Network-Aware Polypharmacology: Multitarget Strategies for the Polysyndromic Heart
Received Date: August 06, 2025 Accepted Date: August 16, 2025 Published Date: August 20, 2025
doi:10.17303/ejmrc.2025.7.102
Citation: Tianqi Duo, Zhiguo Wang (2025) Network-Aware Polypharmacology: Multitarget Strategies for the Polysyndromic Heart. Eur J Med Res Clin Trials 7: 1-9
Abstract
Cardiovascular syndromes such as heart failure with preserved ejection fraction (HFpEF), Cardiovascular-Kidney-Metabolic Syndrome, and metabolic dysfunction–associated steatotic liver disease (MASLD) arise from tightly interwoven biology-metabolic stress, inflammation, fibrosis, and neurohumoral activation-that defies the one-target-one-drug model. In this mini review, we synthesize how network aware polypharmacology-single agents with multi node activity, rational combinations, repurposed drugs, and RNA therapeutics-aligns with the biology of the polysyndromic heart. What is new here is a concise, clinician oriented framework that (i) contrasts polypharmacology with traditional monotherapy and simple combination therapy; (ii) foregrounds the gut-liver-heart axis as a tractable, underused entry point in MASLD related cardiac dysfunction; and (iii) highlights RNA modalities as programmable tools for multi node modulation. We link major drug classes to specific trial outcomes and patient reported benefits to move beyond cataloging toward practical translation. Together, these advances point to integrated, network aware therapeutics capable of addressing the polysyndromic heart.
Keywords: Polypharmacology; HFpEF; CKM Syndrome; MASLD; Cardiometabolic; Multitarget Drugs; Network Pharmacology; RNA-Based Therapeutics
Introduction
From Organ Silos to Polysyndromes
The growing burden of cardiometabolic diseases reflects not only an aging population but also the increasing prevalence of obesity, type 2 diabetes, and hypertension. The clinical presentation of these conditions often overlaps, resulting in diagnostic entities that span multiple organ systems. For example, HFpEF is frequently accompanied by obesity, insulin resistance, atrial fibrillation, and chronic kidney disease-all of which interact through inflammatory, neurohumoral, and fibrotic pathways [1,2]. Similarly, CKM syndrome reflects an interlinked decline in cardiovascular, renal, and metabolic function, with shared risk factors and bidirectional pathophysiological mechanisms [3,4].
The recognition that these are not isolated diseases but integrated syndromes has led to a reevaluation of treatment paradigms. Standard-of-care therapies developed under reductionist models targeting a single pathway (e.g., β-blockade or RAS inhibition) often yield modest results in syndromic populations. This has prompted a shift toward therapeutic models that acknowledge complexity and embrace polypharmacology as both a conceptual and clinical solution.
In modern cardiology, clinicians increasingly face syndromes that transcend traditional organ-based classification. Conditions such as HFpEF [1,2], CKM syndrome [3,4], and MASLD [5-7] represent multisystem disorders with intertwined mechanisms affecting the heart, kidneys, liver, vasculature, and adipose tissue. These syndromes resist simplistic, linear therapeutic interventions. Rather than a single dominant driver, they are fueled by a web of inflammation, fibrosis, metabolic stress, and neurohumoral imbalance.
Traditional monotherapies often target singular pathways. However, for complex diseases with redundant, nonlinear networks, single-target approaches are prone to therapeutic failure or limited efficacy. A shift toward multitarget therapeutic strategies-anchored in polypharmacology-is not only logical but essential [8,9].
Working Premise
When biology is distributed across modules (inflammation-fibrosis-metabolism-hemodynamics), therapies must also be distributed. Polypharmacology-by design or by informed combination-offers that distribution and, crucially, can be tuned to patient phenotype.
Understanding Polypharmacology in the Cardiovascular Context-an Author Perspective
Polypharmacology offers a nuanced understanding of drug behavior, acknowledging that most drugs interact with multiple targets-even if originally designed for a single mechanism [8,9]. Moreover, polypharmacology facilitates a systems approach to disease management. It moves beyond the symptom-by-symptom model and targets the system-wide dysfunction that characterizes syndromic disease, aligning with precision medicine and personalized treatment paradigms.
Definitions of Polypharmacology with Contrasts.
•Monotherapy aims at one dominant pathway. Effective when pathogenesis is concentrated (e.g., HFrEF neurohormonal blockade), less so in HFpEF[1-3].
•Simple combination therapy stacks independent single target agents; effectiveness depends on additive-not necessarily synergistic-benefit and tolerability.
•Network aware polypharmacology intentionally engages multiple, mechanistically connected nodes (via single pleiotropic agents, fixed dose combinations, or sequenced regimens) to intercept feedback and compensatory escape [8, 9].
Clinical Intuition
Cardiovascular diseases and metabolic disorders are particularly suitable for polypharmacologic intervention due to the convergence of numerous molecular cascades. The vasculature, myocardium, and kidneys respond to metabolic, hormonal, and inflammatory cues, often through overlapping signaling networks. Drugs capable of modulating multiple points within these networks may exert enhanced efficacy or resilience against compensatory escape pathways.
In HFpEF, unloading without anti-inflammatory or metabolic effects leaves endothelial dysfunction and skeletal muscle abnormalities untouched [1,2]. In CKM, glycemic control without renal hemodynamic and tubular anti inflammation underperforms [3]. In MASLD, liver only strategies miss adipose and gut mediated drivers [5-7]. Polypharmacology is not an ideology but a practical alignment with these realities.
Tradeoffs
Benefits include pathway synergy and durability; risks include off target effects and complexity. Network guidance (omics, phenomapping) helps tip the balance [8,9].
Syndromic Complexity: Why One Target Is Not Enough
The clinical inertia surrounding HFpEF reflects the limitations of monotherapies in syndromic conditions. Unlike HFrEF, where neurohormonal antagonists provide mortality benefits, HFpEF has seen multiple neutral trials. These include studies of spironolactone (TOPCAT) [10, 11], neprilysin inhibition (PARAGON-HF) [12,13], and endothelin receptor antagonists. One reason is the heterogeneity of HFpEF pathophysiology, where no single pathway predominates.
CKM syndrome similarly defies single-target solutions. Diabetic kidney disease progresses via hyperfiltration, tubulointerstitial inflammation, and RAAS activation. Simultaneous control of glycemia, blood pressure, and inflammation is necessary. MASLD, too, reflects a complex interplay of hepatic fat accumulation, lipotoxicity, oxidative stress, and systemic inflammation that feeds back into cardiovascular dysfunction.
Syndromic Mechanisms at a Glance
HFpEF: endothelial-microvascular dysfunction, obesity-related inflammation, extracellular matrix remodeling, pulmonary hypertension, and skeletal myopathy interact to produce exertional intolerance and congestion [1,2].
CKM: hyperfiltration, tubulointerstitial inflammation, and RAAS activation amplify cardiac congestion and metabolic stress; multicomponent control is required [3].
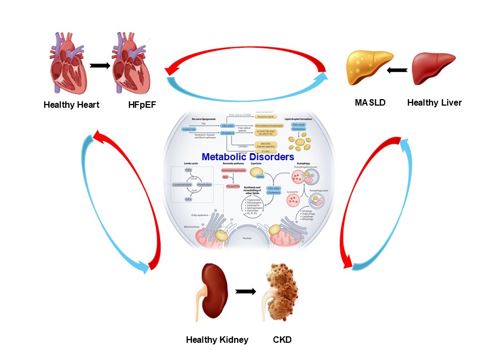
MASLD and the gut-liver-heart axis (expanded): hepatic steatosis and lipotoxicity propagate systemic insulin resistance and low-grade inflammation. Dysbiosis increases gut permeability and endotoxemia, shifts bile acid pools and short-chain fatty acids, and elevates trimethylamine-N-oxide (TMAO)-all linked to vascular dysfunction, diastolic impairment, and renal injury [5-7]. Targeting this axis (weight loss, GLP-1RA, SGLT2i, dietary fiber, and microbiome-informed strategies) is a rational polypharmacologic lever [5-7].
Bidirectional loops connecting HFpEF, CKM, and MASLD (inflammation, fibrosis, neurohumoral activation, metabolic dysfunction), with an inset highlighting the gut-liver-heart axis (Figure 1). In this context, polypharmacologic agents offer the ability to address multiple disease-driving nodes concurrently, with the potential to break the cycle of progressive multi-organ damage.
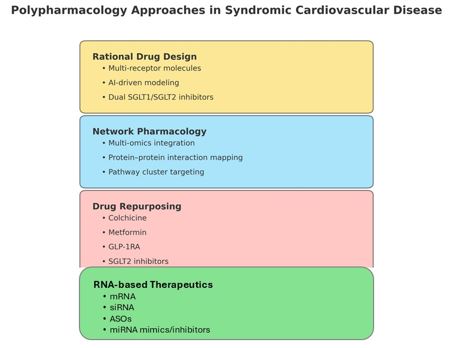
Tools of Polypharmacology: From Design to Repurposing
Network Pharmacology
Disease modules identified from transcriptomics and protein–protein interaction maps expose clusters (e.g., persistent inflammatory-fibrotic signatures in HFpEF) that are best handled by multi node engagement [8,9].
Rational Multi Target Design
Examples include saroglitazar (PPARα/γ agonist) for metabolic-inflammatory coupling and finerenone (selective, non-steroidal MRA) for anti-fibrotic/anti-inflammatory renal-cardiac protection [3]. Exploratory dual SGLT1/SGLT2 designs seek added enteroendocrine effects beyond glycosuria.
Repurposing With Outcomes, Not Catalogs
SGLT2 inhibitors: consistently reduce heart failure hospitalization and slow renal decline across ejection fraction spectra; patients often report better activity tolerance and fewer congestion episodes [21,22].
GLP 1 Receptor Agonists: drive weight loss, lower hepatic fat, and reduce atherosclerotic events; in HFpEF with obesity, improvements in KCCQ health status and 6 minute walk distance parallel symptom relief [2,3].
Colchicine: beyond gout, attenuates post infarct remodeling and lowers recurrent ischemic events [2,3].
Metformin: AMPK mediated mitochondrial and endothelial benefits contribute to greater exercise tolerance and possible anti fibrotic effects independent of glycaemia [2].
Strategy Map: network pharmacology → multitarget design → repurposing → RNA therapeutics; with example targets and readouts.
RNA Therapeutics as Programmable Polypharmacology
Even more exciting and promising is the emergence of RNA-based therapeutics, widely regarded as the “third wave” in pharmaceutical innovation [8,9,14-22]. RNA-based therapeutics are drugs that utilize ribonucleic acid (RNA) molecules to modulate gene expression or encode functional proteins for therapeutic purposes. Unlike traditional drugs that act on proteins after they are produced, RNA therapies intervene earlier in the central dogma-at the level of RNA-offering novel strategies to treat a broad range of diseases.
The key advantages of RNA-based therapeutics, which make them a powerful and versatile modality in modern drug development, include:
Precision and specificity: RNA drugs (e.g., siRNA, ASOs, mRNA) are designed to bind or mimic specific nucleotide sequences, allowing highly targeted modulation of gene expression. This enables isoform-or mutation-specific therapies, especially important in genetic and rare diseases.
Rapid and programmable design: Once a disease-related gene sequence is known, RNA drugs can be quickly designed by altering the nucleotide code, bypassing the long lead times of small-molecule screening or protein engineering. This is why mRNA vaccines for COVID-19 were developed within months.
Access to “undruggable” targets: Traditional small molecules and antibodies can only interact with proteins that have accessible pockets or surfaces. RNA-based therapeutics act upstream, targeting pre-mRNA, mRNA, or noncoding RNAs, allowing access to previously untreatable targets.
Versatility of mechanisms: RNA modalities operate upstream of proteins, enabling precise, reversible control of gene networks that underlie polysyndromes [13-16]:
mRNA: transient expression of therapeutic proteins (e.g., angiogenic factors peri-CABG)[17,18].
siRNA: post-transcriptional gene silencing with sequence specificity[13-16].
Antisense oligonucleotides (ASOs): translation blockade, splice modulation, or RNase H–mediated degradation [13-16].
miRNA therapeutics: mimics or inhibitors to rebalance multi-gene programs; multitarget anti-microRNA antisense oligodeoxyribonucleotide (AMO) can modulate entire micro-networks[19-22].
saRNA: activation of endogenous protective genes[13-16].
Lower risk of genomic integration: Unlike DNA-based gene therapy, RNA does not integrate into the genome, reducing the risk of insertional mutagenesis.
Transience and reversibility: RNA therapies are transient-ideal when temporary modulation is desired (e.g., vaccine response, reversible gene silencing). This reduces long-term risks and enhances safety control.
Potential for personalized medicine: RNA therapeutics can be tailored to an individual’s specific mutation or tumor neoantigen, paving the way for custom cancer vaccines and genotype-specific treatments.
Scalability and manufacturing simplicity: mRNA production is cell-free and can be scaled rapidly, avoiding the complex bioreactor systems needed for protein biologics. The same mRNA platform can be re-used with different sequences (e.g., for new viral variants or tumor targets).
Therapeutic breadth across systems: Applications span multiple domains, including: infectious diseases (e.g., COVID-19, RSV), genetic disorders (e.g., SMA, DMD), oncology (personalized vaccines, immunotherapy), and cardiovascular/metabolic (e.g., Lp(a), PCSK9, ANGPTL3).
Platform potential for multi-indication development: A single RNA delivery platform (e.g., lipid nanoparticles) can serve many indications just by swapping the payload, enabling fast and cost-effective pipelines.
Why relevant: Many HFpEF/CKM/MASLD drivers are polygenic and tissue-distributed. With suitable delivery (e.g., LNPs, GalNAc), RNA tools can engage several nodes within one pathway ensemble-a programmable form of polypharmacology[13-16].
Clinical Translation-Tying Mechanisms to What Patients Feel
Beyond individual trials, large-scale meta-analyses now support the use of multitarget agents across syndromic populations. The DELIVER trial extended the benefits of dapagliflozin to a broad HFpEF cohort, while STEP-HFpEF showed weight loss and improved quality of life with semaglutide.
Furthermore, real-world data registries offer complementary insights into polypharmacologic efficacy. Observational cohorts suggest that patients with diabetes, HFpEF, and chronic kidney disease derive additive benefits from combination therapies such as SGLT2i + GLP-1RA, despite these agents not being co-formulated.
The convergence of mechanistic plausibility, trial evidence, and real-world confirmation strengthens the case for polypharmacology as the next frontier in cardiometabolic care. Adaptive trial designs and enriched biomarker stratification may further accelerate the development of multitarget regimens.
For HFpEF, SGLT2 inhibitors lower HF admissions and improve health status; GLP-1RA in obese HFpEF improves symptoms and exercise capacity alongside weight loss [2,3].
In case of CKD within CKM, finerenone reduces kidney disease progression and cardiovascular events, aligning with its anti-fibrotic signature [3].
For the management of atherosclerosis: low-dose colchicine reduces recurrent ischemic events [2,3].
Real-world registries suggest additive benefits from combinations such as SGLT2i + GLP-1RA, emphasizing practical, phenotype-guided stacking rather than one-size-fits-all regimens [3].
Future Directions and Challenges
To fully realize the potential of polypharmacology, translational frameworks must evolve. This includes:
(1) Developing composite biomarkers that reflect multiorgan improvement (e.g., NT-proBNP + liver stiffness + eGFR).
(2) Regulatory acceptance of multitarget endpoints and mechanisms of action in drug approval.
(3) Stratifying patients using machine learning models to identify those most likely to benefit from specific multitarget profiles.
(4) Mitigating risks of adverse effects through molecular precision and pharmacogenomics.
Additionally, clinical training must adapt to syndromic thinking, encouraging cardiologists, hepatologists, nephrologists, and endocrinologists to engage in shared therapeutic planning based on polypharmacologic strategies [3,5].
Challenges remain (biomarkers, regulation, design complexity), but omics guided targeting and AI assisted discovery are shrinking the gap [8,9].
Conclusion
As cardiometabolic disease presents increasingly as polysyndrome, therapy must be network aware. Polypharmacology offers a scientifically grounded and clinically promising framework to address the multifactorial nature of HFpEF, CKM syndrome, and MASLD. The era of the “silver bullet” is ending. The future lies in rational, network-aware, multitarget therapeutics that recognize the heart not as an isolated organ, but as a hub in a complex biological web [1-9, 13-16].
Funding Source
This work was supported the National Natural Science Foundation of China (Nos. 82404719) to Tianqi Duo, and by the Guangdong Provincial Department of Education (2024KSYS006) to Zhiguo Wang
Author Contributions
Wang Z conceptualized the work and drafted the manuscript. Duo T refined the concept and performed editing, polishing, and proofreading of the manuscript.
Conflict of Interest Statement
The author declares no conflict of interest.
- Paulus WJ, Tschöpe C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 62: 263–71.
- Redfield MM, Borlaug BA (2023) Heart Failure with Preserved Ejection Fraction: A Review. JAMA. 329: 827–38.
- Ndumele CE, Neeland IJ, Tuttle KR, Chow SL, Mathew RO, et al. (2023) American Heart Association. A Synopsis of the Evidence for the Science and Clinical Management of Cardiovascular-Kidney-Metabolic (CKM) Syndrome: A Scientific Statement from the American Heart Association. Circulation. 148: 1636–64.
- Duo T, Wen Y, Bian Y, Zhang XF, Ju J, et al. (2025) Interplay Between Metabolic Dysfunction-Associated Fatty Liver Disease and Cardiovascular-Kidney-Metabolic Syndrome in Asia: A Decadal Analysis and Future Projections from the Global Burden of Disease Study. Metabolism. 2025. (in revision).
- Targher G, Byrne CD, Tilg H (2024) MASLD: a systemic metabolic disorder with cardiovascular and malignant complications. Gut. 73: 691-702.
- Bilson J, Mantovani A, Byrne CD, Targher G (2024) Steatotic liver disease, MASLD and risk of chronic kidney disease. Diabetes Metab. 50: 101506.
- Ha S, Wong VW, Zhang X, Yu J (2024) Interplay between gut microbiome, host genetic and epigenetic modifications in MASLD and MASLD-related hepatocellular carcinoma. Gut. 74: 141-52.
- Wang Z, Yang B (2022) Polypharmacology: Principles and Methodologies. Volumes I & II. Springer Nature (Switzerland AG), Gewerbesrasse 11, 6330 Cham, Switzerland.
- Wang Z (2024) Anti-Aging Polypharmacology. Cambridge Scholars Publishing (UK).
- Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, et al. (2014) TOPCAT Investigators. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 370: 1383-92.
- Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, et al. (2015) Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circulation. 131: 34-42.
- Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, et al. (2019) PARAGON-HF Investigators and Committees. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N Engl J Med. 381: 1609-20.
- Docherty KF, Vaduganathan M, Solomon SD, McMurray JJV (2020) Sacubitril/Valsartan: Neprilysin Inhibition 5 Years After PARADIGM-HF. JACC Heart Fail. 8: 800-10.
- Sparmann A, Vogel J (2023) RNA-based medicine: from molecular mechanisms to therapy. EMBO J. 42: e114760.
- Dzau VJ, Hodgkinson CP (2024) RNA Therapeutics for the Cardiovascular System. Circulation. 149: 707–16.
- Wang Z (2009) MicroRNA Interference Technologies. Springer-Verlag, New York, USA.
- Anttila V, Saraste A, Knuuti J, Hedman M, Jaakkola P, et al. (2023) Direct intramyocardial injection of VEGF mRNA in patients undergoing coronary artery bypass grafting. Mol Ther. 31: 866-74.
- Anttila V, Saraste A, Knuuti J, Jaakkola P, Hedman M, et al. (2020) Synthetic mRNA Encoding VEGF-A in Patients Undergoing Coronary Artery Bypass Grafting: Design of a Phase 2a Clinical Trial. Mol Ther Methods Clin Dev. 18: 464-72.
- Wang Z (2011) The concept of multiple-target anti-miRNA antisense oligonucleotides technology. Methods Mol. Biol. 676: 51–8.
- Lu Y, Xiao J, Lin H, Bai Y, Luo X et al. (2009) A single anti-microRNA antisense oligodeoxyribonucleotide (AMO) targeting multiple microRNAs offers an improved approach for microRNA interference. Nucleic Acids Res. 37: e24–33.
- Wang Z (2011) The principle of miRNA-Masking Antisense Oligonucleotides Technology. Methods Mol Biol. 676: 43–50.
- Wang Z (2011) The guideline of the design and validation of miRNA mimics. Methods Mol Biol. 676: 211-24.

Figures at a glance