Improving Outcomes in Children with HIV: What does Telomere Length Dynamics tell us?
Received Date: August 03, 2024 Accepted Date: September 03, 2024 Published Date: September 06, 2024
doi: 10.17303/jaid.2024.11.104
Citation: Carlota Miranda-Solé, Laura Arencibia-Hernando, Ginevra Pistocchi, Kennedy Otwombe, Mark F Cotton, et al. (2024) Improving Outcomes in Children with HIV: What does Telomere Length Dynamics tell us? J HIV AIDS Infect Dis 11: 1-15
Abstract
Background: Understanding telomere length (TL) dynamics may shed light on mechanisms influencing immunological health and inform treatment strategies for children with perinatally-acquired HIV.
Methods: TL was assessed through PCR using DNA extracted from peripheral blood mononuclear cells from South African children without HIV recruited from an observational study, and participants from the randomised Children with HIV Early antiRetroviral (CHER) trial, where infants aged < 12 weeks with HIV were randomised to early limited versus deferred continuous antiretroviral therapy (ART). TL determinants were evaluated alongside clinical and immunological data using Spearman’s using multi-linear regression models.
Findings: TL was measured in 160 children without HIV (median age = 1.95 [0.98-3.56] years), and 106 children with HIV (n = 79 samples at 2 years, n = 50 at 5 years). At 2 years, the shorter TL negatively correlated with higher thymic output (correlation coefficient = -0.554 (95% CI: -0.824/-0.081), p = 0.026) in children with HIV, who also had shorter TL than age-matched controls (p< 0.0001), but not at 5 years. Early and longer ART duration correlated with longer TL. At 5 years, all children in CHER had TL similar to those without HIV. No relationship between TL and cytomegalovirus or Epstein-Barr virus co-infection, HIV-serostatus or HIV proviral DNA reservoir was identified.
Interpretation: Our findings illustrate the dynamic activity of the thymus in early childhood years, suggesting a positive feedback mechanism in response to immunological stress, as reflected by TL shortening. However, TL is preserved after early HIV diagnosis and ART initiation as recommended in current guidance.
Keywords: Perinatal HIV; Telomere Length; Early Antiretroviral erapy; Cytomegalovirus; Epstein-Barr VirusF
Introduction
Th e survival of children with perinatally-acquired HIV (HIV) has improved signi cantly with the introduc tion of antiretroviral therapy (ART) in the past two decades. Longitudinal studies show a substantial decrease in mortali ty, from 7.2-6.9 (mono-/dual-therapy) to 0.8 deaths per 100 person-year following ART implementation; AIDS-related mortality has similarly declined [1]. Nevertheless, children with HIV experience chronic in ammation leading to non AIDS related complications, including cardiovascular and respiratory diseases, and non-AIDS related cancers [2]. De spite ART, mortality rates are higher, and lifespan is shorter for those with HIV. ese challenges may be linked to pre mature aging and immunosenescence [2,3], de ned by a de cline in the immune system’s e ectiveness and function, with decreasing ability to recognise and respond to patho gens. While CD4 counts, CD4/CD8 ratios, and HIV viral loads monitor virological control and disease progression, few immunological markers re ect the impact of HIV and ART on immunosenescence.
Co-infections such as cytomegalovirus (CMV) and Epstein-Barr Virus (EBV) are prevalent among individ uals with HIV [4,5]. ese co-infections are associated with increased cytokine expression, potentially inuencing HIV viraemic control, disease progression, and the risk of HIV related co-morbidities [6]. Nonetheless, it remains unclear whether CMV and EBV co-infection directly contribute to immunopathogenesis, or if their presence re ects more se vere compromise in immune function.
Telomeres are genomic repeat sequences (3’-T TAGG-5’) the ends of chromosomes that serve as protective caps, preventing genomic instability [7]. Telomeres shorten a er every cell division, until reaching a length where cells become senescent or apoptotic. The association between TL shortening, immunosenescence and ageing is well-recog nized [7,8]. Shorter TL and increased telomere attrition are linked with poorer outcomes in adults with chronic lung dis ease, cardiovascular diseases and age-related [9], prevalent in HIV [3,10]. erefore, analysing TL dynamics in Sub-Sa haran African children with HIV may be key to understand ing which strategies will optimise immunological health of this population.
TL decreases with age, yet its dynamics through out early childhood is not fully explored [11]. It has been suggested that changes in TL exhibit a non-linear trend as one ages [12,13], characterised by a more signi cant short ening in the rst 3 years of life, followed by a relatively steady decline therea er. Cowell et al [12] attribute the ini tial TL decline to the rapid expansion of the haematopoietic stem cell pool during the immune system’s development, supporting the role of TL as a marker of immunological age.
In children, the haematopoietic stem cell pool can be assessed through two key parameters: the thymic and naïve B-cell output. ese, representing the generation of naïve T- and B-cells from the thymus and bone marrow re spectively, play a crucial role in immune system maturation and maintaining immunological health [14]. ey inuence the proportion of naïve/memory immune cells, consequent ly impacting the overall TL. Additionally, demographic char acteristics such as biological sex and ethnicity can also inu ence TL [15].
HIV is associated with chronic immune activation and in ammation, contributors to heightened T-cell di er entiation and proliferation2. Consequently, memory and dif ferentiated T-cell percentages increase, characterised by in creased expression of CD57, lack of CD28 expression (both markers of cellular senescence) and shorter TL [8,16] The impact of HIV extends to the thymus, a crucial source of naïve T-cells [17].The repercussions of HIV on the thymus further alter the naïve/memory T-cell balance [16]. Overall, HIV infection leads to an aged immunophenotype, with in creased immunosenescence and TL attrition8.
Patterns of T-cell exhaustion seen in children with HIV are comparable to those of the elderly [3,18], highlight ing premature ageing of the immune system. Notably, chil dren with HIV have shorter TL than age-matched children without HIV. Con icting results have been found on the im pact of ART on TL. Some studies report shorter TL in ART- naïve compared to ART-treated children, while others show an inhibitory action of NRTIs on telomerase [10,19].The shortened TL in these children is linked with higher di er entiation of T-cell subsets, a lower CD4+/CD8+ ratio, and increased HIV viremia [10,19]. However, there still is a lack of data regarding TL in children with HIV, particularly in high burden settings [4,5].
Recognising the importance of early and sustained ART has been pivotal to reduce mortality and morbidity, as well as lowering the size of the HIV proviral DNA reservoir [20-23]. However, there is a gap in research focusing on op timising the immunological health of these children and rev ersing immunosenescence.
Th is study examines factors that inuence TL in childhood, including children with and without HIV, to provide insight into mechanisms that determine immunological health, and identify individuals at risk of developing pre mature age-related co-morbidities.
Methods
Study Populations
The study used stored samples from two South African populations: children from the Children with HIV Early antiRetroviral (CHER) trial22,23 and children without HIV from a Child Wellness Clinic (CWC).
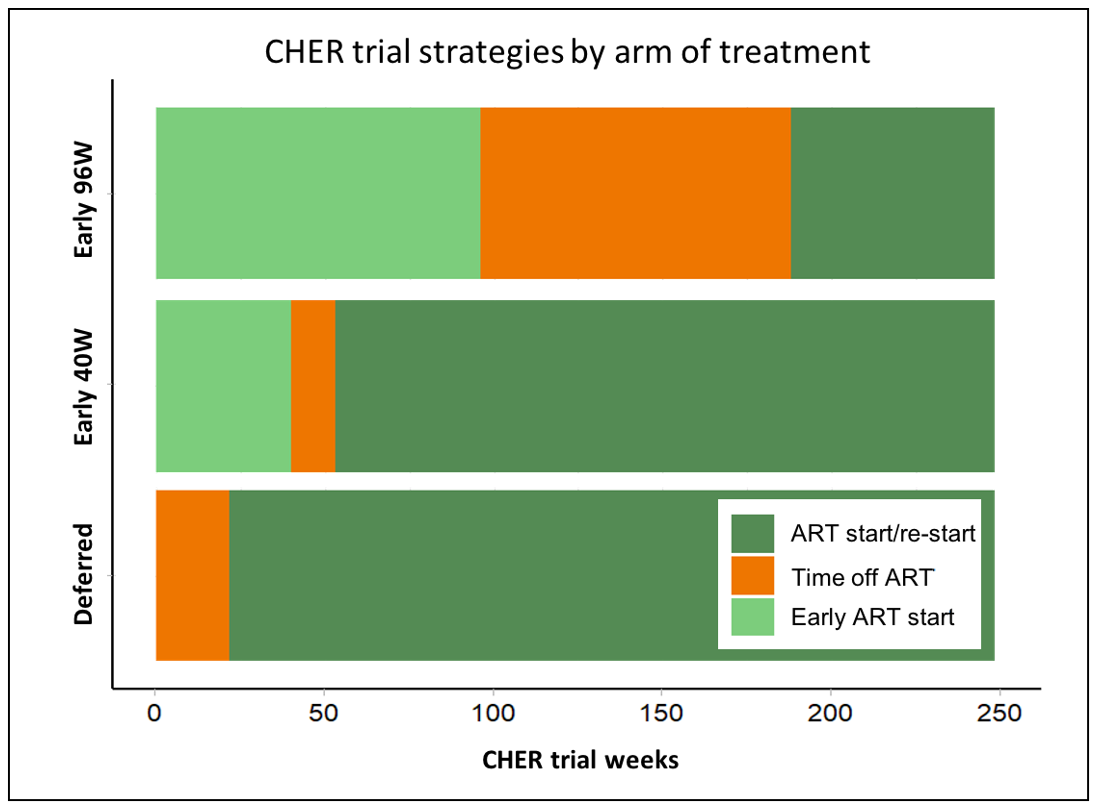
The CHER trial was a phase 3, randomised, open-label, 3-arm study conducted at the Perinatal HIV Research Unit (PHRU), Johannesburg and the Children’s Infectious Diseases Clinical Research Unit (KIDCRU), Cape Town between 2005 and 2011. The trial aimed to prove the superiority of early limited ART (within 12 weeks of life) in delaying disease progression over deferred continuous ART. During ART-free periods, children were monitored clinically and with CD4 counts and percentages. Three hundred and seventy-seven children with HIV under 12 weeks of age were randomised to the three arms (Figure 1): deferred ART start (ART-Def) and early ART start followed by treatment interruption at 40 or 96 weeks (Early-40W and Early-96W, respectively). Participants would restart ART in the event of clinical or immunological deterioration.
The CWC was a research clinic located in an informal settlement in Cape Town which focused on establishing haematological and immunological reference ranges for South African children without HIV aged 0 to13 years; 487 infants were recruited24,25. Children were required to be clinically well at enrolment and without any chronic medical condition, and to attend in the presence of their biological mother and hand-held health record.
Blood Sampling
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood from CWC and CHER trial participants at enrolment. Further samples were taken in the CHER trial. Samples at 96 and 248 weeks from ART introduction, equivalent to 2 and 5 years of age, were used in this study. Genomic DNA (gDNA) extracted from total PBMCs was stored at -20ºC until TL analysis.
IMMUNOPHENOTYPING
Immunophenotypic analysis of CHER and CWC samples was done by flowcytometry batch analysis on cryopreserved PBMCs. The data included measurements of helper (CD4+) and cytotoxic (CD8+) T-cell, as well activated (HLADR+) and proliferating (Ki67+) T-cells at 2 and 5 years of age
THYMIC AND NAÏVE B-CELL OUTPUT
Thymic and naïve B-cell output measures were obtained using a mathematical model combining real-time quantitative PCR (qPCR) measurements of T-cell excision circles (TRECs) and kappa-deleting recombination excision circles (KRECs) respectively, and flowcytometry measurements for naïve and proliferating naïve T- or B-cells11,26.
TELOMERE LENGTH MEASUREMENT
TL were measured using the ScienCell’s Absolute Human Telomere Length Quantification qPCR Assay (AHTLQ) kit on PBMCs gDNA, following the manufacturer’s instructions. The assay simultaneously amplifies a telomere sequence and a single-copy reference (SCR) gene on human chromosome 17. SCR gDNA serves for data normalisation; known TL from the SCR was employed to extrapolate measurements for target samples. Testing included sample duplicates, and triplicates of reference DNA and non-template controls to monitor contamination. Both qPCR reactions were performed on a Bio-Rad CFX96 instrument: 95ºC for 10 min, 32 cycles of 20-sec denaturation at 95ºC, 20-sec annealing at 52ºC and 45-sec extension at 72ºC, followed by an infinite hold at 20ºC. TL were determined by the 2-ΔΔCq method, reported as TL per chromosome end in kilobases (kb).
CMV and EBV Cell-Associated DNA
CMV and EBV cell-associated DNA (CA-DNA) was measured in PBMCs gDNA by QX200 Droplet Digital PCR (ddPCR) System using supermix for probe (no dUTP; BioRad). The reaction mix included 2X ddPCR supermix for probe (1X), CMV- and EBV-specific primers (0.9µM), CMV/EBV probes (HEX, 0.7µM), RPP30 (FAM, 0.9µM) and 150 ng of template DNA. Droplets were generated with a QX200TM droplet generator (Bio-Rad), transferred to a 96-well plate, and amplified by PCR. The plate was transferred to a QX200TM Droplet Reader (Bio-Rad) and analysed using QuantaSoft Version 1.7.4.0917.
STATISTICAL ANALYSIS
Statistical analyses were conducted using RStudio 2021.09.2, Build 382. Frequencies were calculated for categorical variables, i.e. gender, ethnicity, z-score for weight measured using WHO approach. Medians and interquartile range (IQR) values were calculated for continuous variables, i.e. age at sample collection, birthweight, thymic and naïve B-cell output – further log transformed. TL trends in healthy children across age-groups were examined using multi-linear regression. Relationships between TL, sex, ethnicity, and weight were explored for both CWC and CHER groups. The impact of factors such as HIV-seroreversion, HIV proviral DNA (pvDNA) and CMV/EBV CA-DNA on TL was assessed through comparisons using non-parametric tests (Kruskal-Wallis and Dunn's tests) and correlation analysis (Spearman’s linear regression). Age-adjusted TL measures were derived from the difference between observed and expected TL. Boxplots represent median values, with error bars representing the 95% confidence intervals (CI). Statistical significance determined with a threshold of p-values ≤ 0.05.
Results
Stored PBMCs from 160 South African children without HIV from the CWC, and 129 samples from 106 children in the CHER trial were used to analyse the impact of HIV on TL. The characteristics of the study populations grouped by age at the time sample was taken are shown in Table 1. No significant differences were seen between the CWC versus CHER participants for sex, age or thymic output. Grouping children with HIV by age showed no differences between groups for birthweight, age at ART start, proportion of lifetime on ART, seroreversion or estimates of HIV reservoir by pvDNA. The distribution of children across different weight-for-age z-scores varied significantly between the CWC and CHER groups at both 2 and 5 years of age, as indicated in Table 1. Specifically, at 2 years, there was a notable difference in the number of children between the two groups across z-scores of -2, -1, 0 (p < 0.0001), and 1 (p < 0.001). Similarly, at 5 years, significant differences were observed for z-scores of -2 (p = 0.045), -1, 0 (p < 0.0001), and 1 (p = 0.002).
Telomere Length in Children without HIV in a High Disease Burden Setting
Dynamic change in TL, thymic and naïve B-cell output with age
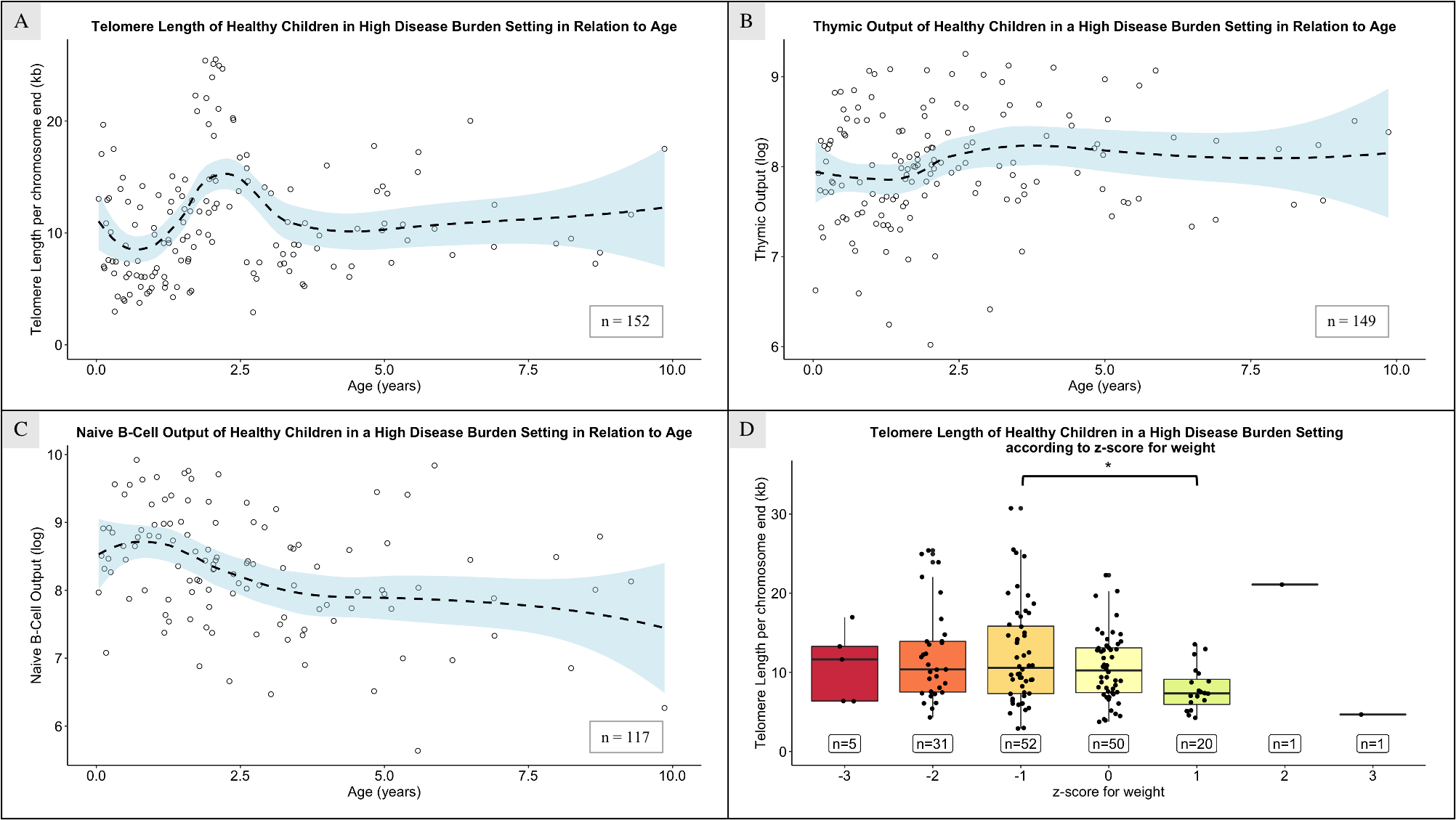
Reference values for TL, thymic and naïve B-cell output in children without HIV in high disease burden setting in relation with age are indicated using the CWC samples. TL peaked at 2.5 years (Figure 2.A), with 0.5-1-year-olds having significantly shorter TL than children aged 1.5-2 and 2-3 years (p < 0.001 and p < 0.0001, respectively; median TL = 10.07, 11.93 and 14.83 at 0.5-1, 1.5-2 and 2-3 years-old). Differences were also significant between 1-1.5 and 2-3 years (median TL = 9.08 and 14.83 respectively, p = 0.009). After age 2-3, TL significantly decreased (p = 0.042). The highest point of thymic output was seen at around 3 years (Figure 2.B). Naïve B-cell output peaked before age 1, followed by a steady decline (Figure 2.C).
Demographics and TL
No difference was seen in TL of healthy children based on gender or ethnicity, at either age group, however shorter TL were seen in children with a z-score for weight of 1 compared to those with a z-score of -1 (p = 0.047; Figure 2.D); no difference was seen in the CHER cohort regarding weight for age z-score.
Telomeres in Children with Perinatally-Acquired HIV
TL difference at 2 years is corrected by the age of 5 years
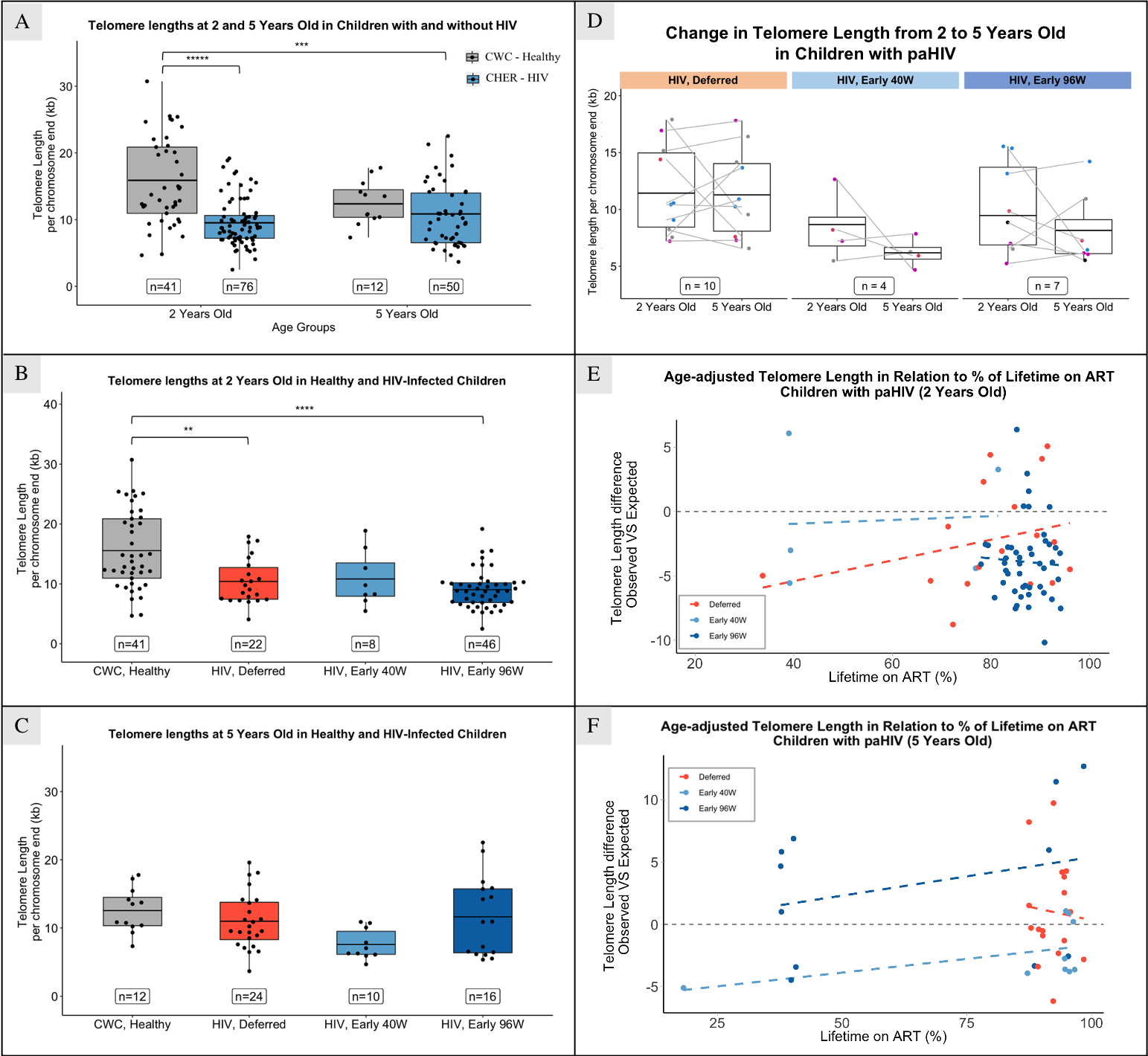
At 2 years, children with HIV had significantly shorter TL than children without HIV (median = 13.74 and 8.86 respectively, p < 0.0001); no difference was seen at 5 years (Figure 3.A). However, no difference in TL was seen at 2 or 5 years of age between children randomized to deferred or early ART (Figure 3.B and 3.C). Thymic output has a significant negative correlation with TL at 2 years (correlation coefficient = -0.554 (95% CI: -0.824/-0.081), p-value = 0.026).
TL change from 2 to 5 years
The progression of TL from 2 to 5 years of age was studied by comparing measurements of the same individual at both time points (n = 21). No clear trend was seen in TL change when analysing all individuals together, nor when stratifying by treatment arm (Figure 3.D) or duration of ART interruption. We found no evidence that age at ART start is associated with TL, in any study arm.
Impact of time of ART on TL
Age-adjusted TL, derived from the difference between observed and expected TL, was used to examine other TL determinants. At 2 and 5 years, 80 and 50 percent of children with HIV had shorter TL than expected in peers without HIV. While not statistically significant, at 2 years, age-adjusted TL correlated positively with the percentage of lifetime on ART in the “HIV, Deferred” arm (correlation coefficient = 0.286, (95% CI: -0.225/0.674), p = 0.27), with no correlation in the “HIV, Early” arms (Figure 3.E). At 5 years, no correlation was seen in the “HIV, Deferred” arm, while a positive correlation existed in both “HIV, Early” arms (correlation coefficient = 0.48 and 0.29 (95 CI: -0.272/0.867 and -0.376/0.758), p = 0.19 and 0.39; in Early 40W and Early 96W, respectively; Figure 3.F).
HIV-serostatus and proviral DNA
TL in children with HIV at 2 and 5 years did not appear related to HIV pvDNA levels as measured in 111/129 samples, or HIV-seroreversion, with 58% (27/46) seropositive, of data available.
Telomere length and immune activation/proliferation
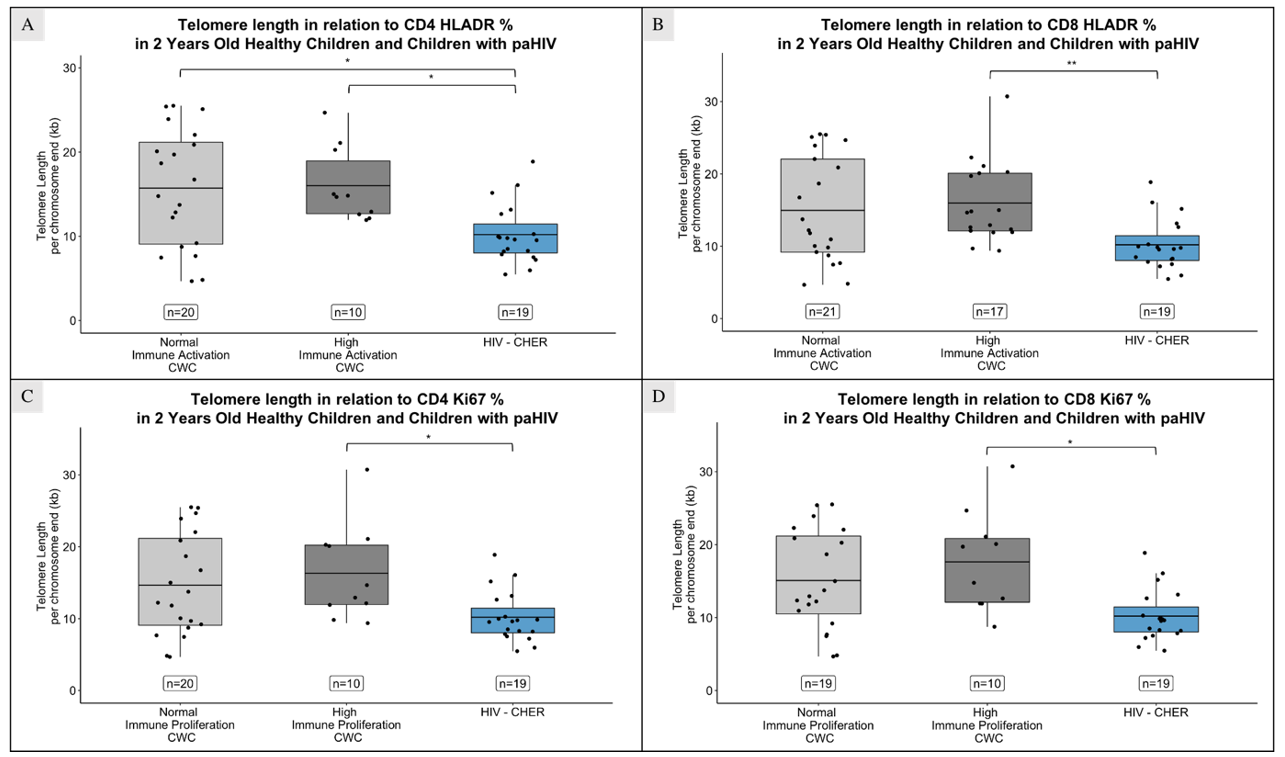
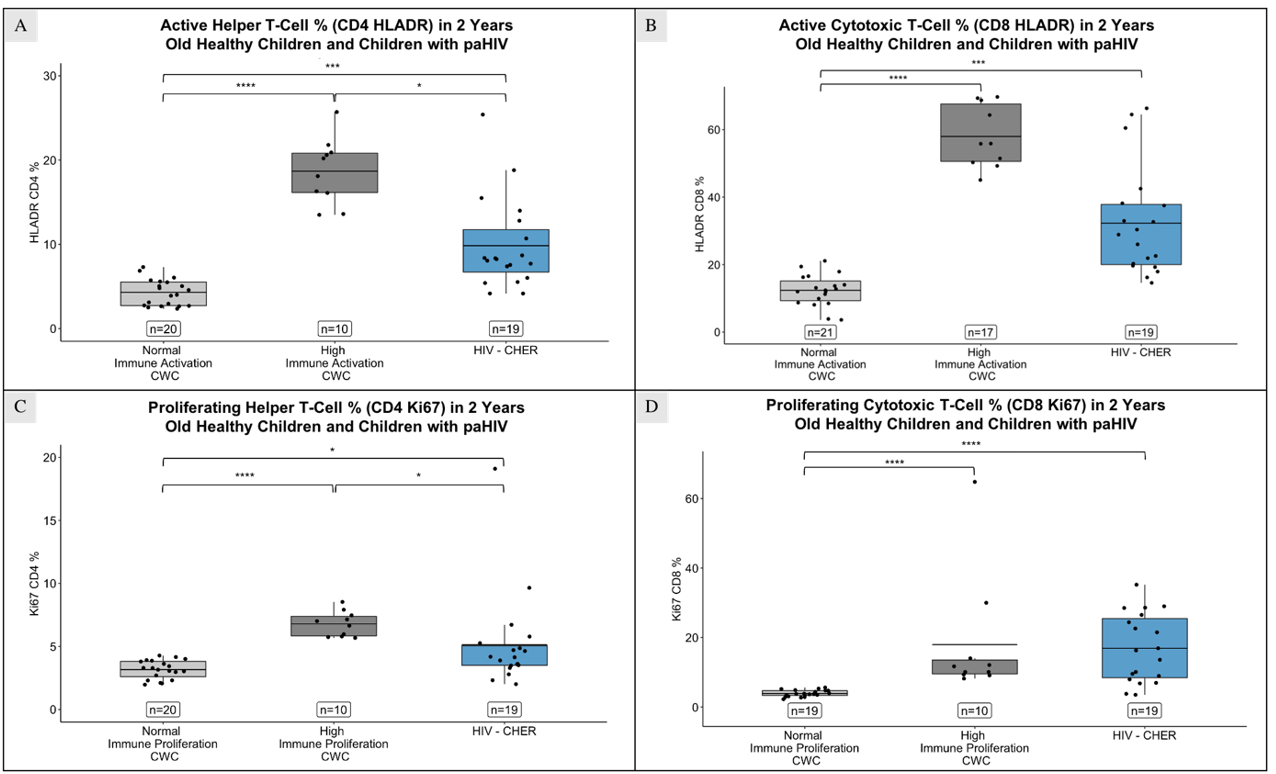
At age 2, children with HIV had shorter TL than those without HIV, regardless of CD4+ and CD8+ immune activation levels (Figure 4.A and 4.B; p = 0.029 and 0.025 in CD4+ T-cells with normal and high immune activation respectively; p = 0.008 in CD8+ T-cells with high immune activation). TL were also shorter compared to children with high CD4+ and CD8+ immune proliferation (Figure 4.C and 4.D; p = 0.034 and 0.013 for CD4+ and CD8+ T-cells respectively).
Children with HIV exhibited higher percentage of activated (HLADR+) and proliferating (Ki67+) CD4+ and CD8+ T-cells than children without HIV with normal activation (p = 0.045 in CD4+ T-cells, and p < 0.001 in CD8+ T-cells; Figure 5.A and 5.B) and normal proliferation (p = 0.033 in CD4+ T-cells, and p < 0.0001 in CD8+ T-cells; Figure 5.C and 5.D), and lower than those with high activation (p < 0.0001 in CD4+ T-cells, and p = 0.07 in CD8+ T-cells) or proliferation (p = 0.02 in CD4+ T-cells).
At 5 years, no significant difference existed on TL nor percentage of activated or proliferating CD4+ T-cells based on HIV infection. Percentage of proliferating CD8+ T-cells was higher in children with HIV than those without HIV with normal proliferation (p = 0.005), and interestingly in children with HIV on ART, TL correlates positively with percentage of proliferating CD8+ T cells (CD8+Ki67+) at 5 years (correlation coefficient = 0.638 (95% CI: 0.015/0.904), p = 0.047).
Presence of CMV and EBV CA-DNA and telomere length
TL from 63 children from the CHER trial and 20 CWC children at 2 years were compared, considering detection of CMV and/or EBV cell-associated (CA) DNA. CMV CA-DNA was detected in eight (12.7%) children with HIV (177.46 [100.56 – 950] copies per million PBMCs) and three (15%) CWC children (40.69 [33.3-50.1] copies per million PBMCs); children with HIV had significantly higher CMV CA-DNA (p = 0.012). EBV CA-DNA was found in 61 (96.8%) children with HIV (735.58 [363.53 – 1383.41] copies per million PBMCs) and 19 (95%) CWC children (425.45 [326.2 – 841.16] copies per million PBMCs); children with HIV had non-significantly higher EBV CA-DNA values. All participants with CMV CA-DNA also had EBV CA-DNA detected (12.7% CHER; 15% CWC). No TL difference existed between those with/without CMV/EBV CA-DNA in either CHER or CWC children, and no correlation was found between TL and CMV/EBV CA-DNA levels.
Discussion
This is the first study to examine the association between TL and HIV in South African children, providing important insights in a high disease burden setting, and represents one of the largest analyses of TL data, including data from a randomised controlled trial. We demonstrated that, at 2 years of age, children with HIV exhibit shorter TL than their peers. However, early ART initiation and an extended proportion of lifetime on ART seem to preserve TL, aligning to levels observed in children without HIV by the age of 5. It appears that the childhood peak in TL may coincide with the peaks of thymic and naïve B-cell output, likely significant TL determinants. Current literature reports on the impact of HIV on telomere shortening, but not on the benefits of early ART.
The TL uctuation across ages observed in chil dren without HIV from a high disease burden setting pro vides insight into the factors inuencing it. Shorter TL from 6 months to 1 year of age compared to older children might re ect heightened cell division, corresponding with in creased environmental antigen-exposure as maternal anti bodies wane [27]. As infants lose maternal immune protec tion, they become more susceptible to infections, which leads to heightened immune activation and proliferation. This, in turn, results in the shortening of leukocyte telom eres, as evidenced by the observed increase in naïve B-cell output.
Between 2 and 3 years of age, corresponding to the cohort’s longest TL, we noted the highest point in thymic output, with a significant TL correlation. Early childhood TL variations are likely due to sample composition differences, as the TL averages all cells in the sample. Increased thymic and naïve B-cell output yield a greater proportion of naïve B- and T-cells, contributing to an overall longer TL in the sample[7,16].
After the initial 2-3 years, thymic and naïve B-cell output plateau[14,26]. Contrary to some studies[12], TL in our population does not notably decrease with age thereafter. This implies potential involvement of other factors, such as a positive feedback mechanism of the thymus and bone-marrow. In an antigen-rich environment due to infectious diseases burden, these organs may continue supplying the immune system with naïve B- and T-cells[17].
Shorter TL observed in children with HIV at 2 years supports findings in American and European cohorts[18,19]. However, by 5 years, the ART strategies used by CHER sustained TL to match the local CWC cohort, regardless of deferred or interrupted therapy. This analysis is limited by fewer numbers of CWC children over 2 years. Moreover, TL variation among CHER participants from 2 to 5 years suggests diverse responses to ART interruption; almost half of individuals show increased TL despite ART interruption. It is plausible that thymic contribution plays a role in maintaining TL, however TL can be influenced by factors such as diet, genetics and psychological stress and these were not assessed in our study[9]. Although literature suggests TL is usually positively associated with nutritional status, no clear association was seen from our analysis using weight-for-age z-score. This may be because weight-for-age z-score do not adequately reflect nutritional status, and there was not a broad distribution for weight-for-age z-score, as most children had a z-score below zero. Immunological follow-up is crucial to understand the relevance of TL and thymic output on the children’s health, given the link between ART interruption and late initiation with higher mortality and larger HIV reservoir.
Our findings underscore the importance of early and prolonged ART, showing an association between early and extended ART with longer TL in the ART-Deferred arm, aligned with existing literature[19–21,23]. Furthermore, the lack of correlation between age at ART start and TL in the early-ART arms suggests that, despite the potential preventive effects of early ART on TL attrition, treatment interruption may still disrupt TL. However, early 40- and 96-weeks ART arms started treatment at a median of 7.3 and 7.8 weeks, respectively. Given that HIV viral load peaks within the first two months of life without ART[28], initiating ART at 7 weeks may not be early enough to halt viral replication and reservoir establishment. Instead, initiation within hours or days of birth would be necessary.
Low-level chronic inflammation and immune activation is recognised in people with HIV, even with virological control on ART[3,10,19], and increased immune activation/proliferation are associated with shortened TL8. In our study, we did not link immune activation/proliferation with TL, possibly due to the immunophenotyping being done at the same timepoint as TL measurement, failing to reflect past immune activity that may have caused TL shortening. Additionally, no differences on TL were noted between children with and without CMV/EBV co-infection, hypothesised to contribute to immune activation and TL shortening[30,31]. This might be explained by thymus plasticity, enhancing thymic activity in response to co-infection, replenishing the T-cell repertoire with naïve circulating T-cells and collectively maintaining longer TL. Mechanistic exploration of thymic activity in children with HIV could unveil compensatory mechanisms and potential therapeutic strategies for TL maintenance. Furthermore, investigations into telomerase activity in HIV children may provide insights into the impact of HIV on TL shortening, given the role of telomerase in telomere elongation.
Our study had several limitations, including uneven sample size and distribution between groups, potentially influencing statistical analysis. Most samples were from single time-point assessments, suggesting that the dynamic TL trend seen across age in South-African children without HIV might reflect variation between individuals rather than depict the trajectory of the same individuals over time. Similarly, only about a quarter of the children with HIV studied at 2 years had subsequent samples at 5 years, and there was a notable high variability in the data amongst individuals from the same study group. The selection was determined by another CHER sub-study selecting children with HIV viraemic control for at least 3 months, limiting the assessment of recent HIV viremia’s impact on TL[20]. Furthermore, TL measurements were done on PBMCs DNA, without knowledge of naïve and memory T-cell subsets proportions. Further work on TL in memory and naïve subsets would likely increase understanding of any interaction between HIV reservoir size and immunosenescence.
Our study is one of the largest data sets analysing the factors that influence TL in children with and without HIV in an African setting. Earlier and longer duration of ART is associated with longer TL, reinforcing current strategies for early diagnosis and effective ART. We demonstrate a potential relationship between TL and lymphoid progenitor organ activity and provide insight into potential feedback mechanisms facilitating immune reconstitution after ART initiation[29]. Moreover, our study found no implication of CMV/EBV co-infection on TL biology. Further longitudinal clinical outcome analysis and prospective TL measurement may contribute to understanding HIV immunopathogenesis.
Funding
Carlota Miranda-Solé is funded by ViiV Health care. The CWC Study was ethically approved by Stellen bosch University (M12/01/005) and the Cape Town Health Department authorised the study. The use of the CHER trial samples was authorised by the University of the Witwa tersrand (040703) and Stellenbosch University (M12/01/005). Regarding open access, the author applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
- Brady, M. T. et al. Declines in Mortality Rates and Changes in Causes of Death in HIV-1-Infected Children During the HAART Era. JAIDS Journal of Acquired Immune Deficiency Syndromes 53, 86–94 (2010).
- Zicari, S. et al. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 11, 200 (2019).
- Chiappini, E. et al. Accelerated aging in perinatally HIV-infected children: clinical manifestations and pathogenetic mechanisms. Aging 10, 3610–3625 (2018).
- Patekar, D., Kheur, S. & More, P. Prevalence of Viral Coinfections with EBV and CMV and Its Correlation with CD4 Count In HIV-1 Serpositive Patients. J AIDS Clin Res 6, (2015).
- Adland, E., Klenerman, P., Goulder, P. & Matthews, P. C. Ongoing burden of disease and mortality from HIV/CMV coinfection in Africa in the antiretroviral therapy era. Front Microbiol 6, (2015).
- Nazim, F., Kayani, H. A., Ali Nathwani, A., Mir, F. & Abidi, S. H. CMV and EBV Co-Infection in HIV-Infected Children: Infection Rates and Analysis of Differential Expression of Cytokines in HIV Mono- and HIV–CMV–EBV Co-Infected Groups. Viruses 14, 1823 (2022).
- Vaiserman, A. & Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front Genet 11, (2021).
- Bellon, M. & Nicot, C. Telomere Dynamics in Immune Senescence and Exhaustion Triggered by Chronic Viral Infection. Viruses 9, 289 (2017).
- Shammas, M. A. Telomeres, lifestyle, cancer, and aging. Curr Opin Clin Nutr Metab Care 14, 28–34 (2011).
- Dalzini, A. et al. Biological Aging and Immune Senescence in Children with Perinatally Acquired HIV. J Immunol Res 2020, 1–15 (2020).
- Lewis, J. et al. Thymic Output and CD4 T-Cell Reconstitution in HIV-Infected Children on Early and Interrupted Antiretroviral Treatment: Evidence from the Children with HIV Early Antiretroviral Therapy Trial. Front Immunol 8, (2017).
- Cowell, W. et al. Telomere dynamics across the early life course: Findings from a longitudinal study in children. Psychoneuroendocrinology 129, 105270 (2021).
- Martens, D. S. et al. Newborn telomere length predicts later life telomere length: Tracking telomere length from birth to child- and adulthood. EBioMedicine 63, 103164 (2021).
- Payne, H. et al. Naive B Cell Output in HIV-Infected and HIV-Uninfected Children. AIDS Res Hum Retroviruses 35, 33–39 (2019).
- Hunt, S. C. et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell 7, 451–458 (2008).
- Rodriguez, I. J. et al. Immunosenescence Study of T Cells: A Systematic Review. Front Immunol 11, (2021).
- Nunes-Alves, C., Nobrega, C., Behar, S. M. & Correia-Neves, M. Tolerance has its limits: how the thymus copes with infection. Trends Immunol 34, 502–510 (2013).
- Gianesin, K. et al. Premature aging and immune senescence in HIV-infected children. AIDS 30, 1363–1373 (2016).
- Dalzini, A. et al. Size of HIV‐1 reservoir is associated with telomere shortening and immunosenescence in early‐treated European children with perinatally acquired HIV‐1. J Int AIDS Soc 24, (2021).
- Payne, H. et al. Early ART-initiation and longer ART duration reduces HIV-1 proviral DNA levels in children from the CHER trial. AIDS Res Ther 18, 63 (2021).
- Tagarro, A. et al. Early and Highly Suppressive Antiretroviral Therapy Are Main Factors Associated With Low Viral Reservoir in European Perinatally HIV-Infected Children. JAIDS Journal of Acquired Immune Deficiency Syndromes 79, 269–276 (2018).
- Cotton, M. F. et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. The Lancet 382, 1555–1563 (2013).
- Violari, A. et al. Early Antiretroviral Therapy and Mortality among HIV-Infected Infants. New England Journal of Medicine 359, 2233–2244 (2008).
- Payne, H. et al. Comparison of Lymphocyte Subset Populations in Children From South Africa, US and Europe. Front Pediatr 8, (2020).
- Lawrie, D., Payne, H., Nieuwoudt, M. & Glencross, D. K. Observed full blood count and lymphocyte subset values in a cohort of clinically healthy South African children from a semi-informal settlement in Cape Town. South African Medical Journal 105, 589 (2015).
- Bains, I., Thiébaut, R., Yates, A. J. & Callard, R. Quantifying Thymic Export: Combining Models of Naive T Cell Proliferation and TCR Excision Circle Dynamics Gives an Explicit Measure of Thymic Output. The Journal of Immunology 183, 4329–4336 (2009).
- Niewiesk, S. Maternal Antibodies: Clinical Significance, Mechanism of Interference with Immune Responses, and Possible Vaccination Strategies. Front Immunol 5, (2014).
- Shearer, W. T. et al. Viral Load and Disease Progression in Infants Infected with Human Immunodeficiency Virus Type 1. New England Journal of Medicine 336, 1337–1342 (1997).
- Chalouni, M. et al. Correlation between blood telomere length and CD4+ CD8+ T-cell subsets changes 96 weeks after initiation of antiretroviral therapy in HIV-1–positive individuals. PLoS One 15, e0230772 (2020).
- Kamranvar, S. A. & Masucci, M. G. The Epstein–Barr virus nuclear antigen-1 promotes telomere dysfunction via induction of oxidative stress. Leukemia 25, 1017–1025 (2011).
- Lin, Z., Gao, H., Wang, B. & Wang, Y. Cytomegalovirus Infection and Its Relationship with Leukocyte Telomere Length: A Cross-Sectional Study. Mediators Inflamm 2021, 1–5 (2021).

Tables at a glance
Figures at a glance