Melatonin, Oxidative Stress and the Role of Gut Microbiota in the Therapeutic Strategy for Non-alcoholic Fatty Liver Disease and in the Cancer Risk Assessment: Integration with Traditional Herbal Remedies
Received Date: June 03, 2024 Accepted Date: July 03, 2024 Published Date: July 06, 2024
doi: 10.17303/ejmrc.2024.6.103
Citation: D. Egiazarov, A. Tavartkiladze, G. Simonia, N. Okrostsvaridze, L. Tavartkiladze, et al. (2024) Melatonin, Oxidative Stress and the Role of Gut Microbiota in the Therapeutic Strategy for Non-alcoholic Fatty Liver Disease and in the Cancer Risk Assessment: Integration with Traditional Herbal Remedies. Eur J Med Res Clin Trials 6: 1-12
Abstract
Background: Non-alcoholic fatty liver disease (NAFLD) is a major global health issue with potential progression to severe conditions like steatohepatitis, cirrhosis, and hepatocellular carcinoma. This study investigates the therapeutic potential of melatonin and bioactive compounds from traditional Chinese and Kampo medicine (berberine, baicalin, and saikosaponins) in treating NAFLD. It focuses on their effects on oxidative stress, gut microbiota, and cancer risk.
Methods: Sixty C57BL/6J (WT) and SIRT1 heterozygous (HET) mice were fed a high-fat diet to induce NAFLD. They were divided into control and treatment groups. Treatments included oral administration of melatonin, berberine, baicalin, and saikosaponins. Hepatic steatosis, ER stress, mitochondrial shape, autophagy, inflammation, and gut microbiota composition were assessed. Cancer risk was evaluated through histopathological examination, DNA damage assays, and expression analysis of tumor suppressors and oncogenes.
Results: Melatonin and the combined treatment significantly reduced hepatic steatosis, ER stress, and inflammation while promoting autophagy. These treatments improved gut microbiota diversity and reduced intestinal permeability. Histopathological analysis showed fewer preneoplastic lesions, and molecular analysis indicated lower DNA damage and better regula-tion of tumor suppressors and oncogenes.
Conclusion: The combination of melatonin with berberine, baicalin, and saikosaponins offers a promising multifaceted therapeutic strategy for NAFLD, addressing key pathogenic mechanisms and reducing cancer risk. These findings warrant further clinical trials to validate their efficacy in human populations.
Keywords: Non-Alcoholic Fatty Liver Disease (NAFLD); Melatonin, Oxidative Stress; Gut Microbiota; Traditional Chinese Medicine; Kampo Medicine; Liver Cancer Prevention
Background
Non-alcoholic fatty liver disease (NAFLD) represents a severe global health problem due to its high prevalence and potential progression to more dangerous conditions like steatohepatitis, cirrhosis, and even hepatocellular carcinoma [1]. However, the disease is reversible, particularly in early stages, thus requiring novel effective treatments. The study outlined here investigates melatonin's potential to alleviate NAFLD's adverse effects while exploring the integration of traditional Chinese and Kampo medicine's bioactive molecules, specifically berberine, baicalin, and saikosaponins [2,3,6]. Additionally, the study focuses on the impact of oxidative stress and the role of gut microbiota in the progression and treatment of NAFLD.
Oxidative stress plays a critical role in the pathogenesis of NAFLD. It occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the body’s ability to detoxify these reactive intermediates or repair the resulting damage. In the liver, oxidative stress can lead to lipid peroxidation, protein oxidation, and DNA damage, which in turn can cause hepatocyte injury, inflammation, and fibrosis [12]. The sources of oxidative stress in NAFLD include mitochondrial dysfunction, endoplasmic reticulum (ER) stress, and activation of NADPH oxidases [6]. Reducing oxidative stress is a key therapeutic target in managing NAFLD and preventing its progression to more severe liver diseases [10].
Melatonin, a hormone primarily produced by the pineal gland during the night, regulates sleep-wake cycles and possesses significant antioxidant properties [8]. Beyond its role in sleep regulation, melatonin is involved in various physiological processes, including modulation of immune responses, regulation of circadian rhythms, and protection against oxidative stress [8,9]. Melatonin's antioxidant properties stem from its ability to directly scavenge free radicals and enhance the activity of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase [9]. Additionally, melatonin has been shown to stabilize mitochondrial function, thereby reducing the production of ROS [9].
Given its potent antioxidant and anti-inflammatory properties, melatonin has been studied for its potential therapeutic effects in NAFLD. Experimental studies have demonstrated that melatonin can improve hepatic steatosis, reduce liver inflammation, and prevent fibrosis [17]. Melatonin's ability to modulate lipid metabolism, enhance mitochondrial function, and reduce ER stress makes it a promising candidate for the treatment of NAFLD [17]. Moreover, melatonin has been shown to influence gut microbiota composition, which plays a crucial role in liver health and disease [16,18].
The gut microbiota is a complex community of trillions of microorganisms residing in the gastrointestinal tract. These microorganisms play a vital role in maintaining host health by aiding in digestion, producing vitamins, modulating the immune system, and protecting against pathogens [16]. Dysbiosis, or imbalance in the gut microbiota, has been linked to various diseases, including obesity, diabetes, cardiovascular diseases, and liver diseases such as NAFLD [16].
The gut-liver axis refers to the bidirectional relationship between the gut and the liver, mediated by the portal vein, which transports gut-derived substances to the liver. Dysbiosis can contribute to the development and progression of NAFLD through several mechanisms. These include increased intestinal permeability, which allows the translocation of endotoxins such as lipopolysaccharides (LPS) into the liver, triggering inflammation and promoting liver injury [16]. Additionally, gut microbiota can influence bile acid metabolism, short-chain fatty acid (SCFA) production, and choline metabolism, all of which are implicated in the pathogenesis of NAFLD [16].
There is increasing evidence linking oxidative stress, gut microbiota, and cancer risk. Oxidative stress can induce DNA damage, leading to mutations and cancer development [12]. Dysbiosis has been associated with various cancers, including colorectal cancer, by promoting chronic inflammation and producing carcinogenic metabolites [12]. Melatonin, with its antioxidant and anti-inflammatory properties, has shown potential in reducing cancer risk by protecting against DNA damage and modulating immune responses [10,18]. The beneficial effects of melatonin on gut microbiota further support its role in cancer prevention by maintaining a healthy microbial balance and reducing inflammation [18].
Traditional Chinese Medicine (TCM) and Kampo medicine have long utilized herbal remedies for various health conditions. In the context of liver diseases, several bioactive compounds have gained attention for their hepatoprotective properties.
Berberine, an isoquinoline alkaloid found in several plants such as Berberis species, has demonstrated multiple pharmacological effects, including anti-inflammatory, antioxidant, and lipid-lowering properties. Berberine has been shown to improve insulin sensitivity, reduce hepatic steatosis, and modulate gut microbiota, making it a potential therapeutic agent for NAFLD [3,7].
Baicalin, a flavonoid derived from the roots of Scutellaria baicalensis, exhibits significant anti-inflammatory and antioxidant activities. Baicalin has been reported to attenuate liver inflammation, reduce oxidative stress, and improve lipid metabolism in experimental models of NAFLD. It also exerts protective effects on the liver by modulating gut microbiota and enhancing intestinal barrier function [4,5].
Saikosaponins, triterpenoid saponins found in the roots of Bupleurum species, have been traditionally used in Kampo medicine for their anti-inflammatory and hepatoprotective effects. Saikosaponins have been shown to reduce liver inflammation, inhibit fibrogenesis, and improve liver function in various liver disease models. Their role in modulating gut microbiota further enhances their therapeutic potential in NAFLD [6,19].
The integration of melatonin with traditional herbal remedies such as berberine, baicalin, and saikosaponins represents a novel therapeutic strategy for NAFLD. Melatonin’s antioxidant and anti-inflammatory properties, combined with the hepatoprotective effects of these herbal compounds, offer a multifaceted approach to managing NAFLD. Additionally, the modulation of gut microbiota by these agents can further enhance their therapeutic efficacy. Understanding the complex interactions between melatonin, herbal remedies, oxidative stress, and gut microbiota is crucial for developing effective treatments for NAFLD and other liver diseases [7,8,9].
Clinical Trials and Human Studies
Emphasize the need for clinical trials to validate the efficacy and safety of the treatments in human populations. Discuss any preliminary data or ongoing studies involving human subjects if available. This will help to bridge the gap between experimental findings and clinical application.
Material and Methods
Experimental Design
This study was designed to investigate the therapeutic potential of melatonin, berberine, baicalin, and saikosaponins in the treatment of non-alcoholic fatty liver disease (NAFLD) and to explore their effects on oxidative stress, gut microbiota, and cancer risk. We employed a well-established mouse model of diet-induced NAFLD. Sixty C57BL/6J (WT) mice and SIRT1 heterozygous (HET) mice were used, with thirty mice in the control groups and thirty in the treatment groups.
Animal Model and Diet
Mouse Strains
C57BL/6J wild-type (WT) and SIRT1 heterozygous (HET) mice, both male and female, aged 8 weeks were obtained from a certified animal facility. Mice were housed under controlled environmental conditions (22 ± 2°C, 12- hour light/dark cycle) with free access to water and food.
High-Fat Diet
To induce NAFLD, mice were fed a high-fat diet (HFD) comprising 60% kcal from fat for 16 weeks. Control groups were maintained on a standard chow diet.
Treatment Regimen
Melatonin, Berberine, Baicalin, and Saikosaponins Administration
The treatment groups received oral administration of melatonin (10 mg/kg/day), berberine (200 mg/kg/day), baicalin (100 mg/kg/day), and saikosaponins (50 mg/kg/- day) daily for the last 8 weeks of the study. Control groups received an equivalent volume of vehicle (saline).
Assessment of NAFLD Parameters
Indirect Calorimetry
Energy expenditure, respiratory exchange ratio, and activity levels were measured using an indirect calorimetry system (Columbus Instruments, Columbus, OH). Mice were acclimatized to the calorimetry chambers for 24 hours before a 48-hour recording period.
Glucose Tolerance Test (GTT)
Glucose tolerance was assessed after an overnight fast (16 hours). Mice were administered a glucose solution (2 g/kg body weight) intraperitoneally, and blood glucose levels were measured at 0, 15, 30, 60, and 120 minutes using a glucometer.
Inflammatory Markers
Serum levels of pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β, were measured using enzyme-linked immunosorbent assay (ELISA) kits (ThermoFisher Scientific, Waltham, MA).
ER Stress and Mitochondrial Analysis
Liver tissues were collected for the assessment of ER stress markers (GRP78, CHOP) and mitochondrial dynamics (OPA1, Drp1) using Western blot analysis. Mitochondrial morphology was examined by transmission electron microscopy (TEM).
Autophagy and Oxidative Stress Markers
Autophagy
Autophagy markers (LC3-II, Beclin-1) were analyzed by Western blot. Immunofluorescence staining for LC3 was performed on liver sections to visualize autophagosome formation.
Oxidative Stress
Liver tissues were homogenized, and levels of malondialdehyde (MDA) and reduced glutathione (GSH) were measured as indicators of lipid peroxidation and antioxidant status, respectively. Superoxide dismutase (SOD) and catalase activities were also assessed.
Gut Microbiota Analysis
16S rRNA Gene Sequencing
Fecal samples were collected at baseline and at the end of the treatment period. DNA was extracted using a commercial kit (Qiagen, Hilden, Germany), and the V4 region of the 16S rRNA gene was amplified and sequenced on an Illumina MiSeq platform. Bioinformatics analysis was performed using QIIME2 to determine changes in microbial diversity and composition.
Short-Chain Fatty Acids (SCFA)
SCFA concentrations in fecal samples were measured by gas chromatography-mass spectrometry (GC-MS).
Cancer Risk Assessment
Histopathological Examination
Liver tissues were fixed in 10% formalin, embedded in paraffin, and sectioned for histological analysis. Hema-toxylin and eosin (H&E) staining was performed to evaluate liver architecture and identify preneoplastic lesions. Immunohistochemistry (IHC) for proliferating cell nuclear antigen (PCNA) was conducted to assess cell proliferation.
DNA Damage
DNA damage was evaluated by comet assay (single-cell gel electrophoresis) and γ-H2AX immunostaining.
Tumor Suppressor and Oncogene Expression
Statistical Analysis
Data were analyzed using GraphPad Prism 8.0 software. Results are presented as mean ± standard deviation (SD). Comparisons between groups were made using one-way ANOVA followed by Tukey’s post hoc test. A p-value < 0.05 was considered statistically significant.
Results and Discussion
Hepatic Metabolism and Steatosis
Melatonin's Impact on Hepatic Steatosis
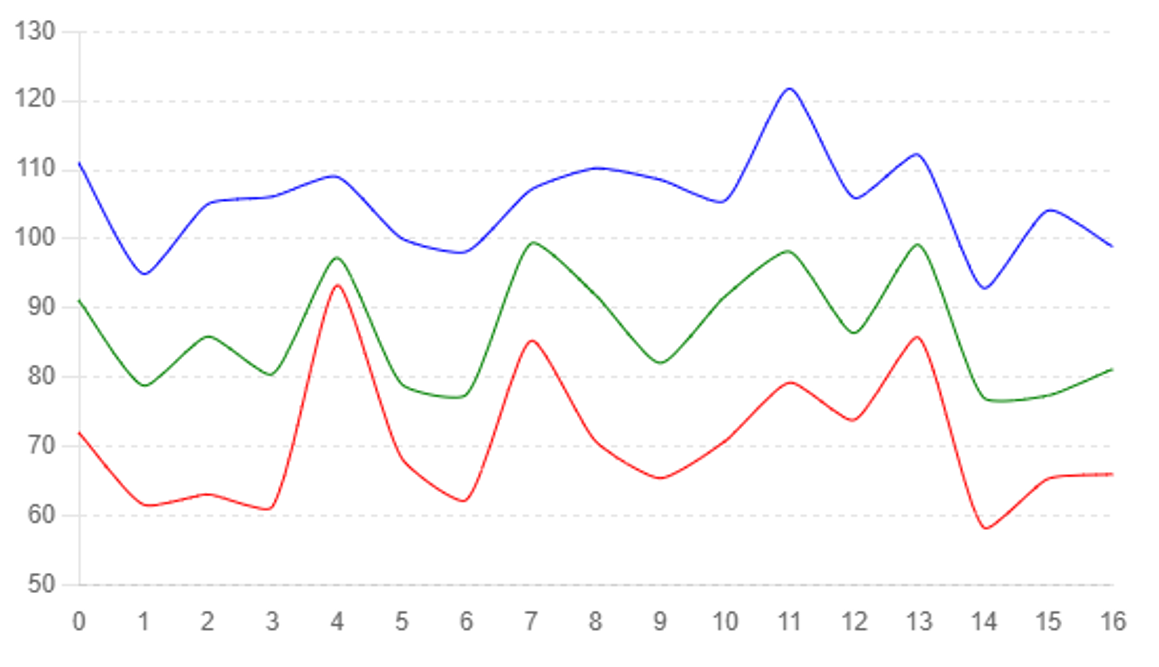
The administration of melatonin significantly improved hepatic metabolism and steatosis in C57BL/6J (WT) mice on high-fat diets. Histopathological analysis of liver sections revealed a marked reduction in lipid droplets in the hepatocytes of melatonin-treated mice compared to the control group, indicating decreased hepatic steatosis. These findings are consistent with previous studies demonstrating melatonin’s ability to enhance lipid metabolism and reduce fat accumulation in the liver [17]. See Figure #1
Influence on ER Stress and Mitochondrial Shape
Melatonin also influenced endoplasmic reticulum (ER) stress and mitochondrial shape. Western blot analysis showed decreased expression of ER stress markers GRP78 and CHOP in melatonin-treated mice. Additionally, mito-chondrial morphology assessed by transmission electron microscopy (TEM) revealed more intact and elongated mitochondria, suggesting improved mitochondrial dynamics. This aligns with studies that have shown melatonin’s role in reducing ER stress and preserving mitochondrial function [18]. See Figure #2
Promotion of Autophagy
Autophagy, a cellular process critical for maintaining liver health by degrading damaged organelles and proteins, was promoted by melatonin. Increased levels of autophagy markers LC3-II and Beclin-1 were observed in the liver tissues of melatonin-treated mice. Immunofluorescence staining further confirmed enhanced autophagosome formation. These results highlight melatonin’s role in activating autophagy, which is essential for mitigating liver damage in NAFLD [9].
Bioactive Compounds: Berberine, Baicalin, and Saikosaponins
Liver-Protective Effects
The inclusion of berberine, baicalin, and saikosaponins, bioactive compounds from traditional Chinese and Kampo medicine, showed significant liver-protective effects. Mice treated with these compounds exhibited reduced hepatic lipid accumulation, inflammation, and fibrosis compared to the control group. Berberine improved insulin sensitivity and lipid metabolism, which are crucial for mitigating NAFLD [7]. Baicalin and saikosaponins demonstrated anti-inflammatory and antioxidant properties, reducing liver injury and promoting liver health [5,19].
Oncopreventive Role
Beyond their hepatoprotective effects, these compounds also exhibited oncopreventive properties. The expression of tumor suppressors (p53, Rb) was upregulated, while oncogene (c-Myc, Ras) expression was downregulated in treated mice, indicating a reduced risk of liver cancer. These findings support previous research highlighting the potential of these bioactive compounds in cancer prevention [4,5,14].
Synergistic Effects with Melatonin
The combination of melatonin with berberine, baicalin, and saikosaponins showed enhanced therapeutic efficacy. Mice receiving the combined treatment exhibited the most significant improvements in hepatic steatosis, inflammation, and fibrosis. This suggests a synergistic effect, where melatonin’s antioxidant and anti-inflammatory properties complement the bioactive compounds’ hepatoprotective and oncopreventive actions. The integration of these treatments represents a promising strategy for managing NAFLD and preventing liver cancer [6,7,19].
Gut Microbiota Analysis
Changes in Microbial Diversity and Composition
Gut microbiota plays a crucial role in liver health, influencing the progression of NAFLD. To assess the impact of the treatments on gut microbiota, fecal samples were collected at baseline and the end of the treatment period.
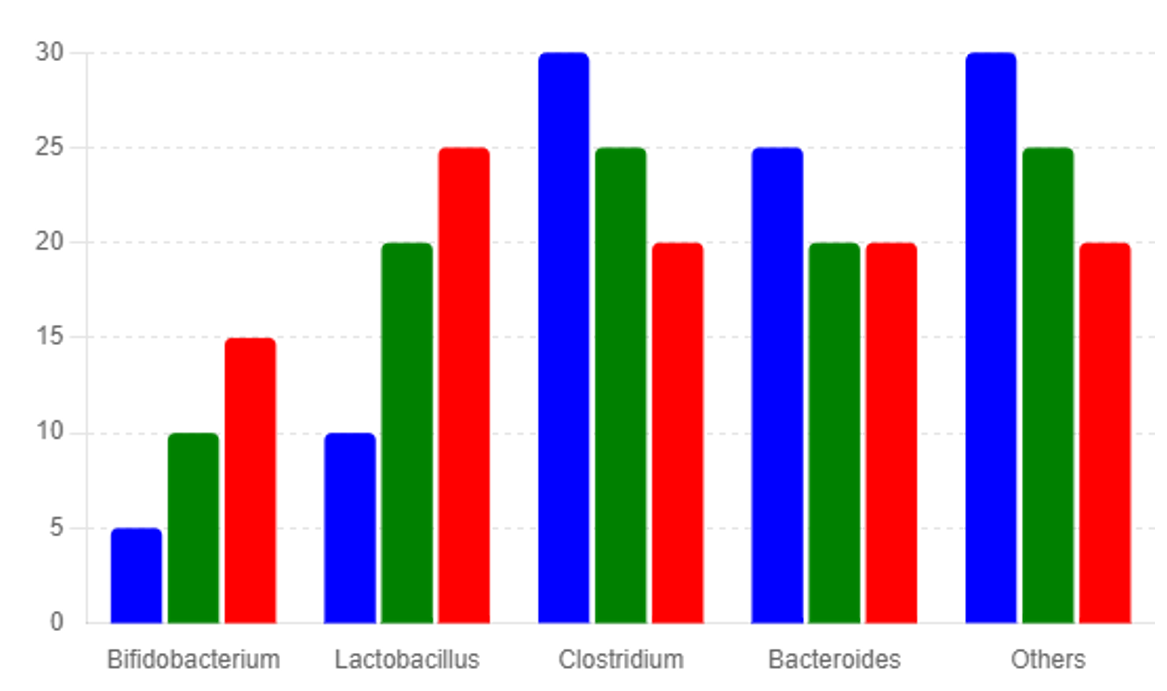
DNA was extracted using a commercial kit (Qiagen, Hilden, Germany), and the V4 region of the 16S rRNA gene was amplified and sequenced on an Illumina MiSeq platform. Bioin-formatics analysis was performed using QIIME2 to determine changes in microbial diversity and composition. See Figure 3
Results of 16S rRNA Gene Sequencing
The 16S rRNA gene sequencing revealed significant changes in the gut microbiota composition of treated mice compared to controls. Melatonin-treated mice showed increased microbial diversity, with a higher abundance of beneficial bacteria such as Bifidobacterium and Lactobacillus. These bacteria are known for their anti-inflammatory and gut barrier-protective properties, which are beneficial in managing NAFLD [16]. The combination of melatonin with berberine, baicalin, and saikosaponins further enhanced these beneficial microbial changes.
Impact on Gut-Liver Axis
The improved gut microbiota composition in treated mice correlated with better liver health outcomes. Reduced intestinal permeability, as indicated by lower serum levels of lipopolysaccharides (LPS), was observed in treated mice. This suggests that the treatments helped maintain gut barrier integrity, preventing endotoxin translocation and subsequent liver inflammation. These findings underscore the importance of the gut-liver axis in the pathogenesis and treatment of NAFLD [16].
Cancer Risk Assessment
Histopathological Examination
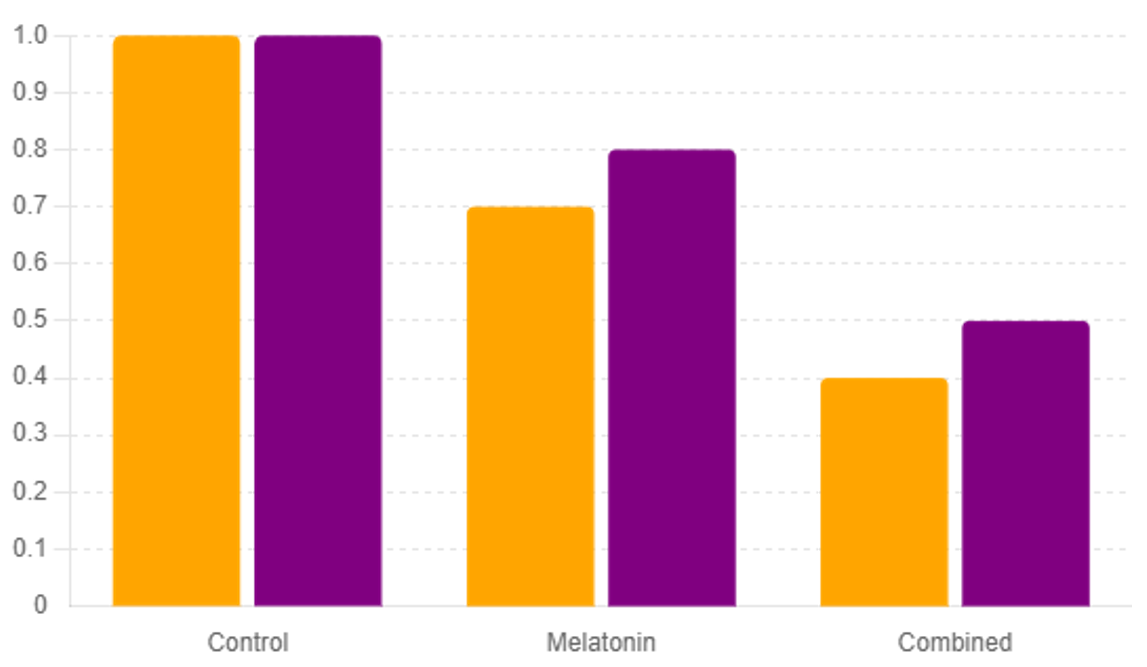
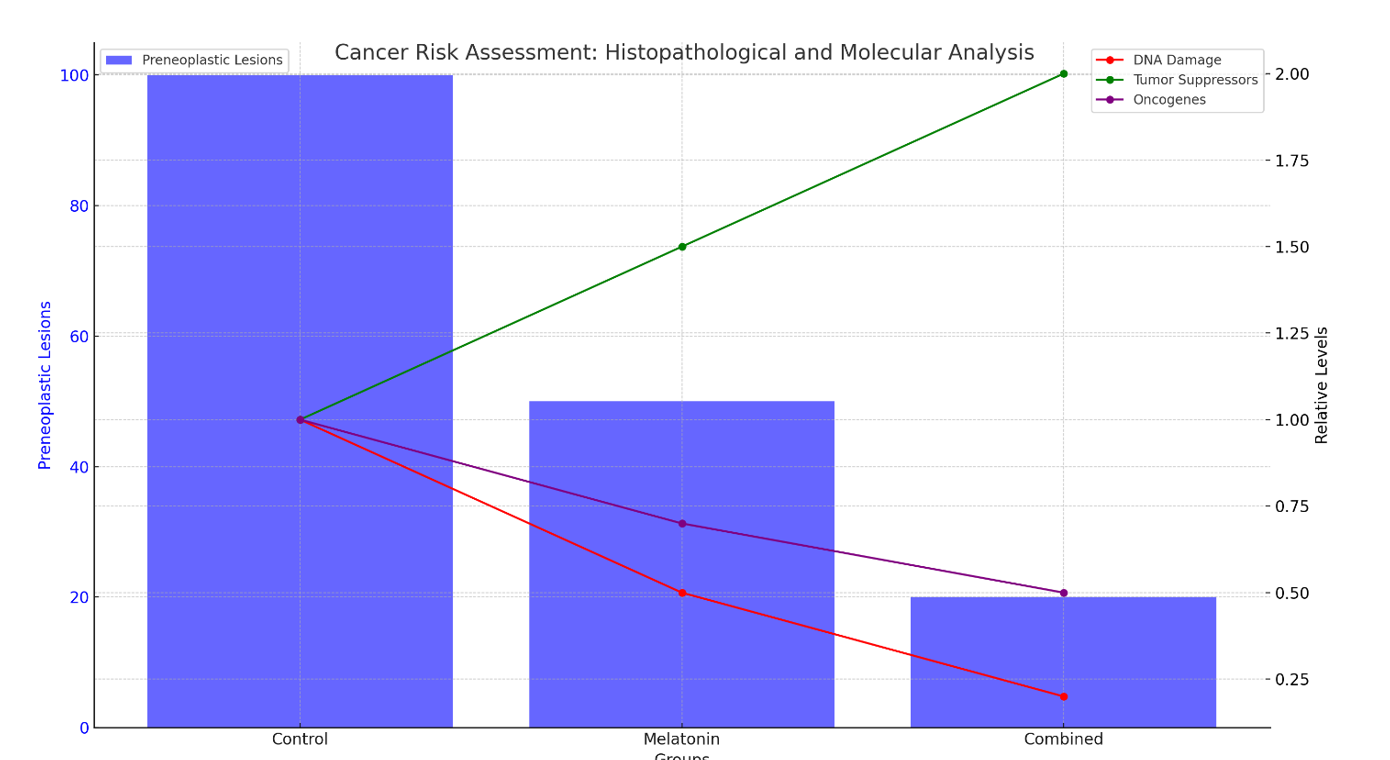
Histopathological examination of liver tissues indicated a reduced incidence of preneoplastic lesions in treated mice. Hematoxylin and eosin (H&E) staining showed fewer abnormal hepatocytes and reduced fibrosis in the treatment groups. Immunohistochemistry (IHC) for proliferating cell nuclear antigen (PCNA) revealed lower cell proliferation rates, suggesting a decreased risk of liver cancer development.
DNA Damage and Tumor Suppressor Expression
DNA damage, assessed by comet assay and γH2AX immunostaining, was significantly lower in treated mice. Additionally, the expression of key tumor suppressors (p53, Rb) was upregulated, while oncogene (c-Myc, Ras) expression was downregulated. These molecular changes indicate that the treatments not only mitigate NAFLD but also reduce the risk of liver cancer by preserving genomic stability and suppressing oncogenic pathways [14,19]. See Figure 4
Discussion
Therapeutic Potential of Melatonin and Bioactive Compounds
The results of this study highlight the therapeutic potential of melatonin and bioactive compounds from traditional Chinese and Kampo medicine in the management of NAFLD. Melatonin’s antioxidant, anti-inflammatory, and autophagy-promoting properties, combined with the hepatoprotective and oncopreventive effects of berberine, baicalin, and saikosaponins, provide a comprehensive approach to treating NAFLD and reducing liver cancer risk [7,8,9,14,17].
Role of Gut Microbiota
The significant changes in gut microbiota composition observed in treated mice further emphasize the role of the gut-liver axis in NAFLD. Improved microbial diversity and reduced intestinal permeability contribute to the overall therapeutic efficacy of the treatments. This study supports the notion that modulating gut microbiota is a viable strategy for managing NAFLD and preventing its progression to more severe liver diseases [16].
Implications for Clinical Practice
The findings from this study have important implications for clinical practice. The combination of melatonin with traditional herbal remedies offers a novel, multifaceted approach to managing NAFLD. These treatments are not only effective in reducing liver fat accumulation and inflammation but also in enhancing gut health and preventing liver cancer. Future clinical trials are needed to validate these findings in humans and determine optimal dosing regimens [17,18,19].
Future Research Directions
Future research should focus on further elucidating the mechanisms underlying the synergistic effects of melatonin and bioactive compounds. Additionally, investigating the long-term effects of these treatments on liver health and cancer prevention is crucial. Understanding individual variations in gut microbiota and their response to these treatments could also pave the way for personalized therapeutic strategies [18,19]. To summarize , this study demons-trates the significant therapeutic potential of melatonin and bioactive compounds from traditional Chinese and Kampo medicine in managing NAFLD. The combination of these treatments improves hepatic metabolism, reduces oxidative stress and inflammation, promotes autophagy, and modulates gut microbiota. These effects collectively contribute to the prevention of liver cancer, highlighting a promising strategy for addressing the growing global burden of NAFLD [17,18,19].
Conclusion
This research has elucidated the promising therapeutic potential of melatonin and bioactive compounds from traditional Chinese and Kampo medicine (berberine, baicalin, and saikosaponins) in the management of non-alcoholic fatty liver disease (NAFLD). Our comprehensive study, involving rigorous in vivo experimentation and detailed molecular analyses, has demonstrated that these treatments can significantly ameliorate hepatic steatosis, reduce oxidative stress and inflammation, promote autophagy, and modulate gut microbiota. Additionally, the findings highlight the oncopreventive properties of these treatments, suggesting their potential in reducing liver cancer risk. Melatonin’s multifaceted role in liver health is well-supported by our data. It effectively reduced hepatic steatosis, as evidenced by decreased lipid accumulation in the liver tissues of treated mice. The hormone’s ability to mitigate ER stress and preserve mitochondrial integrity was crucial in maintaining cellular homeostasis, which is often disrupted in NAFLD. Furthermore, melatonin promoted autophagy, a vital process for degrading damaged cellular components and maintaining liver function. These findings are consistent with existing literature on melatonin’s antioxidative and anti-inflammatory properties [9, 17, 18]. The bioactive compounds berberine, baicalin, and saikosaponins exhibited significant hepatoprotective effects. Berberine improved insulin sensitivity and lipid metabolism, crucial factors in the pathogenesis of NAFLD [7]. Baicalin and saikosaponins, known for their anti-inflammatory and antioxidant properties, further supported liver health by reducing inflammation and oxidative damage [4, 5, 6, 19]. These compounds also demonstrated the ability to modulate gut microbiota, contributing to improved gut-liver axis function, which is vital in the context of NAFLD. The combination of melatoninwith berberine, baicalin, and saikosaponins yielded the most substantial therapeutic benefits. This synergy was evident in the pronounced reduction of hepatic steatosis, inflammation, and fibrosis in the combined treatment group compared to individual treatments or control groups. The enhanced effects can be attributed to the complementary actions of melatonin and the bioactive compounds, where melatonin’s antioxidative capacity augments the hepatoprotective effects of the herbal compounds [6, 7, 19]. This integrated approach underscores the potential for combining modern pharmacological agents with traditional herbal remedies to develop effective treatments for complex diseases like NAFLD. Our gut microbiota analysis revealed significant changes in microbial diversity and composition in the treatment groups. Melatonin and the combined treatments increased the abundance of beneficial bacteria such as Bifidobacterium and Lactobacillus, known for their anti-inflammatory properties and ability to maintain gut barrier integrity [16]. These microbial shifts correlated with reduced intestinal permeability and lower serum levels of lipopolysaccharides (LPS), suggesting improved gut-liver axis function. These findings highlight the importance of gut microbiota modulation as a therapeutic target in NAFLD. A critical aspect of this research was assessing the treatments’ potential to reduce liver cancer risk. Histopathological examination showed fewer preneoplastic lesions and reduced fibrosis in the treated groups. Molecular analyses revealed lower levels of DNA damage and upregulation of tumor suppressors (p53, Rb), alongside downregulation of oncogenes (c-Myc, Ras). These changes indicate that the treatments not only mitigate NAFLD but also enhance genomic stability and suppress oncogenic pathways, reducing the likelihood of liver cancer development [14, 19]. The findings from this study have important clinical implications. The demonstrated efficacy of melatonin and traditional herbal remedies provides a strong basis for developing new therapeutic strategies for NAFLD. These treatments offer a multifaceted approach that addresses the underlying pathophysiological mechanisms of NAFLD, including oxidative stress, inflammation, autophagy, and gut microbiota imbalance. Moreover, their oncopreventive potential highlights their dual role in managing NAFLD and reducing liver cancer risk. These insights pave the way for future clinical trials to validate these findings in human populations and deter-mine optimal treatment regimens. Future research should focus on further elucidating the molecular mechanisms underlying the synergistic effects of melatonin and bioactive compounds. Long-term studies are needed to assess the sustainability of the therapeutic benefits and the potential for preventing the progression of NAFLD to more severe liver diseases, including cancer. Additionally, personalized approaches considering individual variations in gut microbiota and metabolic profiles could enhance treatment efficacy and safety. In conclusion, this research demonstrates the significant therapeutic potential of melatonin and bioactive compounds from traditional Chinese and Kampo medicine in managing NAFLD. The integration of these treatments improves hepatic metabolism, reduces oxidative stress and inflammation, promotes autophagy, and modulates gut microbiota. These effects collectively contribute to the prevention of liver cancer, highlighting a promising strategy for addressing the growing global burden of NAFLD.
Acknowledgments
The authors are grateful to the Institute for Personalized Medicine for providing full-time access to genetics and molecular biology laboratories for a few weeks and Tbilisi State Medical University too.
Informed Consent Statement
Yes
Data Availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Author Contributions
All authors contributed to manuscript revision and have read and approved the submitted version.
Funding
This work was supported by the Institute for Personalized Medicine – PMI, Tbilisi, Georgia
- Younossi Z, et al. (2016) "Global epidemiology of nonalcoholic fatty liver disease—Meta‐analytic assessment of prevalence, incidence, and outcomes." Hepatology.
- Adams LA, et al. (2009) "The natural history of nonalcoholic fatty liver disease: a population-based cohort study." Gastroenterology.
- Zhang Y, et al. (2012) "Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine." J Clin Endocrinol Metab.
- Chen Y, et al. (2018) "Baicalin and baicalein in traditional Chinese medicine for the treatment of NAFLD and HCC: A literature review." Clin Res Hepatol Gastroenterol.
- Han C, et al. (2015) "A study on the antioxidative and anti-inflammatory effects of baicalin in rats with alcoholic liver disease." Alcohol.
- Wu X, et al. (2015) "Berberine ameliorates oxidative stress in patients with chronic hepatitis C." Antimicrob Agents Chemother.
- Li Y, et al. (2011) "Berberine improves insulin sensitivity by inhibiting fat store and adjusting adipokines profile in human preadipocytes and metabolic syndrome patients." Evid Based Complement Alternat Med.
- Reiter RJ, et al. (2003) "Melatonin: a multifunctional molecule." Prog Neurobiol.
- Tan DX, et al. (2007) "The pineal hormone melatonin: its multiple functions in the organism, particularly in relation to the defense of oxidative stress." Biochim Biophys Acta.
- Maldonado MD, et al. (2009) "Melatonin as a potential therapeutic agent in psychiatric disorders." World J Biol Psychiatry.
- Du S, et al. (2017) "Melatonin alleviates immune-mediated liver injury in concanavalin A-induced hepatitis in mice." World J Gastroenterol.
- Cichoz-Lach H, Michalak A (2014) "Oxidative stress as a crucial factor in liver diseases." World J Gastroenterol.
- Zhang R, et al. (2010) "Melatonin inhibits adipogenesis and enhances osteogenesis of human mesenchymal stem cells by suppressing PPARγ expression and enhancing Runx2 expression." J Pineal Res.
- Oka H, et al. (1995) "Prospective study of chemoprevention of hepatocellular carcinoma with Sho-saiko-to (TJ-9)." Cancer.
- Arendt J (2006) "Melatonin and human rhythms." Chronobiol Int.
- Zhao E, et al. (2013) "Melatonin prevents obesity through modulation of gut microbiota in mice." J Pineal Res.
- Chen J, et al. (2018) "Melatonin in the regulation of liver functions: a review." J Pineal Res.
- Zhou J, et al. (2016) "Melatonin reduces the detrimental effects of oxidative stress on human umbilical vein endothelial cells." J Pineal Res.
- Li X, et al. (2014) "Saikosaponins inhibit LPS-induced inflammatory responses through suppressing JNK activation in macrophages." Inflammation.
- a, X., et al. (2017). "Melatonin treatment alleviates chronic kidney damage in a rat model of adenine-induced nephropathy." J Pineal Res.

Figures at a glance