Becker JC, Stang A, DeCaprio JA, Cerroni L, Lebbé C et al. (2017) Merkel cell carcinoma. Nat Rev Dis Primers 3:17077.
- Busam KJ, Walsh N, Wood BA, Merkel cell carcinoma. In: Elder DE, Massi D et al. (2018) WHO Classification of Skin Tumours. 4th ed. Lyon, France: IARC;2018:47–50. World Health Organization Classification of Tumours 11.
- Turshudzhyan A, Hadfield M, Grant-Kels J (2021) Updates on the diagnosis, current and future therapeutic options in Merkel-cell carcinoma. Melanoma Res 31: 421-5.
- Walsh NM, Cerroni L (2020) Merkel cell carcinoma:A review. J Cutan Pathol 48: 411-21.
- Barksdale SK (2017) Advances in Merkel cell carcinoma from a pathologist's perspective. Pathology 49:568-74.
- Harms KL, Healy MA, Nghiem P, Sober AJ, Johnson TM et al. (2016) Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann Surg Oncol 23: 3564-71.
- Maloney NJ, Nguyen KA, Bach DQ, Zaba LC (2021) Sites of distant metastasis in Merkel cell carcinoma differ by primary tumor site and are of prognostic significance: A population-based study in the Surveillance, Epidemiology, and End Results database from 2010 to 2016. J Am Acad Dermatol 84: 568-70.
- Boyer M, Cayrefourcq L, Garima F, Foulongne V, Dereure O et al. (2020) Circulating Tumor Cell Detection and Polyomavirus Status in Merkel Cell Carcinoma. Sci Rep 10: 1612.
- Lin PP (2020) Aneuploid Circulating Tumor-Derived Endothelial Cell (CTEC): A Novel Versatile Player in Tumor Neovascularization and Cancer Metastasis. Cells 9:1539.
- Zhang T, Zhang L, Gao Y, Wang Y, Liu Y et al. (2021) Role of aneuploid circulating tumor cells and CD31+circulating tumor endothelial cells in predicting and monitoring anti-angiogenic therapy efficacy in advanced NSCLC. Mol Oncol 15: 2891-909.
- da Silva Chaves SN, Dutra Costa BP, Vidal Gomes GC, Lima-Maximino M, Pacheco Rico E et al. (2020) NOS-2 participates in the behavioral effects of ethanol withdrawal in zebrafish. Neurosci Lett 728: 134952.
- Anderson NM, Simon MC (2020) The tumor microenvironment. Curr Biol 30: R921-R5.
- Lin D, Shen L, Luo M, Zhang K, Li J et al. (2021) Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther 6: 404.
- Wei C, Yang C, Wang S, Shi D, Zhang C et al. (2019) Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer 18:64.
- Allen JE, Saroya BS, Kunkel M, Dicker DT, Das A et al. (2014) Apoptotic circulating tumor cells (CTCs) in the peripheral blood of metastatic colorectal cancer patients are associated with liver metastasis but not CTCs. Oncotarget 5:1753-60.
- Gires O, Pan M, Schinke H, Canis M, Baeuerle PA (2020) Expression and function of epithelial cell adhesion molecule EpCAM: where are we after 40 years? Cancer Metastasis Rev 39: 969-87.
- Yang J, Antin P, Berx G, Blanpain C, Brabletz T, et al. (2021) EMT International Association (TEMTIA). Guidelines and definitions for research on epithelialmesenchymal transition. Nat Rev Mol Cell Biol 21: 341-52.
- Wang L, Li Y, Xu J, Zhang A, Wang X et al. (2018) Quantified postsurgical small cell size CTCs and EpCAM+ circulating tumor stem cells with cytogenetic abnormalities in hepatocellular carcinoma patients determine cancer relapse. Cancer Lett 412: 99-107.
- Liu X, Li J, Cadilha BL, Markota A, Voigt C et al.(2019) Epithelial-type systemic breast carcinoma cells with a restricted mesenchymal transition are a major source of metastasis. Sci Adv 5: eaav4275.
- Li LT, Jiang G, Chen Q, Zheng JN (2015) Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep 11: 1566-72.
- Sun X, Kaufman PD (2018) Ki-67: more than a proliferation marker. Chromosoma 127: 175-86.
- Krajcovic M, Overholtzer M (2012) Mechanisms of ploidy increase in human cancers: a new role for cell cannibalism. Cancer Res 72: 1596-601.
- Riethdorf S, Hildebrandt L, Heinzerling L, Heitzer E,Fischer N et al. (2019) Detection and Characterization of Circulating Tumor Cells in Patients with Merkel Cell Carcinoma. Clin Chem 65: 462-72.
- Gauci ML, Aristei C, Becker JC, Blom A, Bataille V et al. (2022) European Dermatology Forum (EDF), the European Association of Dermato-Oncology (EADO) and the European Organization for Research and Treatment of Cancer (EORTC). Diagnosis and treatment of Merkel cell carcinoma: European consensus-based interdisciplinary guideline - Update 2022. Eur J Cancer 171: 203-31.
- Terushkin V, Brodland DG, Sharon DJ, Zitelli JA (2021) Mohs surgery for early-stage Merkel cell carcinoma (MCC) achieves local control better than wide local excision ± radiation therapy with no increase in MCC-specific death.Int J Dermatol 60: 1010-2.
- Hong AM, Stretch JR, Thompson JF (2021) Treatment of primary Merkel cell carcinoma: Radiotherapy can be an effective, less morbid alternative to surgery. Eur J Surg Oncol 47: 483-5.
- Harounian JA, Molin N, Galloway TJ, Ridge D,Bauman J et al. (2021) Effect of Sentinel Lymph Node Biopsy and LVI on Merkel Cell Carcinoma Prognosis and Treatment. Laryngoscope 131: E828-E35.
- D'Angelo SP, Russell J, Lebbé C, Chmielowski B, Gambichler T et al. (2018) Efficacy and Safety of First-line Avelumab Treatment in Patients with Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol 4: e180077.
- Topalian SL, Bhatia S, Amin A, Kudchadkar RR,Sharfman WH et al. (2020) Neoadjuvant Nivolumab forPatients with Resectable Merkel Cell Carcinoma in the CheckMate 358 Trial. J Clin Oncol 38: 2476-87.

Figures at a glance