Impaired Kidney Function and Associated Factors in Patients Attending HIV Clinic at Korle-Bu Teaching Hospital in Accra, Ghana
Received Date: August 13, 2023 Accepted Date: September 13, 2023 Published Date: September 16, 2023
doi: 10.17303/jaid.2023.10.102
Citation: Edmund Tetteh Nartey, Peter Puplampu, Raymond Ashalley Tetteh, Vincent Boima, Vincent Ganu et al. (2023) Impaired Kidney Function and Associated Factors in Patients Attending HIV Clinic at Korle-Bu Teaching Hospital in Accra, Ghana. J HIV AIDS Infect Dis 10: 1-14
Abstract
Introduction
The aging people living with HIV (PLWH) are at risk for several age-related diseases including cardiovascular diseases and kidney disease with both HIV infection and antiretroviral therapy being risk factors. Studies have indicated that PLWH have a higher risk of developing CKD compared with the general population. This study aimed to assess the magnitude of renal function impairment and its associated factors among adult patients attending HIV clinic in the Korle-bu Teaching Hospital in Accra, Ghana
Methods
A single-center hospital-based cross-sectional study was conducted from September 2022 to February 2023. A total of 444 PLWH were recruited as study participants. Baseline clinical history, laboratory investigations and demographic characteristics were obtained from the patients’ folders or through a questionnaire. Kidney function was defined by estimated Glomerular Filtration Rate (eGFR), which was calculated using the new Creatinine- Based Equations to Estimate GFR without Race. The primary outcome of kidney function impairment was defined using the threshold of eGFR < 60 mL/min/1.73m2 . A logistic regression analysis was carried out to determine the factors associated with kidney function impairment A two-tailed pvalues < 0.05 were considered statistically significant.
Results
Majority of the study participants were classified as having either normal kidney function (n=188; 42.3%) or mild kidney function impairment (n=185; 42.3%) and the proportion of participants with eGFR< 60 mL/min/1.73 m2 was 16.0% (n=71). Increasing age, male gender and clinically diagnosed hypertension were significantly associated with impaired kidney function (p< 0.05) in the multivariable logistic regression.
Conclusion
An integrated care model of HIV and non-communicable diseases appears to be a good direction to follow in Sub-Saharan Africa and emphasis on kidney disease preventive services among the HIV population may reduce the burden of impaired kidney function in PLWH in low- and middle-income countries.
Keywords: HIV; ART; Impaired Kidney function; Hypertension
Introduction
The introduction of newer anti-retroviral drugs in the form of anti-retroviral therapy (ART) for the management of HIV has led to a better health care for people living with HIV (PLWH). HIV infection is now considered a chronic disease. Antiretroviral therapy has substantially increased the survival of PLWH, thus generating a new epidemiological setting of older PLWH [1]. The aging PLWH are at risk for several age-related diseases including cardiovascular diseases and kidney disease with both HIV infection and ART being risk factors [2]. Both acute kidney injury (AKI) and chronic kidney disease (CKD) are important emerging comorbidities of clinical significance amongst PLWH both in developed countries [3] and in resource-limited settings [4]. Studies have indicated that PLWH have a higher risk of developing CKD compared with the general population. In addition, PLWH diagnosed with CKD do have 2-20 fold greater risk of developing end-stage renal disease (ESRD), compared with the general population [2,5,6]. Reports have suggested that ESRD occur at a younger age in PLWH compared with HIV-negative individuals [7], and PLWH have worse outcomes on dialysis [8,9]. Associated factors for kidney disease include viral damage to podocytes causing glomerulopathy and tubular damage which can lead to acute tubular necrosis or interstitial nephritis. Other factors include use of nephrotoxic medications including ARTs such as adefovir, tenofovir and indinavir; comorbidities (like dyslipidemia, cognitive decline, bone disorders, cardiovascular diseases) and modifiable factors [10-12]. Thus, the WHO recommends the regular screening for kidney disease among PLWH.
Reported CKD prevalence in PLWH varies widely between WHO geographical regions (ranging from 2-38%) depending on the methods and definition of CKD [13]. In a systematic review and meta-analysis of 60 studies on PLWH, the cumulative incidence of estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73m2 c among PLWH ranged from 0.3 to 19.5%; the pooled incidence rate was 12.50 (95%CI: 9.00-17.36) per 1000 PYs [14]. With only 5 studies included from Africa, subgroup analysis indicated a pooled incidence of 13.21 (3.73-46.84) per 1000 PYs [14]. An earlier published systematic review and meta-analysis has indicated pooled prevalence of CKD in PLWH worldwide to be 4.8% and in PLWH in sub-Saharan Africa (SSA) to be 7.0% with West Africa sub-region having the highest prevalence of 9.2% [15]. A systematic review and meta-analysis of studies in SSA indicate a prevalence of CKD of 3.1% [16]. However, some studies in SSA have reported a high prevalence of CKD of 44.4% [17].
In Ghana, a study reported a prevalence of CKD in PLWH (eGFR < 60 ml/min/1.73m2 ) of 3.7% [18]. Another study in Ghana also reported a prevalence of impaired kidney function among ART-exposed PLWH as 12.6% (8.15% in males and 14.0% in females) and suggested a need for dose adjustments, especially before onset of therapy [19]. A retrospective cohort study in Ghana also reported a prevalence of impaired kidney function of 13.9% (eGFR < 60 ml/min/1.73m2 ) [20]. The importance of research in HIV related kidney disease must be emphasized as the burden of non-communicable diseases is on the increase among PLWH in SSA, the epicenter of HIV infection [14]. This study aimed to assess the magnitude of renal function impairment and its associated factors among adult patients attending HIV clinic in the Korle-bu Teaching Hospital (KBTH) in Accra, Ghana.
Methods
Study Design and Settings
A single-center hospital-based cross-sectional study was conducted from September 2022 to February 2023 at the HIV-clinic of the Korle-bu Teaching Hospital (KBTH) in Accra, Ghana. The clinic operates three time a week for adult HIV patients. The study population comprised about 20,000 adult PLWH who attends the HIV clinic, and the sampling frame was the electronic register of patients. Each patient is scheduled to attend the clinic at least once every three months for clinical assessment and medication dispensing. All consenting PLWH aged 18 years and above, on Tenofovir Disoproxil Fumarate (TDF), and with estimated creatinine clearance > 60 mL/min/1.73 m2 at initiation of TDF-based ART were eligible for inclusion in the study. All PLWH who were hospitalized at time of the study, had diabetes as a comorbidity, had known renal malformations or recurrent nephrolithiasis or on nephrotoxic drugs (ganciclovir, cyclosporine, etc.), being managed currently for advanced HIV disease (as indicated in medical records), and pregnant or given birth in the last 6 months from the start of the study (for females) were excluded from the study.
Sample Size, Data Collection and Management
A sample size of 435 was calculated based on estimating a population parameter for cross-sectional studies as described by Charan and Biswas (2013) using a prevalence of kidney function impairment in PLWH previously reported as 19.5% [21], a standard normal variance of 1.96, an error rate of 4.0% and a 15% adjustment for missing data. A total of 444 PLWH were recruited as study participants.
A simple random sampling technique was used to select study participants. The minimum cycle of a patient attending the HIV clinic is once every three months. The number of patients sampled per clinic day was 12 for 37 clinic days. Computer generated random sequence of 12 unique code numbers was generated from the sampling frame (list of patients booked for a clinic day) for each clinic day for 37 clinic days, and these were the patients recruited into the study.
Baseline clinical history, laboratory investigations and demographic characteristics were obtained from the patients’ folders or through a questionnaire and recorded in a structured data collection sheet. HIV-related information, including the date of HIV diagnosis, date of ART initiation, and ART regimen, Clusters of differentiation 4 (CD4) count, co-morbidities present were extracted from patients’ folders. Participants’ weight and height were measured, and their body mass index (BMI) calculated in Kg/m2 . BMI values < 18.5 were classified as underweight, 18.5 - 24.9 were classified as normal weight; 25.0 - 29.9 was overweight and values ≥30 was obese. Extracted CD4 cell count from clinical folders was categorized into; CD4 cell counts < 200 cell/mm3 or ≥200 cells/mm3 . Presence of comorbidity was defined as patients who had other chronic non-communicable disease(s) such as hypertension, diabetes mellitus, heart disease or stroke as extracted from their clinical folders. Current smoking refers to patients smoking at least one cigarette per day in the past 30 days. Regular alcohol use was defined as alcohol use at least 3 times per week.
Laboratory Measurements
About 5 mL venous blood sample was taken from each participant and 3 ml was dispensed into vacutainer plain tube and allowed to clot. Clotted blood was centrifuged at 3000 rpm for 5 minutes at room temperature and serum was kept frozen at -20o C and analyzed later for creatinine and other biochemical measurements using HumaStar 100 autoanalyser.
Estimation of Glomerular Filtration Rate (Egfr) and Categorization of Kidney Disease
Kidney function was defined by estimated Glomerular Filtration Rate (eGFR), which was calculated using the new Creatinine- Based Equations to Estimate GFR without Race by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [22]. eGFR classification of CKD was graded according to Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group recommendation [23] as: normal kidney function (eGFR ≥90 mL/min/1.73m2), mild kidney function impairment (eGFR, 60-89mL/min/1.73m2), moderate kidney function impairment(eGFR, 30-59 mL/min/1.73m2), severe kidney function impairment (eGFR, 15-29 mL/min/1.73m2) and kidney failure (eGFR < 15 mL/min/1.73m2). In this study, the primary outcome of kidney function impairment was defined using the threshold of eGFR < 60 mL/min/1.73m2.
Statistical Analysis
Stata 14 software was used to analyse the data. Continuous variables were reported as mean ± SD or median with interquartile range if not normally distributed. Categorical variables were presented as frequency and percentage. A logistic regression analysis was carried out to determine the factors associated with kidney function impairment. The preliminary bivariable analysis was designed to determine the associated factors as grouped under socio-demographic, clinical and HIV /ART-related factors. Thereafter, a multivariable logistic regression model was generated using the purposeful selection of variables method [24]. The performance of the final model was assessed on "calibration" using the Hosmer-Lemeshow goodness-of-fit test statistic and on discrimination using the Receiver Operating Characteristics (ROC) area under the curve (AUC). The discrimination of the variables was considered; poor if AUC < 0.6, moderate if AUC is 0.60-0.80, good if AUC is 0.81-0.99 and perfect if AUC=1.00 [25]. For any variablewith incomplete data, multiple imputation by chain equation was used to impute for missing observations. A two-- tailed p- values < 0.05 were considered statistically significant.
Ethics
Ethical clearance for the study was obtained from the Institutional Review Board of the Korle-Bu Teaching Hospital (KBTH-STC/IRB/000105/2020) and the Ethical and Protocol Review Committee of the University Of Ghana College Of Health Sciences (CHS-Et/M.3-4.3 /2020- -2021). Informed written consent was also obtained from each individual study participants recruited into the study. The study was conducted under the guidance of the 1964 Helsinki Declaration and its later amendments.
Results
A total of 444 PLWH were recruited as study participants with a mean age of 46.3 ± 9.4 years and majority were female (n=309; 69.6%) and married (n=222; 50.0%) (Table 1). Most of the study participants do not drink alcohol (n=263; 59.2%) or smoke tobacco/cigarettes (n=401, 90.3%) (Table 2). Table 2 indicates a description of the clinical characteristic of the study participants.). For BMI assessment, 249 (56.1%)of the study participants were overweight/obese) and 186 (41.9%) had normal body weight.(Table 2). A total of 24.8% (n=110) of the study participants had clinically diagnosed hypertension as extracted from their clinical folders (Table 2). Majority of the study participants were classified as having either normal kidney function (n=188; 42.3%) or mild kidney function impairment (n=185; 42.3%) (Table 2) and the proportion of participants with eGFR< 60 mL/min/1.73 m2 was 16.0% (n=71) (Table 2).
Tables 3 and 4 show the results of bivariable logistic regression analysis of factors associated with impaired kidney function. Increasing age, gender, educational level, religion, marital status, smoking of cigarettes and alcohol consumption were associated with impaired kidney function in the bivariable analysis (Table 3). Table 4 shows that duration on TDF-based ART and clinical diagnosis of hypertension were associated with impaired kidney function bivariable analysis.
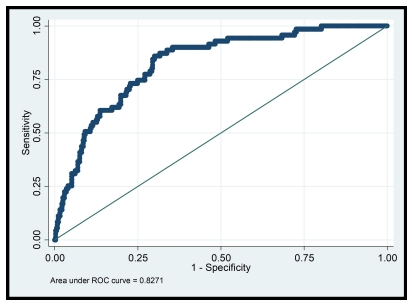
Table 5 shows results of the multiple logistic regression of factors associated with impaired kidney function. Among the socio-demographic factors studied, increasing age and male gender were significantly associated with impaired kidney function (p< 0.05) (Table 5). Study participants who were clinically diagnosed of hypertension had increased odds of impaired kidney function compared with those who were not (aOR=3.89 [95% CI, 2.03-7.45]; p< 0.001) and the odds of impaired kidney function increases by 18% (95% CI, 1.03-1.35, p=019) for every one-year increase of TDF-based ART administration (Table 5). Post-estimation analysis indicated that the generated model was "good" on "discrimination" with an area under the receiver operating characteristics curve of 0.83 (95% CI, 0.78-0.86; p< 0.001) (Figure 1). In terms of "calibration", the generated model gave a Hosmer-Lemeshow goodness-of-fit test χ2 value of 10.52 (p=0.230) indicating no evidence of lack of goodness of fit between the predicted probabilities and the "true" probabilities.
Post-estimation analysis indicated that the generated model was "good" on "discrimination" with an area under the receiver operating characteristics curve of 0.83 (95% CI, 0.78-0.86; p< 0.001) (Figure 1). In terms of "calibration", the generated model gave a Hosmer-Lemeshow goodness-of-fit test χ2 value of 10.52 (p=0.230) indicating no evidence of lack of goodness of fit between the predicted probabilities and the "true" probabilities.
Discussion
The present study revealed that the prevalence of impaired kidney function in persons attending HIV clinic at KBTH in Accra was 16.0%. Impaired kidney function is an important comorbidity in human immunodeficiency virus (HIV) infected patients and is associated with poor outcomes [26,27]. Several prevalence figures of impaired kidney function in PLWH have been reported globally depending on methods and definitions used. In Ghana, reported prevalence of impaired kidney function among PLWH ranged from 3.7% to 13.9% [18-20]. In the present study, the 16.0% prevalence of impaired kidney function is comparable with other studies reported in SSA of 16.1%and 20.7% in Ethiopia [28,29] and 13.9% in Ghana [20]. Renal impairment is recognized as a global public health problem in PLWH with huge health care cost burden [15]. With a global prevalence of CKD in PLWH of 4.8% [15] using the CKD-EPI formula in a systematic review, the current study prevalence of impaired kidney function of 16.0% indicates a higher proportion of the burden of this disease in a typical SSA country and efforts must be made to address it. This is supported by evidence from other countries in SSA where a high prevalence of CKD in PLWH of 44.4% was reported in Cameroon using the CKD-EPI formula [17] as used in this study. With studies showing that the increasing incidence of kidney disease is higher in PLWH compared with the general population [30]and being associated with increased mortality and morbidity [20,31], there is a need to institute measures such as policies and protocols that will enable early detection of impaired kidney function among PLWH patients as early interventions may slow down the progression of kidney disease.
Results from the present study indicate that factors associated with impaired kidney function were increasing age, male gender, increasing duration on TDF-based ART and presence of hypertension as a co-morbidity. Increasing age is a well-noted risk factor for CKD and impaired kidney function [2,32]. In the present study, the odds of impaired kidney function increases by 3% for every additional one-year increase in age. This result is comparable and consistent with other studies conducted in PLWH in both developed [14,33] and developing countries [34,35] including Ghana [20]. From the multivariate analysis, the contribution of increasing age to impaired kidney function suggests that impaired kidney function is likely to become an even more common comorbidity among PLWH in this era of ART.
Results from this study indicate a strong association between gender and impaired kidney function with males having 8-fold increased odds of impaired kidney function compared with females. Although this is inconsistent with earlier studies reporting of females being associated with impaired kidney function both in developed [14,36] and developing countries [34,37], other studies [20,29] including a systematic review and meta-analysis of 61 articles from 60 countries [15] reported no association between gender and impaired kidney function. Nonetheless, the current results are consistent with other report of males being associated with impaired kidney function in PLWH [28,33]. In the present females were more likely to have impaired kidney function compared to males. The effect, if any, of gender difference on prevalence of impaired kidney function remains unclear and the inconsistency may be explained by sociocultural differences, in sample size, and population variation among studies [29].
Several epidemiological studies have reporteda strong association between TDF and increase in risk of impaired kidney function in PLWH [21] and long-term exposure to ART has been reported to contribute to the burden of renal disease in PLWH [2]. Results from the present study indicate 18% increase in odds for impaired kidney function per each consecutive year of being on TDF-based ART. This is consistent with other studies which have reported that longer cumulative exposure to TDF-based ART is associated with impaired kidney function in PLWH [38]. It is worth noting that, in the light of the WHO guidelines which recommend the use of TDF-based regimen as both first line (TDF in combination with either emtricitabine (FTC) or lamivudine (3TC) and a non-nucleotide reverse transcriptase inhibitor (NNRTI) or an integrase inhibitor) and second line (TDF in combination with ritonavir-boosted protease inhibitors (bPI)-either atazanavir (ATV/r) or lopinavir (LPV/r)), serum creatinine determination and assessment of kidney function in PLWH on TDF-based ART should be part of routine clinical care in HIV clinics. Despite this, some earlier studies have suggested that ART can be delivered safely in SSA without laboratory monitoring [39].
Concerning the comorbidities investigated in this study, clinically diagnosed hypertension was found to be associated with impaired kidney function. Results of the present study which indicated an increased odds of impaired kidney function of 3.89 ([95% CI, 2.03-7.45], p< 0.001) in hypertensive patients with PLWH is comparable with results of most recent studies [37,40,41] including a systematic review and meta-analysis [15]. Hypertension is an age-related condition and with the increasing age of HIV-positive patients, a higher prevalence of impaired kidney function might be predicted in future in PLWH [15]. Thus, it is imperative that comorbidities such as hypertension when diagnosed should be managed assiduously and efficiently to prevent onset of renal impairment or CKD and delay progression to ESRD.
Results of the current study suggest that there may be a changing pattern of chronic co-morbidities including kidney disease among PLWH which will thus require health policies and interventions aimed at incorporating the integration of chronic disease management into HIV care [42,43]. An integrated care model appears to have achieved good results in parts of SSA [44]. While a routine kidney function assessment might not be practicable in resource limited setting like SSA, strategies to identify patients at risk of impaired kidney function is warranted and thus targeted monitoring of kidney function prior to and during ART is recommended in order to prevent severe nephrotoxicity. The experience garnered from chronic care management of HIV should be leveraged as a platform for integration of NCDs services into HIV populations [15]. Although, data on the cost-benefit analysis of such a policy direction is absent, suggestions indicate that integrating chronic disease management into existing HIV care may in the long term be more cost effective compared with the traditional vertical approach and single disease management [45].
Study Limitations
● The current study used a cross sectional study design to determine prevalence of impaired kidney function and associated factors in patients attending HIV clinic at the KBTH in Accra. Inferences from this study should be done in the context of the study design as causality cannot be established. Also, since we conducted an observational study, there may be unmeasured confounders that we are unable to account for hence may have affected our results. Also, we cannot exclude the possibility that the observed associations may be due in part to unknown or unmeasured factors such as eating habits, dietary intake, or any other potentially nephrotoxic drugs.
● Impaired kidney function in this study was defined by eGFR < 60 mL/min/1.73 m2 at only a single time point. Given the fact that small, normal variations could result in crossing the threshold from moderate to mildly impaired kidney function, measurement of GFR might overestimate the prevalence of impaired kidney function. In addition, the differentiation of impaired kidney function into CKD and AKI was not possible due to lack of systematic repeat of creatinine measurements in this cross-sectional study design. In addition, urinalysis, which would permit us to assess proteinuria or albuminuria that reflect the kidney damage, was not done.
● Some variables used in the analysis were extracted from the clinical folders of study participants and hence their appropriateness depends on the extent of correctness attached to these data when they were collected.
Conclusion
The outcome of this study clearly shows a high prevalence of impaired kidney function in patients attending the HIV clinic at the KBTH. This observed prevalence of impaired kidney function could be due to an "unmasking" of an already high predisposition to impaired kidney function by the HIV infection itself, the administration of TDF-based ART and an ageing HIV cohort with its accompanying risk of hypertension. Multivariable regression modeling indicated that in addition to the known traditional risk factors for impaired kidney function (including hypertension and age), duration of exposure to TDF-based ART is also associated with impaired kidney function. An integrated care model of HIV and non-communicable diseases appears to be a good direction to follow in SSA and emphasis on CKD preventive services among the HIV population may reduce the burden of impaired kidney function in PLWH in low and middle income countries.
Declarations
Funding
Funds for this project was made available through a grant from BANGA, University of Ghana [UGBA/TRG-013/2022-2023]
Ethics Approval and Consent to Participate
Ethical clearance for the study was obtained from the Institutional Review Board of the Korle-Bu Teaching Hospital (KBTH-STC/IRB/000105/2020) and the Ethical and Protocol Review Committee of the University of Ghana College of Health Sciences (CHS-Et/M.3-4.3/2020-2021). Informed written consent was also obtained from each individual study participants recruited into the study. The study was conducted under the guidance of the 1964 Helsinki Declaration and its later amendments. Patients' data were de-identified during data capture, entry, analysis and storage by ensuring only study codes were used consistently throughout the project cycle.
Availability of Data and Materials
The datasets used and/or analysed during the current study are available from the corresponding author or the lead author on reasonable request.
Competing Interests
The authors declare that they have no competing interests.
- WHO (2019) Data and Statistics.
- Heron JE, CI Bagnis, DM Gracey (2020) Contemporary issues and new challenges in chronic kidney disease amongst people living with HIV. AIDS research and therapy 17: 1-13.
- Abraham AG et al. (2015) End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis 60: 941-9.
- Kalyesubula R et al. (2018) impaired renal function in a rural Ugandan population cohort. Welcome open research 3.
- Bansi L et al. (2009) Clinical epidemiology of HIV-associated end-stage renal failure in the UK. Aids 23: 2517-21.
- Lucas GM et al. (2007) End-stage renal disease and chronic kidney disease in a cohort of African-American HIVinfected and at-risk HIV-seronegative participants followed between 1988 and 2004. Aids 21: 2435-43.
- Althoff KN et al. (2015) Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis 60: 627-38.
- Razzak Chaudhary S et al. (2015) Trends in the outcomes of end-stage renal disease secondary to human immunodeficiency virus-associated nephropathy. Nephrol Dial Transplant 30: 1734-40.
- Trullàs JC et al. (2011) Outcome and prognostic factors in HIV-1-infected patients on dialysis in the cART era: a GESIDA/SEN cohort study. J Acquir Immune Defic Syndr 57: 276-83.
- Mocroft A et al. (2014) Deteriorating renal function and clinical outcomes in HIV-positive persons. Aids 28: 727-37
- GBD (2020) Chronic Kidney Disease Collaboration, Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395: 709-33.
- Ryom L et al. (2019) Serious clinical events in HIV-- positive persons with chronic kidney disease. Aids 33: 2173-88.
- Rosenberg AZ et al. (2015) HIV-associated nephropathies: epidemiology, pathology, mechanisms and treatment. Nature Reviews Nephrology 11: 150-60.
- Shi R et al. (2022) Interaction of sex and HIV infection on renal impairment: baseline evidence from the CHART cohort. Int J Infect Dis 116: 182-8.
- Ekrikpo UE et al. (2018) Chronic kidney disease in the global adult HIV-infected population: A systematic review and meta-analysis. PLoS One 13: e0195443.
- Baynes HW et al. (2019) Chronic kidney disease among human immunodeficiency virus positive patients on antiretroviral therapy in sub-Saharan Africa: A systematic review and meta-analysis. Saudi J Kidney Dis Transpl 30: 1190-1200.
- Patrice HM et al. (2018) Prevalence and associated factors of chronic kidney disease among patients infected with human immunodeficiency virus in Cameroon. Iran J Kidney Dis 12: 268-274.
- Obirikorang C et al. (2014) Renal function in Ghanaian HIV-infected patients on highly active antiretroviral therapy: a case-control study. PloS one 9: e99469.
- Owiredu W et al. (2013) Renal insufficiency in Ghanaian HIV infected patients: need for dose adjustment. African health sciences 13: 101-11.
- Sarfo FS et al. (2013) High prevalence of renal dysfunction and association with risk of death amongst HIV-infected Ghanaians. J Infect 67: 43-50.
- Nartey ET et al. (2019) Tenofovir-associated renal toxicity in a cohort of HIV infected patients in Ghana. BMC research notes 12: 1-6.
- Inker LA et al. (2021) New Creatinine- and Cystatin C-Based Equations to Estimate GFR without Race. N Engl J Med 385: 1737-49.
- Group (2013) K.D.I.G.O.K.C.W., KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. . Kidney inter., Suppl 3: 1-150.
- Bursac Z et al. (2008) Purposeful selection of variables in logistic regression. Source Code Biol Med 3: 17.
- Dodoo AN et al. (2014) Profile of adverse events in patients receiving treatment for malaria in urban Ghana: a cohort-event monitoring study. Drug safety 37: 433-48.
- Campos P, A Ortiz, K Soto (2016) HIV and kidney diseases: 35 years of history and consequences. Clin Kidney J 9: 772-81.
- Hentzien M et al. (2016) Impact of Age-related Comorbidities on Five-year Overall Mortality among Elderly HIV-Infected Patients in the Late HAART Era--Role of Chronic Renal Disease. J Nutr Health Aging 20: 408-14.
- Manaye GA, DD Abateneh, W Niguse (2020) Chronic Kidney Disease and Associated Factors Among HIV/AIDS Patients on HAART in Ethiopia. HIV AIDS (Auckl) 12: 591-9.
- Kefeni BT, KW Hajito, M Getnet (2021) Renal Function Impairment and Associated Factors Among Adult HIV-- Positive Patients Attending Antiretroviral Therapy Clinic in Mettu Karl Referral Hospital: Cross-Sectional Study. HIV AIDS (Auckl) 13: 631-40.
- Islam FM et al. (2012) Relative risk of renal disease among people living with HIV: a systematic review and meta-- analysis. BMC Public Health 12: 234.
- Webster AC et al. (2017) Chronic Kidney Disease. Lancet 389: 1238-52
- Weinstein JR, S Anderson (2010) The aging kidney: physiological changes. Adv Chronic Kidney Dis 17: 302-7.
- Pinto Neto LF et al. (2016) Nephrotoxicity during tenofovir treatment: a three-year follow-up study in a Brazilian reference clinic. Braz J Infect Dis 20: 14-8.
- Pry JM et al. (2022) Evaluation of kidney function among people living with HIV initiating antiretroviral therapy in Zambia. PLOS Glob Public Health 2: e0000124.
- Mapesi H et al. (2021) Prevalence, incidence and predictors of renal impairment in persons with HIV receiving protease-inhibitors in rural Tanzania. PLoS One 16: e0261367
- Petersen N et al. (2019) Prevalence of impaired renal function in virologically suppressed people living with HIV compared with controls: the Copenhagen Comorbidity in HIV Infection (COCOMO) study. HIV medicine 20: 639-47.
- Cailhol J et al. (2011) Prevalence of chronic kidney disease among people living with HIV/AIDS in Burundi: a cross-sectional study. BMC Nephrol 12: 40.
- Suzuki S et al. (2017) Effect of Tenofovir Disoproxil Fumarate on Incidence of Chronic Kidney Disease and Rate of Estimated Glomerular Filtration Rate Decrement in HIV-1-Infected Treatment-Naive Asian Patients: Results from 12-Year Observational Cohort. AIDS Patient Care STDS 31: 105-12.
- Team DT et al. (2010) Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomised non-inferiority trial. Lancet 375: 123-31.
- Bonjoch A et al. (2014) High prevalence of signs of renal damage despite normal renal function in a cohort of HIV-infected patients: evaluation of associated factors. AIDS Patient Care STDS 28: 524-9.
- Calza L et al. (2014) Prevalence of renal disease within an urban HIV-infected cohort in northern Italy. Clin Exp Nephrol 18: 104-12.
- Bloomfield GS et al. (2014) HIV and noncommunicable cardiovascular and pulmonary diseases in low- and middle-income countries in the ART era: what we know and best directions for future research. J Acquir Immune Defic Syndr 67: S40-53.
- Oni T et al. (2014) Chronic diseases and multi-morbidity--a conceptual modification to the WHO ICCC model for countries in health transition. BMC Public Health 14: 575.
- Edwards JK et al. (2015) HIV with non-communicable diseases in primary care in Kibera, Nairobi, Kenya: characteristics and outcomes 2010-2013. Trans R Soc Trop MedHyg 109: 440-6
- Hyle EP et al. (2017) The association between HIV and atherosclerotic cardiovascular disease in sub-Saharan Africa: a systematic review. BMC Public Health 17: 954.

Tables at a glance
Figures at a glance