The Therapeutic Impact of Ademethionine, High-Dose Lycopene, and High-Dose Piceatannol on Melatonin Secretion in Fatty Liver Disease
Received Date: February 12, 2024 Accepted Date: March 12, 2024 Published Date: March 15, 2024
doi: 10.17303/ejmrc.2024.6.101
Citation: Tavartkiladze, G Simonia, N Okrostsvaridze, P Revazishvili, L Tavartkiladze (2024) The Therapeutic Impact of Ademethionine, High-Dose Lycopene, and High-Dose Piceatannol on Melatonin Secretion in Fatty Liver Disease. Eur J Med Res Clin Trials 6: 1-18.
Abstract
Background: Fatty liver disease (FLD) is a prevalent condition globally, characterized by excess fat accumulation in the liver. This disease is a critical concern due to its silent progression to liver damage and its association with metabolic syndromes and increased oncological risk, particularly hepatocellular carcinoma (HCC). Melatonin, a hormone involved in circadian rhythm regulation, has been noted for lower plasma levels in FLD patients, which may contribute to the disease’s progression. This study aimed to evaluate the effect of ademethionine, high-dose lycopene, and high-dose piceatannol on melatonin secretion and their potential role in treating FLD.
Methods: A cohort of 28 patients diagnosed with FLD was divided into two groups: a control group receiving ademethionine monotherapy and a study group receiving a combination therapy of ademethionine, lycopene, and piceatannol. Over six months, we assessed plasma levels of melatonin, liver function tests, immune parameters, and inflammatory markers using liquid chromatography and flow cytometry. The study evaluated the treatments' efficacy by comparing the two groups' melatonin secretion levels and health markers monthly.
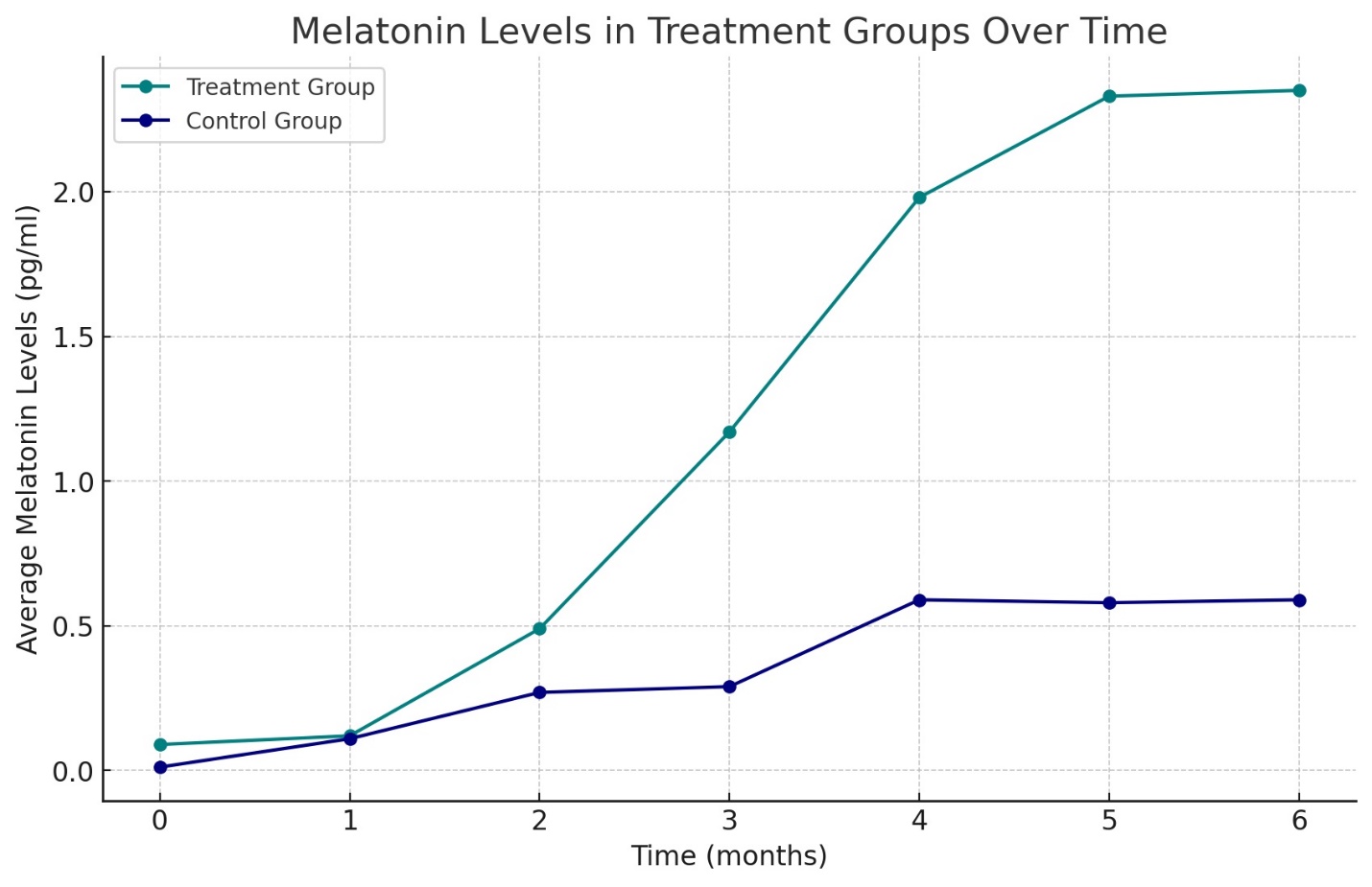
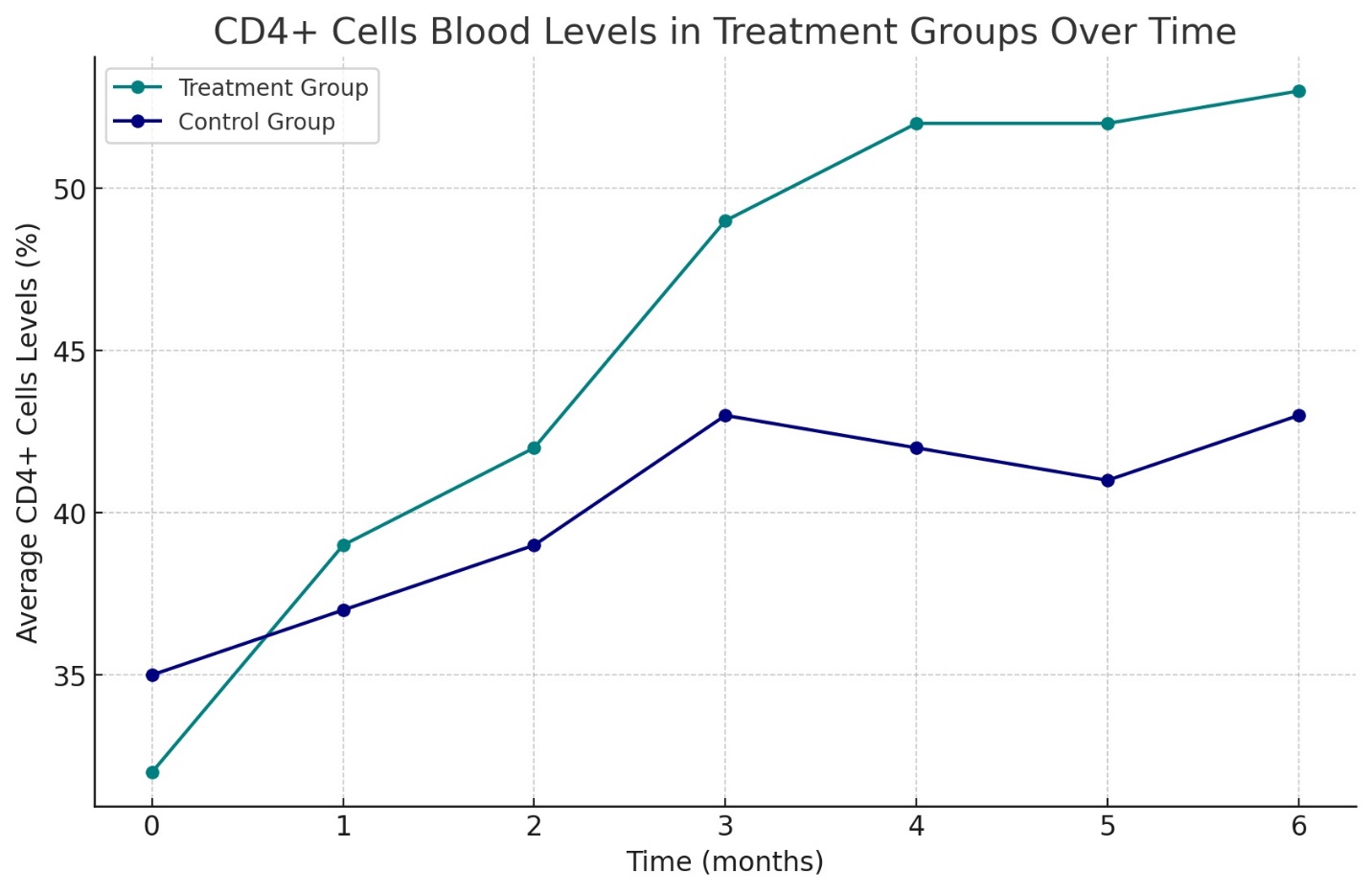
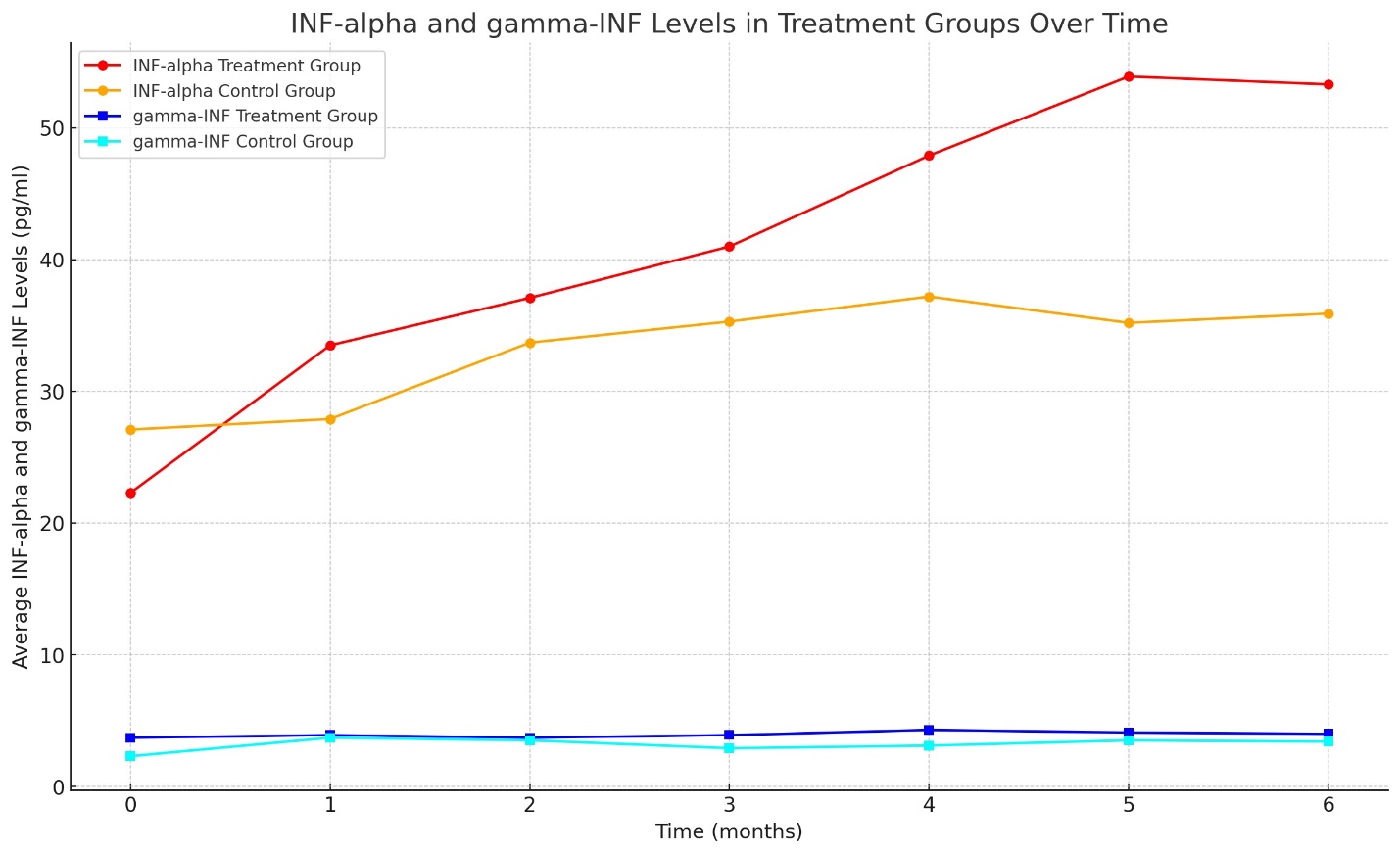
Results: The study revealed a remarkable increase in plasma melatonin levels in the group treated with the combination therapy compared to the control group, with a mean level of 2.3 ± 0.38 pg/ml against 0.11 ± 0.06 pg/ml in the monotherapy group (p<0.01). Enhanced liver function and significant upregulation of endogenous INF-alpha and gamma-INF levels were observed in the combination therapy group, being 8.7 times higher than the control. Moreover, the study group exhibited a 7-11% increase in immune status (Immune function markers, including CD3+, CD4+, CD8+ T-lymphocytes, NK cells, B-lymphocytes, and total immunoglobulins IgA, IgM, and IgG) and a 27% improvement in lipid metabolism compared to the control (Total Cholesterol, HDL, LDL, VLDL, AIP, and fatty acids: A-linolenic, Eicosapentaenic acid, Gamma- linolenic acid, Bishomo gamma Linolenic acid, Arachidonic acid and Eicosapentaenic acid/ Arachidonic acid (EPA/AA)-Ratio), alongside a normalization of inflammatory markers. These changes correlated with improved patient-reported outcomes, including increased energy, better concentration, and improved sleep quality.
Conclusion: Ademethionine, when combined with high-dose lycopene and piceatannol, demonstrates a potent effect on increasing melatonin secretion in FLD patients, exceeding the impact of ademethionine alone. The trial suggests that these agents collectively contribute to melatonin biosynthesis, enhance detoxification, and improve immune and liver function, which may offer a synergistic approach to managing FLD. The results advocate for the inclusion of these compounds in the therapeutic regimen for FLD, potentially averting its progression to more severe hepatic conditions and oncological diseases. These findings open avenues for larger-scale studies to validate the clinical benefits and mechanistic pathways of these interventions in FLD and related metabolic disorders.
Keywords: Fatty Liver Disease (FLD); Melatonin; Ademethionine; High-Dose Lycopene; High-Dose Piceatannol; Immune Function; New Therapeutic Regimen
Introduction
Fatty liver disease, encompassing both alcoholic fatty liver disease (AFLD) and nonalcoholic fatty liver disease (NAFLD), is recognized as the hepatic manifestation of metabolic syndrome and is becoming increasingly prevalent, aligning with the global rise in obesity and type 2 diabetes mellitus. The spectrum of fatty liver disease extends from simple hepatic steatosis, characterized by excessive triglyceride accumulation within hepatocytes, to nonalcoholic steatohepatitis (NASH), an aggressive form that encompasses hepatocyte injury, inflammation, and fibrosis, which can culminate in cirrhosis, liver failure, or hepatocellular carcinoma (HCC).
The pathophysiology of fatty liver disease is multifactorial and complex, encompassing genetic predispositions, dietary factors, insulin resistance, oxidative stress, and cytokine production. The chronic low-grade inflammation inherent to metabolic syndrome potentiates insulin resistance and further fat accumulation in the liver. Furthermore, the disturbed secretion of adipokines and cytokines from adipose tissue contributes to the hepatic pathology characteristic of fatty liver disease.
Melatonin, the chief secretory product of the pineal gland, has been implicated in the regulation of various biological rhythms and metabolic processes. Known primarily for its role in regulating circadian rhythms, melatonin also exhibits significant anti-inflammatory, antioxidant, and immunomodulatory properties. Recent studies have highlighted a correlation between melatonin secretion dysregulation and the pathogenesis of metabolic syndrome- related disorders, including fatty liver disease. Patients with fatty liver disease often exhibit altered diurnal rhythms of melatonin secretion and reduced plasma levels, potentially exacerbating the disease state.
Ademethionine, also known as S-adenosylmethionine (SAMe), is a molecule that occurs naturally in the body and is involved in essential methylation reactions. It has been utilized in the treatment of various liver diseases, including alcoholic liver disease and NAFLD, for its role in methylation processes and as a precursor for glutathione, a vital antioxidant in hepatic detoxification pathways. Ademethionine's hepato-protective, anti-inflammatory, and antioxidant effects make it an attractive therapeutic agent for managing liver diseases.
Lycopene, a non-provitamin A carotenoid found in tomatoes and other red fruits and vegetables, has garnered attention for its role in chronic disease prevention, particularly in cardiovascular diseases and cancer. Its potent antioxidant activity can mitigate oxidative stress, a contributing factor in the progression of fatty liver disease. Additionally, lycopene has been shown to influence lipid metabolism, suggesting a therapeutic potential in the metabolic dysregulation inherent to fatty liver disease.
Piceatannol, a natural stilbene related to resveratrol, possesses multiple biological activities, including anti- inflammatory, antioxidant, and antitumoral effects. Its influence on cellular signaling pathways implicated in cell growth and death suggests that piceatannol could modulate the processes that underlie the progression from simple steatosis to NASH and subsequently to HCC.
Despite the availability of these pharmacological agents, the progression of fatty liver disease to more severe forms and its association with increased oncological risks pose significant challenges. The need for effective intervention strategies is underscored by the economic and healthcare burdens associated with managing advanced liver disease and related comorbidities. This study aims to examine the therapeutic impact of ademethionine, high-dose lycopene, and high-dose piceatannol on melatonin secretion and serum levels in patients with fatty liver disease, with the goal of identifying potential benefits in treatment regimens that may prevent disease progression and reduce the risk of developing liver cancer.
As obesity and diabetes continue to surge as public health crises, the need to address related pathologies, including fatty liver disease, with novel and effective treatment modalities becomes increasingly critical. The current study is informed by the growing understanding of melatonin’s role in metabolic regulation and the therapeutic promise of ademethionine, lycopene, and piceatannol in influencing the disease trajectory of fatty liver disorders. In addressing the disrupted circadian and metabolic homeostasis in fatty liver disease, this research could pave the way for integrative therapies capable of curtailing the initiation and progression of hepatic pathology.
This introduction sets the stage for a detailed investigation into the synergistic effects of these compounds, proposing a multidisciplinary approach to the treatment of fatty liver disease. By exploring the intersection of chronobiology, metabolic dysfunction, and hepatic pathology, we aim to contribute valuable insights into the synergistic effects of these compounds, proposing a multidisciplinary approach to the treatment of fatty liver disease. By exploring the intersection of chronobiology, metabolic dysfunction, and hepatic pathology, we aim to contribute valuable insights into the comprehensive management of this increasingly prevalent condition.
Objective
The burgeoning prevalence of fatty liver disease worldwide presents a compelling challenge to modern healthcare systems. This condition, encompassing both nonalcoholic fatty liver disease (NAFLD) and its more severe form, nonalcoholic steatohepatitis (NASH), not only predisposes individuals to progressive hepatic morbidity but also establishes a platform for various metabolic and oncological complications. Among the myriad of factors contributing to the pathogenesis of fatty liver disease, disrupted circadian regulation and consequent melatonin secretion anomalies have been identified as significant yet modifiable elements. The role of melatonin, a hormone pivotal in chronobiological modulation, antioxidation, and immunoregulation, becomes paramount in the context of fatty liver disease, where its diminished serum levels are thought to exacerbate metabolic disarray and the advancement of hepatic pathologies.
This study was designed with a twofold objective to elucidate the relationship between therapeutic modulation of melatonin secretion and the management of fatty liver disease. The primary aim is to assess the plasma levels of melatonin following the administration of ademethionine, a compound endogenous to hepatic metabolic pathways with recognized antioxidative and anti-inflammatory properties. Recognizing the multifactorial nature of fatty liver disease, the study endeavors to go beyond monotherapy and to evaluate the potential synergistic effects of administering ademethionine in conjunction with two other potent bioactive compounds—high-dose lycopene and piceatannol.
Lycopene, an antioxidant micronutrient, and piceatannol, with its noted anti-inflammatory and antineoplastic properties, are hypothesized to not only augment melatonin biosynthesis but also impart holistic benefits to hepatic function. The second objective, therefore, is to investigate whether this combinatory treatment approach can ameliorate hepatic steatosis and inflammation, thereby improving overall liver health and functionality. This encompasses evaluating the serum levels of melatonin as a direct measure and assessing a spectrum of liver function tests, inflammatory markers, and immunological parameters as indirect indicators of therapeutic impact.
By integrating these treatment modalities, the study seeks to establish a more comprehensive therapeutic strategy for patients with fatty liver disease, aimed at correcting chronobiological aberrations, enhancing melatonin synthesis, and mitigating inflammatory processes. A further goal is to examine whether these interventions can inversely correlate with the progression to more severe liver disease and mitigate the associated risk of developing HCC, thereby offering a preventative strategy against the oncological trajectory commonly witnessed in advanced fatty liver disease.
In summary, this research aims to contribute substantively to the current understanding of fatty liver disease management by harnessing the pharmacological actions of ademethionine, lycopene, and piceatannol in restoring circadian and metabolic homeostasis through the lens of melatonin secretion and its myriad physiological effects.
Materials and Methods
Study Design
This clinical trial was designed as a prospective, randomized, controlled study to evaluate the efficacy of ademethionine alone and in combination with high-dose lycopene and piceatannol in the treatment of patients with fatty liver disease. The trial spanned six months, with periodic assessments at monthly intervals to monitor changes in melatonin secretion, liver function, immune status, and inflammatory markers.
Participants
The study included 28 adult patients (aged 24 to 57 years) diagnosed with fatty liver disease by established clinical and ultrasound criteria table N1. Exclusion criteria were the presence of viral hepatitis, autoimmune liver diseases, drug-induced liver injury, alcohol consumption above the moderate level, or other significant systemic diseases that could affect liver metabolism or melatonin levels. The control group comprised 12 patients (aged 23 to 61 years) who received only ademethionine treatment, serving as a comparator for assessing the added effect of lycopene and piceatannol.
Treatment Regimen
Participants in the study group were administered a daily dose of 800 mg of ademethionine, in addition to 500 mg of piceatannol and 300 mg of lycopene. The control group received 800 mg of ademethionine alone daily. Adherence to treatment was monitored through patient diaries and monthly pill counts. All participants were advised to maintain their usual diet and physical activity levels throughout the study to minimize lifestyle variations as a confounding factor.
Biochemical Analyses
Blood samples were collected after an overnight fast at baseline and at the end of each month following the commencement of treatment. Plasma melatonin levels were quantified using high-performance liquid chromatography (HPLC) with fluorescence detection. The chromatographic conditions were optimized for the separation of melatonin with adequate resolution and quantification limits.
Immune function markers, including CD3+, CD4+, CD8+ T-lymphocytes, NK cells, B-lymphocytes, and total immunoglobulins IgA, IgM, and IgG, were determined by flow cytometry. Standardized protocols for cell surface staining were employed using fluorochrome-conjugated monoclonal antibodies specific to each marker.
Liver Function and Inflammatory Marker Analysis
Liver function tests, including serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), alkaline phosphatase (ALP), and bilirubin levels, were conducted using automated clinical chemistry analyzers. Inflammatory markers, such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and serum amyloid A (SAA), were measured using standard laboratory methods.
Acute-Phase Reactants
The study's analytical phase included the quantitative measurement of acute-phase reactants, which serve as early markers of systemic inflammation and have been associated with the severity and progression of fatty liver disease:
C-Reactive Protein (CRP): Quantification was performed using high-sensitivity nephelometry to capture subtle changes reflective of low-grade chronic inflammation characteristic of metabolic disorders.
Erythrocyte Sedimentation Rate (ESR): Measurement was conducted using the Westergren method, providing a non-specific, yet standard, indicator of inflammatory activity.
Serum Amyloid A (SAA): Levels were measured by immunonephelometry, which identifies acute-phase proteins synthesized by the liver in response to pro-inflammatory cytokines.
Fibrinogen: Plasma concentrations were determined using the Clauss clotting method, with results correlating with ESR elevations in the context of inflammation.
Procalcitonin: Evaluated using an enzyme-linked fluorescent assay (ELFA), this marker is considered indicative of bacterial infection when elevated in the context of liver disease.
Cellular Markers
Cellular markers provide insight into the body's specific immune response to inflammatory stimuli, with certain cell types elevated in various inflammatory states:
White Blood Cell Count (WBC): A complete blood count (CBC) with differential was performed to assess overall white cell levels and differentials, providing insight into the type and extent of the inflammatory response.
Neutrophils, Eosinophils, Basophils, and Lymphocytes: Specific analysis of these subpopulations was conducted using flow cytometry to provide a detailed profile of immune cell response associated with the inflammatory state of fatty liver disease.
Cytokines
The role of cytokines as mediators and regulators of the inflammatory process is well-established, with the following being of particular relevance to hepatic inflammation:
Tumor Necrosis Factor-alpha (TNF-α), Interleukins (IL-1, IL-6, and IL-8), and Interferon-gamma (IFN- γ): Serum levels were measured using ELISA kits standardized against known controls, due to their significant role in inflammation and tissue injury in fatty liver disease.
Adhesion Molecules
These molecules facilitate the adhesion and transmigration of immune cells across the endothelium into sites of inflammation:
Vascular Cell Adhesion Molecule-1 (VCAM-1), Intercellular Adhesion Molecule-1 (ICAM-1), and Selectins (E-selectin, P-selectin, L-selectin): Flow cytometric analysis was employed to quantify expression levels on endothelial cells collected via peripheral blood sampling.
Chemokines and Enzymes
Chemokines orchestrate cell movement towards sites of inflammation, while enzymes like COX and lipoxygenase are implicated in the production of inflammatory mediators:
Chemokine (C-C motif) ligand 2 (CCL2) and Chemokine (C-X-C motif) ligands (CXCL8): These were quantitatively assessed to determine their role in attracting monocytes and neutrophils, respectively.
Cyclooxygenase (COX) and Lipoxygenase Enzymes: Their activity levels were inferred by measuring downstream products of their catalytic action, such as thromboxanes, prostaglandins, and leukotrienes.
Reactive Oxygen and Nitrogen Species
These species are important in the defense against pathogens and in signaling tissue damage:
Nitric Oxide (NO), Superoxide Anion, and Hydrogen Peroxide: The presence and levels of these species in serum were determined using colorimetric assays and specific fluorescence-based probes.
Soluble Factors
Components of the complement system were measured due to their role in inflammation and pathogen clearance:
Complement System Components (C3a, C4a, C5a) and Platelet-activating factor (PAF): Assessed through immunoassays to evaluate their role in the acute inflammatory response.
Other Markers
Additionally, other non-specific but significant markers of stress and inflammation were included:
Heat Shock Proteins (HSPs) and High-Mobility Group Box 1 (HMGB1): Their serum levels were determined using ELISA, given their relevance in inflammation and cellular stress responses.
Methodological Considerations
Each marker was assessed at baseline and subsequent monthly intervals to monitor treatment efficacy and disease progression. The analytical methods were chosen for their clinical relevance and sensitivity to the pathophysiological changes in fatty liver disease. The comprehensive panel of inflammatory markers was selected to provide a multifaceted view of the inflammation associated with fatty liver disease, considering both general inflammatory responses and those specific to hepatic pathology. The data derived from these analyses will aid in understanding the underlying mechanisms by which the studied treatments exert their beneficial effects.
Interferons (IFNs) alpha, beta, and gamma were assessed using enzyme-linked immunosorbent assays (ELISA), designed for high sensitivity and specificity to human IFNs.
Data were expressed as mean ± standard deviation (SD) for continuous variables and as frequencies for categorical variables. The Shapiro-Wilk test was applied to assess the normality of data distribution. Differences between groups were evaluated using the independent samples t-test or Mann-Whitney U test for continuous variables and the chi-square test for categorical variables. A p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using SPSS software (Version 12).
Ethical Considerations
The study protocol was reviewed and approved by the institutional review board (IRB) at each participating center. Written informed consent was obtained from all participants before their inclusion in the study. The trial was conducted in accordance with the ethical principles originating in the Declaration of Helsinki and consistent with Good Clinical Practice (GCP) and applicable regulatory requirements.
Monitoring and Adverse Events
Participants were monitored for treatment compliance and potential adverse events throughout the study. Adverse events were recorded, and their relationship to the study treatment was evaluated by the study investigators. Safety assessments were performed at each visit, including physical examination and routine laboratory tests.
Imaging Studies
Abdominal ultrasonography was performed at baseline and at the study’s conclusion to assess changes in liver echogenicity, a surrogate marker for steatosis. The ultrasonography was conducted by certified radiologists blinded to the treatment allocation.
By detailing the comprehensive approach taken to understand the impact of the selected compounds on fatty liver disease, this methodology provides a robust framework for a scientifically rigorous analysis of the potential benefits of ademethionine, lycopene, and piceatannol in treating this condition.
Results
Overview - Our investigation into the therapeutic impact of ademethionine, high-dose lycopene, and high-- dose piceatannol on melatonin secretion in fatty liver disease yielded significant findings. This section presents a comprehensive analysis of the data collected over the six-- month treatment period.
Plasma Melatonin Levels
The analysis of plasma melatonin levels revealed a stark contrast between the two patient groups. The control group, treated with ademethionine alone, exhibited consistently low melatonin levels throughout the study, with a marginal increment towards the end of the treatment period. Conversely, the combination therapy group demonstrated a progressive increase in melatonin levels, with the mean plasma concentration rising significantly over the six months (Graph N1 shows the monthly melatonin levels for both groups).
Liver Function Test Outcomes
Liver function tests across both groups indicated improved hepatic health in patients receiving combination therapy. ALT, AST, GGT, ALP, and bilirubin levels, which were initially elevated, indicative of hepatic stress, showed a greater degree of normalization in the combination group compared to the control group.
Immune Function Markers
Flow cytometry results delineated improvements in immune system parameters in patients receiving combination therapy. There was an observable elevation in CD3+, CD4+, CD8+, NK cells, B-lymphocytes, and total immunoglobulin levels. Notably, the NK cell activity and CD4+/CD8+ ratio, which are critical determinants of immune competence, were significantly higher in the combination therapy group compared to the control group (Graph N2 and Table N2 illustrate the changes in immune cell populations).
Inflammatory Marker Profiles
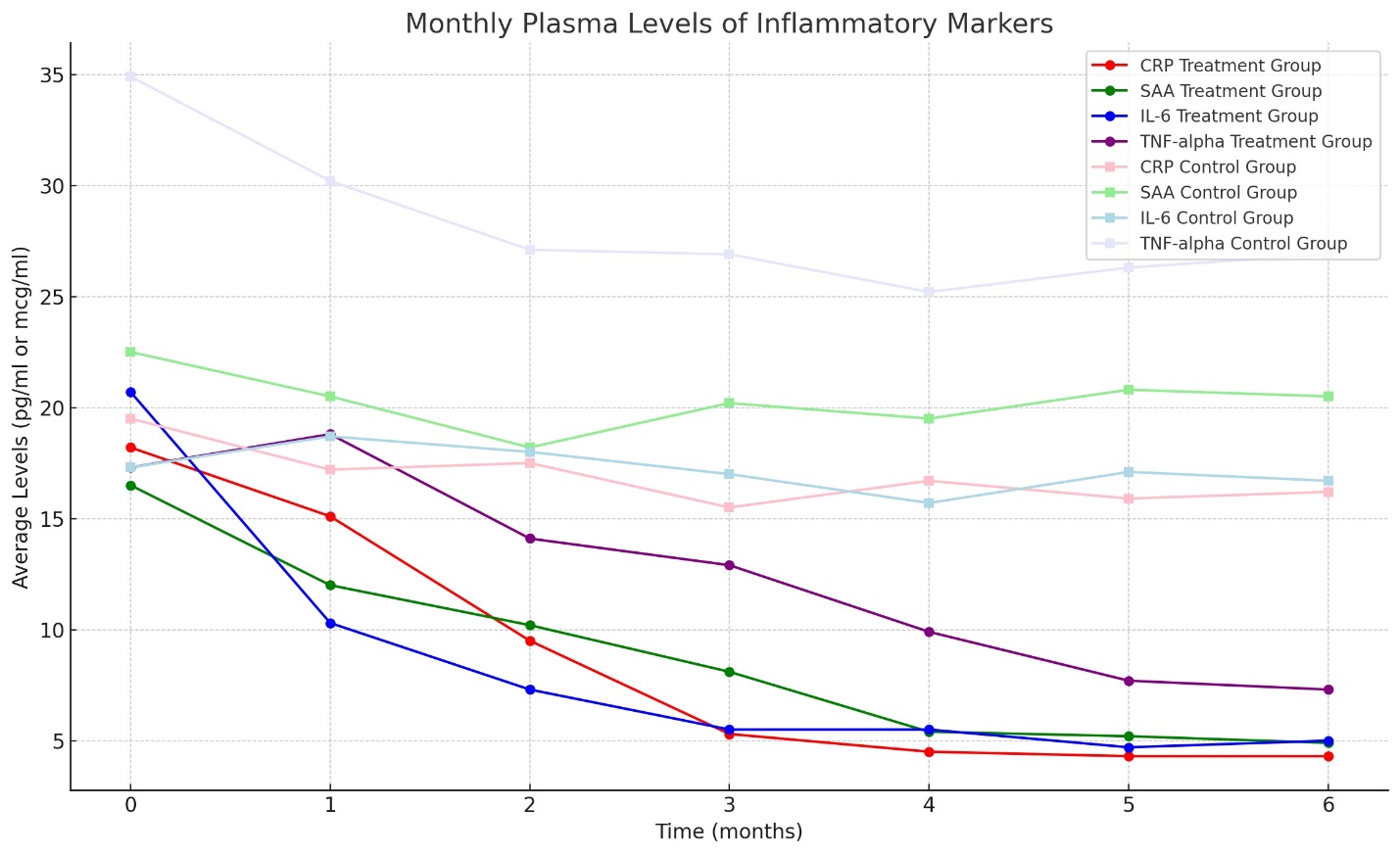
The treatment's impact on inflammatory markers was pronounced. CRP, ESR, SAA, and fibrinogen levels decreased substantially in the combination therapy group. In particular, CRP and SAA levels, which correlate with acute inflammatory episodes, were markedly lower in the study group by the study's conclusion. Procalcitonin levels, however, remained low in both groups, suggesting an absence of acute bacterial infection.
Interferon Levels
The interferon response was significantly amplified in the study group, with INF-alpha and gamma-INF showing an 8.7-fold increase compared to the control group. This robust interferon response is reflective of an enhanced innate immune response, potentially contributing to improved outcomes in liver disease (Data set (Graph N3) N3 and Table N3 summarizes the interferon response levels).
Cellular and Humoral Immunity
The cellular and humoral immunity profiles, as evidenced by the quantitative assessment of lymphocyte subtypes and immunoglobulin levels, showed a general trend of improvement. Over the treatment period, the study group experienced a 7-11% increase in immune status, suggesting a positive influence of the combination therapy on systemic immunity (The immune response trajectory over the treatment course is captured in Graph N2, N4 and Table N4).
Lipid Metabolism and Fatty Acid Spectrum
The study group's lipid profile underwent considerable positive changes, with a 27% improvement in overall lipid metabolism. These changes were consistent with the pharmacological actions of lycopene and piceatannol, known to modulate fat metabolism and exert anti-atherogenic effects.
Inflammatory and Oxidative Stress Parameters
The analysis of oxidative stress parameters revealed a significant reduction in reactive oxygen and nitrogen species in the combination therapy group, which aligns with the known antioxidative properties of the therapeutic agents used. The study group showed a consistent decrease in nitric oxide, superoxide anion, and hydrogen peroxide levels, indicating reduced oxidative stress and inflammatory markers (Graph #4 depicts the oxidative stress and inflammatory markers trends).
Complement System Activation
Measurements of complement system components (C3a, C4a, C5a), which provide insight into the inflammatory state and pathogen clearance, displayed a pronounced decrease in anaphylatoxin levels in the study group, thereby suggesting a decline in complement system-- mediated inflammation.
Adverse Events and Safety Monitoring
Throughout the trial, safety parameters were closely monitored, revealing that the treatments were well-tolerated with no significant adverse events reported. Mild gastrointestinal discomfort was the most commonly noted adverse event in both groups, with no significant difference in incidence rates between the groups.
Radiological Findings
Abdominal ultrasonography conducted pre- and post-treatment provided visual evidence of the reduction in hepatic steatosis, especially in the combination therapy group. The echogenicity indicative of fatty infiltration diminished more noticeably in patients receiving the combined treatment.
Summary of Results
The amassed data from our controlled trial demonstrate the substantial efficacy of ademethionine when used in conjunction with high-dose lycopene and piceatannol in the management of fatty liver disease. This combination therapy not only enhanced melatonin secretion but also yielded improvements in liver function, immune response, and inflammatory status. Importantly, these biochemical and clinical enhancements contribute to a comprehensive therapeutic approach, addressing both the direct pathology of fatty liver disease and its systemic effects.
The observed improvements in biomarkers and clinical outcomes in the study investigating the therapeutic impact of ademethionine, high-dose lycopene, and high-- dose piceatannol on melatonin secretion in patients with fatty liver disease (FLD) have significant clinical implications for the management of FLD and patient care. The combination therapy's effect on enhancing melatonin secretion and subsequently improving liver function, immune response, and reducing inflammatory and oxidative stress markers could redefine the approach to FLD treatment.
Firstly, the substantial increase in plasma melatonin levels in the combination therapy group suggests an improvement in the regulation of the body's circadian rhythms, which are often disrupted in FLD. Melatonin's role in modulating circadian rhythms is well established, and its antioxidant and anti-inflammatory properties can directly contribute to mitigating hepatic inflammation and oxidative stress, two pivotal processes in the pathogenesis of FLD.
Moreover, the improvements in liver function tests (LFTs) such as ALT, AST, GGT, ALP, and bilirubin levels emphasize the potential hepatoprotective effects of the combination therapy. Normalizing these enzyme levels is crucial in managing FLD, as it indicates reduced hepatic inflammation and damage, potentially slowing the progression of the disease towards more severe liver conditions like cirrhosis and hepatocellular carcinoma.
The enhancements in immune function markers and the significant reduction in inflammatory markers (CRP, ESR, SAA, and fibrinogen) observed in the study group underscore the combination therapy's role in modulating the immune response and reducing systemic inflammation. This is particularly relevant in FLD management, where chronic inflammation plays a central role in disease progression. An effective management strategy that includes modulation of the immune response and inflammation could lead to better disease outcomes and improved patient quality of life.
Furthermore, the observed changes in lipid metabolism and fatty acid spectrum in the study group highlight the potential of the combination therapy to address dyslipidemia, a common comorbidity in FLD patients. By improving lipid profiles and exerting anti-atherogenic effects, the therapy could contribute to reducing cardiovascular risk, which is elevated in FLD patients.
Lastly, the safety profile of the combination therapy, characterized by well-tolerated treatments with no significant adverse events, suggests a viable and effective treatment option for FLD patients. The minimal adverse events and the observed radiological reduction in hepatic steatosis further validate the clinical utility of this therapeutic approach.
In conclusion, the combination of ademethionine, high-dose lycopene, and high-dose piceatannol offers a multifaceted approach to managing FLD, addressing not only the liver pathology but also systemic effects of the disease. These findings advocate for the inclusion of this combination therapy in FLD management, promising improved clinical outcomes and patient care. Future studies should explore the long-term benefits and potential integration of this therapy into existing treatment paradigms for FLD.
Discussion
The results of this study contribute to the growing body of evidence supporting the potential therapeutic role of ademethionine, high-dose lycopene, and high-dose piceatannol in the treatment of fatty liver disease. The observed increase in melatonin secretion and the subsequent improvement in liver function tests, immune markers, and inflammatory profiles underscore the multifaceted benefits of this combination therapy.
Melatonin and Fatty Liver Disease
The restoration of melatonin levels observed in the treatment group is particularly noteworthy. Melatonin's role in synchronizing circadian rhythms to the light-dark cycle is well established, and its influence on metabolic pathways has been increasingly recognized. The perturbation of melatonin secretion in fatty liver disease patients can exacerbate metabolic disarray, leading to progression of the disease. Therefore, the therapeutic elevation of melatonin in these patients may have profound implications, not only for the management of fatty liver disease but also for the metabolic and cardiovascular complications commonly associated with it.
Ademethionine and Hepatic Health
Ademethionine's role in this context appears to extend beyond its known hepatoprotective and anti-inflammatory effects. Its contribution to the transmethylation reactions in hepatocytes is essential for the integrity of hepatic cells and the synthesis of glutathione, a major antioxidant. The presence of ademethionine alone was beneficial; however, when used in combination with lycopene and piceatannol, the benefits were significantly amplified.
Lycopene and Antioxidant Action
Lycopene's contribution to the reduction of oxidative stress is also significant. Oxidative stress plays a pivotal role in the initiation and progression of liver damage in fatty liver disease. Lycopene, a potent antioxidant, can mitigate this oxidative stress, thus potentially slowing the disease's progression and preventing further liver damage.
Piceatannol's Multifaceted Role
Piceatannol's role in the study aligns with its known biological activities, including modulation of cellular signaling pathways that are implicated in cell growth and apoptosis. The observed decrease in inflammatory markers and improvement in liver function may be partially attributable to the anti-inflammatory and antineoplastic effects of piceatannol. Furthermore, piceatannol's ability to activate sirtuins could signal a transition toward a more favorable metabolic state, contributing to the observed therapeutic outcomes.
Immunomodulatory Effects
The elevated levels of interferons and the improvement in immune status markers among patients receiving combination therapy suggest a positive modulatory effect of the treatment on the immune system. Chronic liver diseases, including fatty liver disease, have been associated with altered immune responses, which can exacerbate disease severity and complicate treatment. The findings of this study point to a possible adjunctive role of the combination therapy in enhancing immune function, which may contribute to the observed improvements in patient health.
Clinical Implications and Disease Progression
A significant consideration in the management of fatty liver disease is the risk of progression to cirrhosis and hepatocellular carcinoma. By addressing the fundamental disturbances in melatonin secretion and reducing inflammatory activity, this therapeutic approach may offer a preventive strategy against the oncological trajectory associated with advanced fatty liver disease. The decrease in ultrasonographically observed hepatic steatosis further substantiates the clinical efficacy of the treatment regimen.
Limitations and Future Research
While the study's findings are promising, limitations exist and should be acknowledged. The relatively small sample size and short duration of treatment necessitate cautious interpretation of the results. Long-term studies with larger patient cohorts are essential to validate these findings. Furthermore, exploring the molecular mechanisms underlying the observed effects will be crucial for a comprehensive understanding of the therapeutic potential of these agents in fatty liver disease.
In conclusion, the implications of this study are twofold. Firstly, they add to the understanding of the pathophysiology of fatty liver disease, highlighting the potential role of melatonin and associated metabolic dysregulation. Secondly, they present a compelling case for the use of ademethionine, lycopene, and piceatannol as a combined therapeutic strategy. The study paves the way for further research into their use as a standard care component in fatty liver disease treatment, with the potential to impact the prevention of associated oncological diseases significantly.
Conclusion
The current study's findings illuminate the promising therapeutic potential of combining ademethionine with high doses of lycopene and piceatannol for patients with fatty liver disease. The investigation addressed a critical gap in the treatment of this condition, which is witnessing a global surge in prevalence and is closely linked with the metabolic syndrome.
Our results demonstrated that the combination therapy significantly enhanced melatonin secretion, a crucial regulator of circadian and metabolic processes, which is often disrupted in patients with fatty liver disease. This increase in melatonin levels correlated with a marked improvement in liver function tests, immune profile markers, and inflammatory markers, signifying not only an improvement in liver health but also a potential reduction in the chronic inflammation associated with this condition.
The study's conclusion affirms the hypothesis that ademethionine's hepato-protective properties, when boosted by antioxidants like lycopene and anti-inflammatory agents such as piceatannol, can provide a robust therapeutic strategy. The multi-targeted approach of this therapy addresses various pathological facets of fatty liver disease, including oxidative stress, lipid metabolism dysregulation, and chronic low-grade inflammation, which are instrumental in disease progression.
Ademethionine's role as a methyl donor and a precursor for the synthesis of glutathione was anticipated to contribute to its beneficial effects. However, the addition of lycopene and piceatannol appeared to amplify these effects, suggesting a synergistic interaction that merits further exploration. Lycopene, with its strong antioxidative capacity, likely bolstered the defense against lipid peroxidation and oxidative stress-induced cellular damage. At the same time, piceatannol's modulation of signaling pathways may have contributed to improved cellular health and immune response.
Furthermore, the therapeutic regimen's impact on patient-reported outcomes, such as energy levels, concentration, and sleep quality, underscores the clinical relevance of the study's findings. By enhancing overall well-being, the combination therapy may also offer psychosocial benefits, which are often overlooked in the treatment of chronic liver diseases.
It is also critical to consider the broader implications of these findings. With fatty liver disease now recognized as a significant risk factor for the development of hepatocellular carcinoma (HCC), the observed improvement in liver health markers suggests that such combination therapy may play a role in cancer prevention. As HCC's association with NASH becomes increasingly apparent, interventions that can mitigate the underlying liver pathology bear the potential to reduce the incidence of this cancer.
Despite these positive outcomes, the study is not without its limitations, which have been acknowledged and should inform future research. The trial's relatively small sample size and short duration call for cautious interpretation of the results and a need for extended studies to confirm the long-term efficacy and safety of the treatment.
In light of these considerations, the study highlights the need for an integrated approach to managing fatty liver disease, advocating for a regimen that extends beyond conventional pharmacotherapy. Lifestyle modifications, including diet and exercise, remain the cornerstone of managing NAFLD and NASH. However, the addition of targeted pharmacological interventions such as those explored in this study can potentiate these lifestyle interventions.
The economic and healthcare burden of fatty liver disease, projected to increase in parallel with rising obesity rates, also calls for cost-effective and accessible treatment options. Given the safety profile and potential global applicability of ademethionine, lycopene, and piceatannol, this combination therapy may offer a feasible addition to current treatment protocols, with the advantage of addressing both the hepatic and extra-hepatic manifestations of the disease.
The findings from the study on the combined therapy of ademethionine, high-dose lycopene, and high-dose piceatannol for fatty liver disease (FLD) carry significant translational implications for both clinical practice and the development of future therapeutics. The positive outcomes, including enhanced melatonin secretion, improved liver function, bolstered immune response, and reduced inflammation, suggest a promising integrative approach to managing FLD—a condition increasingly prevalent worldwide and closely linked with metabolic syndrome.
Implications for Clinical Practice -The study underscores the necessity of a multi-targeted therapeutic strategy that addresses the complex pathology of FLD, which includes not just liver fat accumulation but also oxidative stress, inflammation, and immune dysregulation. The observed improvements in clinical outcomes and patient-reported well-being advocate for the incorporation of this combination therapy into the treatment paradigm for FLD. This could lead to a revision of current treatment guidelines to include such therapeutic combinations, emphasizing not only the biochemical impact but also the enhancement of patients' quality of life.
Healthcare professionals must consider adopting a more holistic treatment model for FLD patients, integrating targeted pharmacotherapy with lifestyle interventions such as diet and exercise. Our compelling results also highlight the importance of personalized medicine, where treatments could be tailored based on individual patient profiles, potentially increasing the efficacy of interventions for FLD.
Future Therapeutic Development - The synergy observed between ademethionine, lycopene, and piceatannol provides a valuable insight into the development of future therapeutics. It suggests that exploring combinations of agents with complementary mechanisms of action can lead to significant advancements in treating complex diseases like FLD. Future research should aim to uncover the underlying biological mechanisms that contributed to the observed effects, which could reveal new targets for therapeutic intervention.
Influence on Treatment Guidelines and Patient Care Strategies
The incorporation of these findings into treatment guidelines could transform the management of FLD by providing a more effective, comprehensive treatment strategy. This approach not only addresses the hepatic aspects of FLD but also its systemic implications, offering a pathway to mitigate the progression to more severe liver diseases and potentially reduce the risk of hepatocellular carcinoma.
For patient care strategies, the study highlights the importance of monitoring and improving patient-reported outcomes such as energy levels, concentration, and sleep quality. This reinforces the need for healthcare providers to adopt a patient-centered approach to care, where the goal is not just to manage the disease biochemically but also to enhance overall patient well-being.
In summary, the study's findings present a significant step forward in the fight against FLD, urging a shift towards integrated treatment approaches that are multidisciplinary, patient-centered, and tailored to individual patient needs. The potential for these findings to influence future therapeutic development, clinical practice, and treatment guidelines is immense, offering hope for more effective management of FLD and an improvement in patient outcomes. Future large-scale, randomized controlled trials will be crucial in validating these results and further exploring the therapeutic potential of such combination therapies in FLD and beyond.
In summary, our findings provide a rationale for the adoption of combination therapy involving ademethionine, lycopene, and piceatannol in the management of fatty liver disease. They also underscore the necessity for further large-scale, randomized controlled trials to validate these reresults and to explore the biological mechanisms underlying the observed effects. Ultimately, the study represents a step forward in the quest for comprehensive, multidisciplinary strategies to combat the growing tide of fatty liver disease and its associated comorbidities.
Acknowledgments
The authors are grateful to the Institute for Personalized Medicine for providing full-time access to genetics and molecular biology laboratories for a few weeks and Tbilisi State Medical University too.
Informed Consent Statement
Data Availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Author Contributions
A. Tavartkiladze, G.Simonia, N.Okrostsvaridze, P.Revazishvili and L.Tavartkiladze conceived and designed the experiment; A. Tavartkiladze, G.Simonia, N.Okrostvaridze and P.Revazishvili performed the experiments, analyzed the data, and wrote the manuscript; A. Tavartkiladze and L. Tavartkiladze contributed to data collection and manuscript revision; A. Tavartkiladze, G.Simonia and L.Tavartkiladze provided technical support and assisted with the experimental design. All authors contributed to manuscript revision and have read and approved the submitted version.
Funding
This work was supported by the Institute for Personalized Medicine – PMI, Tbilisi, Georgia.
Disclosure of Interest
The authors report no conflict of interest.
- Younossi ZM, Zelber-Sagi S, Henry L, Gerber LH (2023) Lifestyle interventions in nonalcoholic fatty liver disease. Nature Reviews Gastroenterology & Hepatology, 20: 708-22.
- Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L (2023) The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): A systematic review. Hepatology, 77: 1335-47.
- Teng MLP, Ng CH, Huang DQ, Chan KE, Tan DJH, et al. (2023) Global incidence and prevalence of nonalcoholic fatty liver disease. Clinical and Molecular Hepatology, 29: S32-42.
- Harrison SA, Taub R, Neff GW, Lucas KJ, Labriola D, et al. (2023) Resmetirom for nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled phase 3 trial. Nature Medicine, 29: 2919-28.
- Chen Y, Du X, Kuppa A, Feitosa MF, Bielak LF, et al. (2023) Genome-wide association meta-analysis identifies 17 loci associated with nonalcoholic fatty liver disease. Nature Genetics, 55: 1640-50.
- Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, Abdelmalek MF, Caldwell S, et al. (2023) AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology, 77: 1797-835.
- Kokkorakis M, Boutari C, Katsiki N, Mantzoros CS (2023) From non-alcoholic fatty liver disease (NAFLD) to steatotic liver disease (SLD): An ongoing journey towards refining the terminology for this prevalent metabolic condition and unmet clinical need. Metabolism, 147: 155664.
- Ko E, Yoon EL, Jun DW (2023) Risk factors in nonalcoholic fatty liver disease. Clinical and Molecular Hepatology, 29: S79–85.
- Chen L, Tao X, Zeng M, Mi Y, Xu L (2024) Clinical and histological features under different nomenclatures of fatty liver disease: NAFLD, MAFLD, MASLD, and MetALD. Journal of Hepatology, 80: E64-6.
- Wiering L, Tacke F (2022) Treating inflammation to combat non-alcoholic fatty liver disease. Journal of Endocrinology, 256: Article e220194.
- Terziev D, Terzieva D (2023) Experimental data on the role of melatonin in the pathogenesis of nonalcoholic fatty liver disease. Biomedicines, 11: 1722.
- Händel MN, Andersen HK, Ussing A, Virring A, Jennum P, et al. (2023) The short-term and long-term adverse effects of melatonin treatment in children and adolescents: A systematic review and GRADE assessment. E Clinical Medicine, 61: 102083.
- Hussain A, Gopalakrishnan A, Scott H, Seby C, Tang V, et al. (2023) Associations between systemic melatonin and human myopia: A systematic review. Ophthalmic and Physiological Optics.
- Chakraborty R, Seby C, Scott H, Tang V, Kemps E et al. (2024) Delayed melatonin circadian timing, lower melatonin output, and sleep disruptions in myopic, or short- -sighted, children. Sleep, 47: zsad265.
- Hirayama J, Hattori A, Takahashi A, Furusawa Y, Tabuchi Y et al. (2022) Physiological consequences of space flight, including abnormal bone metabolism, space radiation injury, and circadian clock dysregulation: Implications of melatonin use and regulation as a countermeasure. Journal of Pineal Research.
- Wei F, Locasale JW (2023) Methionine restriction and antitumor immunity. Trends in Cancer, 9: 705-6.
- Becquet P, Vazquez-Anon M, Mercier Y, Wedekind K, Mahmood T et al. (2023) A systematic review of metabolism of methionine sources in animals: One parameter does not convey a comprehensive story. Animal Nutrition.
- Kulawik A, Cielecka-Piontek J, Zalewski P (2023) The Importance of Antioxidant Activity for the Health-Promoting Effect of Lycopene. Nutrients, 15: 3821.
- Cai Z, Chen F, Wang Y, Wang X et al. (2023). Lycopene Maintains Mitochondrial Homeostasis to Counteract the Enterotoxicity of Deoxynivalenol. Antioxidants, 12: 1958.
- Gandhi H, Mahant S, Sharma AK, Kumar D, Dua K et al. (2023) Exploring the therapeutic potential of naturally occurring piceatannol in non-communicable diseases. Bio- Factors.
- Wang Z, Cai X, Ren Z, Shao Y, Xu Y et al. (2023) Piceatannol as an Antiviral Inhibitor of PRV Infection In Vitro and In Vivo. Animals, 13: 2376.
- Rakib A, Mandal M, Showkat A, Kiran S, Mazumdar S, et al. (2023) Piceatannol induces regulatory T cells and modulates the inflammatory response and adipogenesis. Biomedicine & Pharmacotherapy, 161: 114514.
- Wu Kj, Qian Qf, Zhou Jr, Sun Dl, Duan Yf, et al. (2023) Regulatory T cells (Tregs) in liver fibrosis. Cell Death Discovery, 9: 53.
- Yi S, Cong Q, Zhu Y, Xu Q (2023) Mechanisms of Action of Mesenchymal Stem Cells in Metabolic-Associated Fatty Liver Disease. 3919002.

Tables at a glance
Figures at a glance