The Risk of Weight Gain in Hiv Treatment-Naïve Individuals Antiretroviral Therapy Insti-Based and Non-Insti-Based Regimeds: A Retrospective Cohort Study
Received Date: July 23, 2025 Accepted Date: August 12, 2025 Published Date: August 15, 2025
doi:10.17303/jaid.2025.12.102
Citation: Taisheng Li, Ling Chen, Jia Tang, Leidan Zhang, Liyuan Zheng, et al. (2025) The Risk of Weight Gain in Hiv Treatment-Naïve Individuals Antiretroviral Therapy Insti-Based and Non-Insti-Based Regimeds: A Retrospective Cohort Study. J HIV AIDS Infect Dis 12: 1-14
Abstract
Objective
Integrase strand transfer inhibitors (INSTIs) have emerged as frontline HIV/AIDS treatment agents. Although weight gain has been observed in some patients following the administration of INSTIs during clinical management, it remains unclear whether this weight gain is truly medication-related. Understanding whether individuals on INSTI-based regimens acquire significant weight gain might thus give useful reference criteria for selecting antiretroviral treatment (ART) regimens for clinical HIV/AIDS management.
Methods
A retrospective analysis was carried out in this study. We compared longitudinal weight changes between individuals in the INSTIs and non-INSTIs groups, excluding those with advanced disease stages. Multivariable regression analysis determined the risk factors affecting severe weight gain.
Results
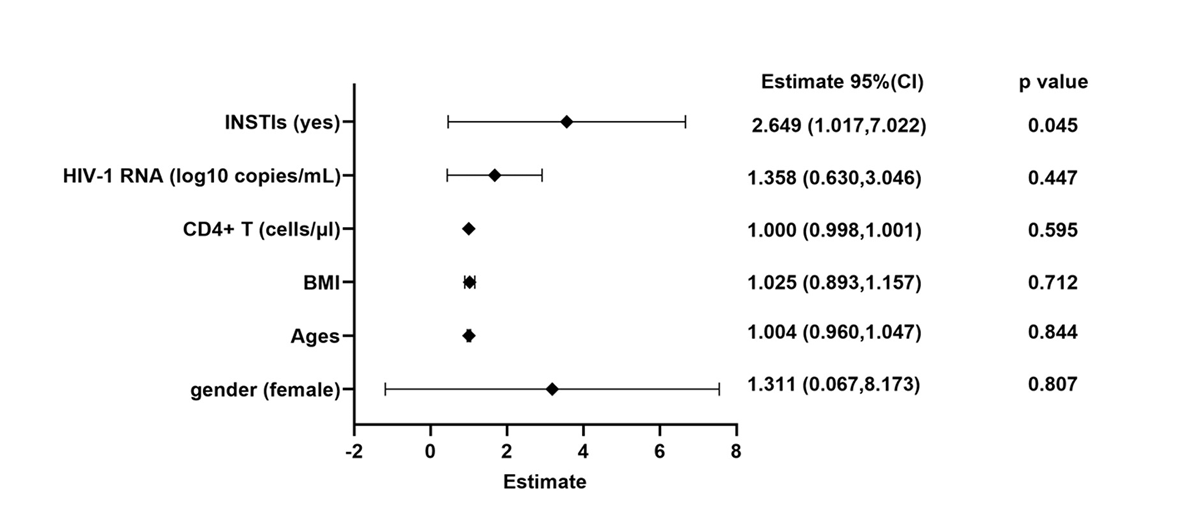
A total of 355 treat-naïve people living with HIV (PLWH) were included. Compared to the non-INSTIs group, PLWH in the INSTIs group showed a noticeably greater gain in body weight and a higher prevalence of obesity, especially the bictegravir (BIC)-based and dolutegravir (DTG)-based ART regimens. In addition, compared to the non-INSTIs group, the INSTIs group had a significantly greater incidence of severe weight gain (19.4% vs. 8.9%, χ2=7.838). Moreover, INSTIs were found to be a risk factor for severe weight gain (OR: 2.649; 95%CI: 1.017-7.022).
Conclusions
In our study, PLWH on INSTIs-based ART were more likely to experience weight gain, and this weight gain was independent of recovery from advanced disease status. We need to monitor body weight change and potential metabolic complications among PLWH initiating ART with INSTIs. Further dietary control and intensified exercise are essential for PLWH experiencing significant weight gain.
Abbreviations
INSTIs: Integrase strand transfer inhibitors; HIV: Human immunodeficiency virus; AIDS: Acquired immunodeficiency syndrome; ART: Antiretroviral therapy; PUMCH: Peking Union Medical College Hospital; BMI: body mass index; VL: viral load; PLWH: People living with HIV; RNA: Ribonucleic acid; OR: Odds ratio; CI: Confidence interval; BMI: Body mass index; DTG: dolutegravir; BIC: Bictegravir; FTC: Emtricitabine; TAF: Tenofovir alafenamide; ABC: Abacavir; 3TC: Lamivudine; EVG: elvitegravir; C: cobicistat; RAL: Raltegravir; EFV: Efavirenz; LPV/r: Lopinavir/ritonavir; d4T: Stavudine; IDV: Indinavir, TDF: Tenofovir disoproxil fumarate; NVP: Nevirapine; WHO: World Health Organization; IQR: Interquartile range; HBV: Hepatitis B virus; HCV: Hepatitis C virus; NRTIs: Nucleoside reverse transcriptase inhibitors; NNRTIs: Non-nucleoside reverse transcriptase inhibitors, PIs: Protease inhibitors; DOR: Doravirine, CAMS: Chinese Academy of Medical Sciences
Keywords: HIV-Infected; Integrase Strand Transfer Inhibitors; Antiretroviral Therapy; Advanced Disease Status; Weight Gain
Introduction
Although antiretroviral therapy (ART) has slashed AIDS-related illness and death, people living with HIV (PLWH) still contend with a new set of long-term challenges—chief among them metabolic disorders that pave the way for cardiovascular disease and diabetes. Smoking, dyslipidemia, and the lingering inflammation and immune activation driven by HIV itself remain the key culprits behind this heightened cardiometabolic risk [1]. However, obesity and weight gain after ART initiation are becoming increasingly recognized problems in our modern HIV treatment paradigm [2, 3].
Weight gain after ART initiation is a typical phenomenon among PLWH and can occur with all antiretroviral classes [4, 5]. Weight gain among PLWH, especially among individuals with a low baseline body mass index (BMI), low CD4+ T count, and excessive HIV-1 RNA, was related to enhanced survival and immunologic recovery early in the HIV epidemic [5, 6] and therefore was considered a “return to health”. However, the median BMI and occurrence of baseline weight problems amongst PLWH-initiating ART have been steadily increasing [4, 5], and many human beings achieve an excess amount of weight leading to post-treatment obesity [7]. In this situation, weight gain raises the hazard of linked co-morbidities such as diabetes and cardiovascular disorders [8-11].
The classification of antiretrovirals regarded as integrase strand transfer inhibitors (INSTIs) contributes to the improved protection and efficacy of modern ART regimens [12]. INSTIs are currently blanketed in desired or encouraged ART regimens in HIV cure suggestions around the world, based totally on research indicating efficacy, safety, and comfort of administration [13, 14]. However, there is mounting evidence that the type of INSTIs, in particular dolutegravir (DTG) and bictegravir (BIC) are associated with more weight gain than other classes of antiretrovirals [15-17]. Further research printed that, compared to Raltegravir (RAL), Elvitegravir (EVG), and non-INSTIs, PLWH based-DTG regimen exhibited the fastest rate of weight gain inside 2 years of ART, even the average body weight increase to 6 kg [18]. Paul E Sax et.al observed that PLWH on the BIC/emtricitabine (FTC)/ tenofovir alafenamide (TAF) regimen skilled a more reported extend in physique weight in contrast to abacavir (ABC)/ lamivudine (3TC)/DTG, with the most sizeable weight gain stated in Black females. Furthermore, a comparative evaluation published no extensive difference in weight attain between the BIC/FTC/TAF regimen and the DTG+FTC/TAF regimen [5]. However, whether or not these reviews of weight gain associated with INSTIs genuinely mirror an association with the tablets themselves remains uncertain. Our goal is to inspect the impact of INSTIs on weight changes by using specifically excluding late-stage PLWH,
Materials and Methods
Study Design and Population
Data from follow-up outpatients at the HIV/AIDS clinic at Peking Union Medical College Hospital (PUMCH) in Beijing, China, from October 2008 to June 2023 was retrospectively analyzed. All PLWH with a baseline body mass index (BMI) of ages ≥ 18 years were screened and enrolled. We primarily documented their blood lipid level, body weight, CD4+ T cell count, and viral load (VL). PLWH with a pre-treatment CD4⁺ T cell count ≥ 200 cells/µL were included in this study according World Health Organization (WHO) clinical staging system [19]. Individuals without a baseline body weight, AIDS stage according WHO clinical staging system [19], switching ART regimen during treatment (from INSTIs to non-INSTIs or vice versa) and those with severe renal or hepatic impairment were excluded. This study was reviewed and exempted by the institutional review board of the PUMCH, Beijing, China.
Procedures and Measures
We collected and recorded basic information about PLWH at AIDS clinic follow-up, including gender, age, BMI, ART initiation regimens, ART initiation time, baseline hepatitis surface B antigen and hepatitis C antibody status, viral load and CD4+ T cell count over time. The BMI was calculated according the following formula: BMI=Weight (kg) / height (m)2. BMI values were generally divided into four groups based on the criteria of WHO: < 18.5 kg/m2, 18.5~23.9 kg/m2, 24~27.9 kg/m2, and ≥ 28 kg/m2, which represented low weight, normal weight, overweight and obesity, respectively [20]. The measures of CD4+ T cell count and VL were adopted flow cytometry and real‑time RT‑PCR assay as described in previous study, respectively [21].
All PLWH ART regimens including bictegravir (BIC)/emtricitabine (FTC)/tenofovir alafenamide (TAF), dolutegravir (DTG)-based regimens, elvitegravir (EVG)/cobicistat (C)/emtricitabine (FTC)/tenofovir alafenamide (TAF), Raltegravir (RAL)-based regimens. Other antiretroviral drugs consist of lamivudine (3TC), efavirenz (EFV) 600 mg/400 mg, lopinavir/ritonavir (LPV/r), Stavudine (d4T), zidovudine (AZT), Indinavir (IDV), tenofovir disoproxil fumarate (TDF), abacavir (ABC), and Nevirapine (NVP). ART regimens containing BIC, DTG, RAL, or EVG were classified into the INSTIs group, while other regimens were classified into the non-INSTIs group.
Statistical Analyses
The tabulated descriptive statistics are displayed as frequencies, median with IQR, and mean with standard deviation. To compare the clinical features of patients in various groups, we employed the t-test for parametric continuous variables, the Mann-Whitney U test for non-parametric continuous data, and the Chi-squared test or Fisher's exact test for categorical variables. A mixed-effects model was used to analyze the data with repeated measures and missing values. A multivariate logistic regression model was used to assess the factors associated with severe weight increase during week 96 (compare to baseline), taking into account age, gender, BMI, CD4+ T cell count, HIV-1 RNA, and the use of INSTIs. Prism version 9 (GraphPad Inc., La Jolla, CA) and SPSS 26.0 (IBM Corporation, Armonk, New York, USA) were used for all statistical analyses. A significance level of p<0.05 was applied to all tests.
Results
Characteristics of the Study Population
Overall, we screened and enrolled 355 eligible outpatients from HIV/AIDS clinics in PUMCH for this analysis, including non-INSTIs group (n=247) and INSTIs group (n=108). Demographics and baseline characteristics are shown in Table 1.
The median age was 31 years. A majority of participants were male. The vast majority are transmitted through sexual contact, with the majority of cases being transmitted homosexually. The median CD4+ T cell count and HIV-1 RNA were 346 cells/µL (IQR: 280-456) and 4.5 log10 copies/mL (IQR: 4.2-4.9), respectively. These data did not show significant differences between the INSTIs group and the non-INSTIs group. The ART regimens in the non-INSTIs group primarily consist of two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitors (NNRTIs), with a few regimens including protease inhibitors (PIs). In contrast, the ART regimens in the INSTIs group mainly comprise first- and second-generation integrase inhibitors, including BIC, DTG, RAL, and EVG-based regimens. In the non-INSTIs group, the proportions of positive hepatitis B surface antigen and positive hepatitis C antibody were 4.5% and 1.2%, respectively, while in the INSTIs group, they were 2.8% and 0.9%, respectively.
Longitudinal Changes in Body Weight in the Non-Instis and Instis Group
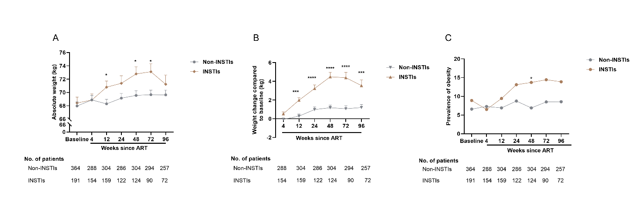
The statistical results within 96 weeks of ART demonstrated that the absolute weight of PLWH in INSTIs group was higher than those non-INSTI-based regimens, and weight gain in INSTIs group was much more than the other group. Similarly, the incidence of obesity was higher in the INSTIs group compared to the non-INSTIs group, especially after 12 weeks of ART, where the trend becomes more pronounced. Notably, during 48 weeks, the INSTIs group weight gain showed a linear upward trend, but after 48 weeks, there was a downward trend in weight gain, which means that the weight gain slowed down. The weight gain of non-INSTIs group had a slow upward trend during ART 2 years (Figure 1). These results were consistent with the statistics for PLWH including advanced disease status and non-advanced disease stage (Supplementary figure 1).
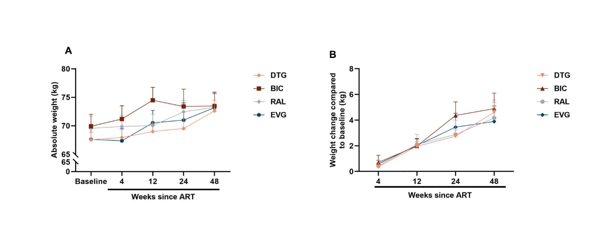
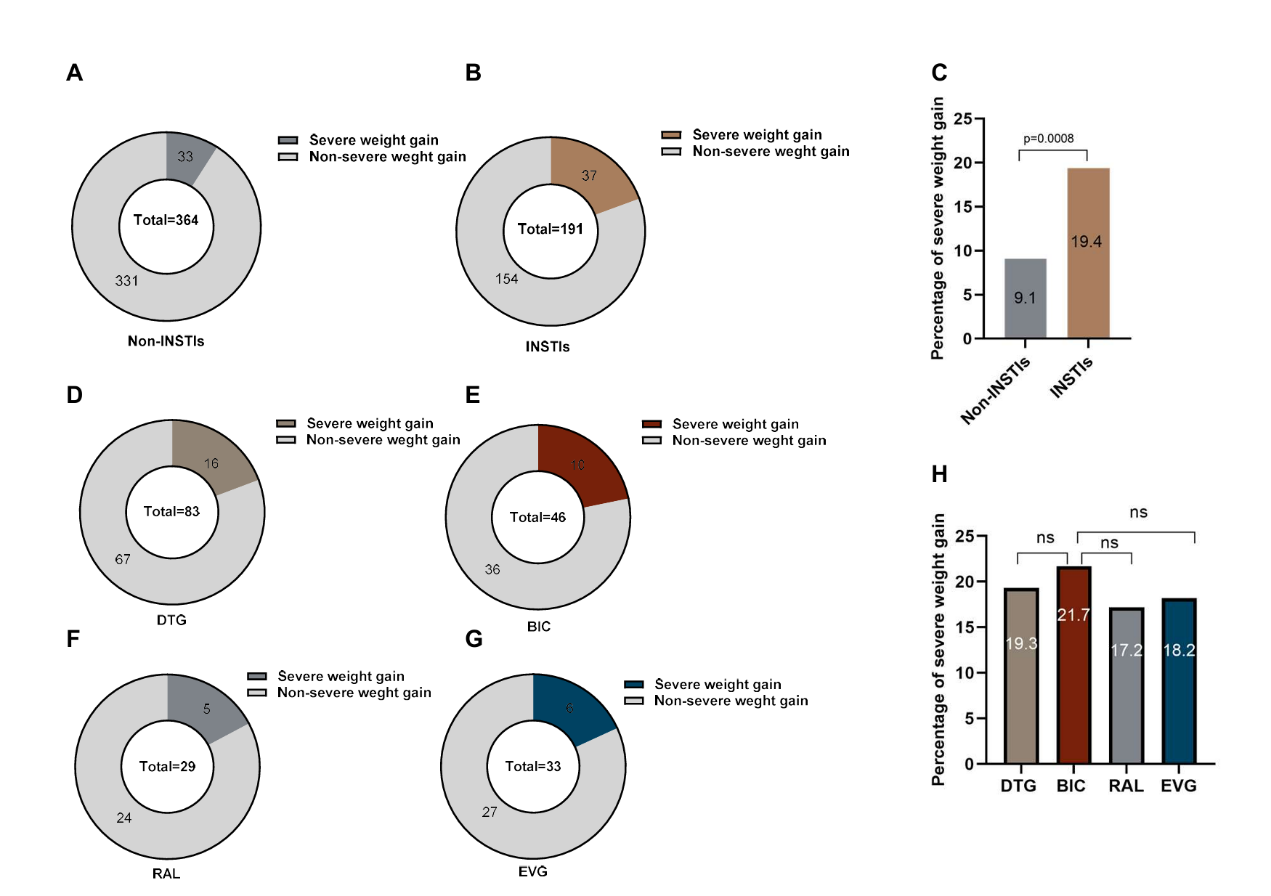
We further analyzed the changes in body weight with specific INSTI regimens, as shown in Table 2. The Subgroup analysis showed that different INSTI regimens generally led to sustained weight gain within 48 weeks of ART, especially the DTG-based and BIC-based regimens, in which the weight gain at 48 weeks of ART reached 2.88±3.35 kg and 3.46±6.26 kg, respectively. However, the weight gain of the EVG-based regimen and the RAL-based regimen at week 48 were more modest, with increases of 2.68±3.82 kg and 2.20±4.30 kg, respectively. The two subgroups, BIC-based regimens and DTG-based regimens, with the most noticeable weight gain, were no significant differences in the longitudinal weight gain. These results align with the statistics findings for PLWH with AIDS stage and non-AIDS stage. (Supplementary figure 2 and table 1).
Severe Weight Gain
To understand factors associated with more extreme weight gain, we introduced the concept of significant weight gain, defined as ≥ 10% weight gain over 96 weeks [15]. We found that 19.4% of PLWH in INSTIs group had experienced severe weight gain, which much higher than non-INSTIs group (19.4(21/108) vs. 9.1(22/247), χ2=7.838, p=0.005) (Figure 2 A-C). Similarly, statistical results for PLWH with baseline CD4+ T cell count of both <200 cells/µL and ≥200 cells/µL also indicated that the proportion of severe weight gain in the INSTIs group was significantly higher than that in the non-INSTIs group (19.4% vs. 9.1%, p=0.0008) (Supplementary figure 3). Furthermore, there were no significant differences in age, sex, baseline BMI, and baseline HIV-1 VL between the two groups. However, baseline CD4+ T cell count was significantly higher in the INSTIs group compared to the non-INSTIs group (395 cells/µL (IQR: 251, 652) vs.340 cells/µL (IQR: 294-421), p=0.028) (Table 3).
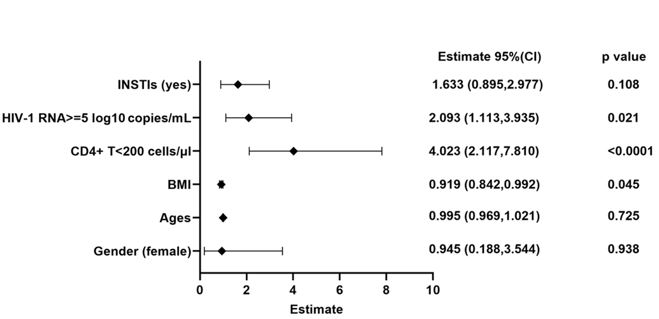
We further explored the risk factors associated with severe weight gain. The logistic regression models were employed to evaluate the variables might influencing the weight such as sex, ages, baseline BMI, baseline CD4+ T cell count, baseline VL, and use INSTIs. Our results suggested that INSTIs use is a risk factor for severe weight gain (OR: 2.649, 95%CI: 1.017-7.022) (Figure 3).
Discussion
Since the widespread clinical use of INSTIs, weight gain has become a major complaint during the follow-up of PLWH on ART. Previous studies have also supported that INSTIs may lead to weight gain, but whether the weight gain is drug-induced remains inconclusive. In our study, we excluded PLWH with advanced-stage disease, for weight gain due to recovery from the disease. The results showed that weight gain induced by INSTIs much greater than older NRTIs, NNRTIs, and protease inhibitors (PIs) during the first 2 years of therapy (Figure 1 and supplementary figure 1). A mix of HIV disease–specific, baseline BMI, and ART-specific factors were associated with more extreme (≥10%) weight gain (Figure 3 and supplementary figure 4). These results filled the gap in knowledge regarding the increase in body weight among PLWH INSTIs-based regimens ART initiation in China.
Many studies have examined the role of ART medications in weight gain among PLWH [4, 22, 23]. Older NRTIs and NNRTIs have not been associated with differential weight gain [5, 24, 25]. Similar to these reports, our results also revealed that non-INSTIs contributed to slight weight gain, which no more than 1.0 kg in ART 2 years (Figure 1B). But the weight gain caused by INSTIs more remarkable, with average weight gain exceed 3.0 kg at week 48 and the greatest rate of weight gain occurring during the initial 48 weeks, which was consistent with previous report and recent study[5, 26, 27] (Figure 1B). And the prevalence of obesity was growing over time (Figure 1C). Furthermore, Different INSTIs, including BIC, DTG, RAL, and EVG, show a more significant weight gain over time with prolonged ART, with this effect being more pronounced in the BIC-based regimen and DTG-based regimen (Table 2). And weight gain induced by BIC appears similar to DTG, consistent with other studies [5, 28, 29]. The smaller weight gains observed with RAL and EVG, compared with their next-generation counterparts DTG and BIC, may be attributable to a synergistic effect between DTG or BIC and TAF [30, 31].
To further explore the impact of ART medications on body weight, we calculated the incidence of severe weight gain between non-INSTIs and INSTIs. We found that 19.4% of PLWH suffered from severe weight gain during the first 2 years of INSTI-based regimens, which was higher previous study [5]. Our study and another report [32] observed strong associations between weight gain and HIV disease characteristics. Disease stage, as reflected by lower baseline CD4+ T cell count and higher baseline HIV RNA, correlated with weight gain in our models of ≥10% weight gain (Supplementary figure 4). However, we also found that in the two groups with significant weight gain, excluding those with a CD4+ T cell count<200 cells/µL, the baseline CD4+ T cell count in the INSTIs group was significantly higher than in the non-INSTIs group. Additionally, the multivariable analysis identified INSTI use as the primary risk factor for severe weight gain. This suggests that individuals’ pre-ART disease status played a significant role in weight gain after treatment, further supporting the conclusion that, in our study, weight gain was more likely to be induced by the medication, rather than a return to health effect, which was aligned with recent study [33].
The cause of the differential weight gain with INSTIs is unknown. One proposed explanation has been the rapid reduction in HIV-1 RNA seen with INSTIs, given the correlation between HIV-1 RNA and resting energy expenditure (REE) [34]. Similarly, in some studies, larger decreases in plasma levels of inflammation and immune activation are more evident with INSTIs compared to other antiretrovirals [35, 36]. Another possible hypothesis is that the weight gain and subsequent metabolic complications may be due to altered gut integrity, potentially due to changes in the intestinal microbiome [37]. Recent study indicated that INSTIs induced adipocyte hypertrophy primarily through the hypoxia pathway (HIF-1-α gene expression), participating in adipose fibrosis. Through positive feedback, this further promotes adipocyte hypertrophy and associated insulin resistance [23, 38]. After the administration of INSTIs, clinicians frequently observe an increase in appetite among HIV-infected individuals. This phenomenon may be associated with the ability of INSTIs to improve metabolic processes in the body. Regardless of the possibility, there are currently no effective interventions in clinical practice for the weight gain caused by INSTIs.
The INSTIs as a newly recommended first-line ART regimen have led to their broader clinical use. Regarding the management of weight gain impacting the quality of life in PLWH on initial INSTI therapy, in addition to standard dietary control and increased exercise [39, 40], are there other solutions, such as switching ART regimens? A recent study has shown that the choice of initial ART regimen, including EFV and TDF, is not associated with weight gain [41]. Many studies have shown that switching from NNRTIs or PIs to INSTIs leads to weight gain [42, 43], but does not increase the risk of long-term complications such as diabetes [44]. This suggests that initially considering a non-INSTIs regimen may provide clinical benefits for PLWH in non-AIDS stages. Recently, Arianna E Kousari et al., thought that switching from an INSTIs-based regimen to a Doravirine (DOR)-based, non-INSTI regimen can result in a modest weight loss, with a decrease of about 2.6% after one year [45]. The impact of switching from a INSTIs regimen to a non-INSTIs regimen on the weight of PLWH requires more data and clinical research for further support. Drawing on mounting evidence that ART drives weight gain in PLWH, an expert consortium—comprising clinicians, public-health specialists, and patient advocates—has issued guidance on when to modify antiretroviral therapy in clinical-trial participants[46]. Under this framework, any enrollee whose weight rises by more than 10 % from baseline or whose BMI exceeds 30 kg/m2 should prompt an immediate reassessment, with consideration given to discontinuing or switching the current regimen.
There are several limitations to our analyses. Firstly, this study is a retrospective investigation with a significant amount of missing data, preventing further statistical analysis of the impact of weight gain on metabolism. Secondly, due to the substantial differences in group sizes, we do not further analyze the weight changes in subgroups of the non-INSTIs group or the data for the INSTIs group at 96 weeks of ART initiation. At the same time, considering the small sample size, we retrospectively included all PLWH non-AIDS stages who were initially treated with INSTIs and non-INSTIs, without conducting formal sample size calculations, which may introduce some bias into the statistical results. Considering China's situation, INSTIs were only included in the medical insurance system in 2022 and began widely used in clinical practice. Therefore, we cannot further analyze the long-term effects of INSTIs on PLWH body weight changes and long-term complications. Finally, our analyses do not evaluate other potential factors to weight gain such as psychiatric comorbidities, concomitant medications, diet, physical activity, or smoking, which may contribute to weight gain in PLWH. All in all, we are unable to identify specific PLWH experiencing severe weight gain after INSTI-based regimens of therapy.
Conclusions
Collectively, our results suggest that INSTIs are more likely to lead to weight gain in PLWH compared to traditional NRTIs, NNRTIs, and PIs, not the effect of disease recovery. Furthermore, they are identified as a risk factor for severe weight gain. This result further corroborates the phenomenon of patients clinically complaining about weight gain associated with the use of INSTIs. For PLWH with INSTIs, we should pay closer attention and monitor to their body weight change. For individuals experiencing significant weight gain, it is recommended to manage their diet and engage in regular exercise. Clarifying the specific HIV-infected population prone to severe weight gain due to the use of INSTIs to facilitate the provision of precise and personalized treatment is a direction that requires further investigation.
Acknowledgments
We thank the patients and their families for their participation and support during this study and thank the staff of the PUMCH HIV/AIDS Clinical Center for their contribution to this work. We also thank all the manuscript authors for reviewing and revising the article. This study was funded by the Special Research Fund for the Central High-level Hospitals of Peking Union Medical College Hospital (2022-PUMCH-D-008), the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2021-I2M-1-037) and National Key Technologies R&D Program for the 13th Five-year Plan (2017ZX10202101-001). We also appreciate the support provided by these organizations.
Supplementary Materials
- Lake JE and JS Currier, (2013) Metabolic disease in HIV infection. Lancet Infect Dis.13: 964-75.
- Gallant J, et al., (2017) Comorbidities among US Patients with Prevalent HIV Infection-A Trend Analysis. J Infect Dis. 216: 1525-33.
- Godfrey C, et al., (2019) Obesity and Fat Metabolism in Human Immunodeficiency Virus-Infected Individuals: Immunopathogenic Mechanisms and Clinical Implications. J Infect Dis. 220: 420-31.
- Koethe JR, et al., (2016) Rising Obesity Prevalence and Weight Gain among Adults Starting Antiretroviral Therapy in the United States and Canada. AIDS Res Hum Retroviruses. 32: 50-8.
- Sax PE, et al., (2020) Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin Infect Dis. 71: 1379-89.
- Yuh B, et al., (2013) Weight change after antiretroviral therapy and mortality. Clin Infect Dis. 60: 1852-9.
- Lakey W, et al., (2015) Short communication: from wasting to obesity: initial antiretroviral therapy and weight gain in HIV-infected persons. AIDS Res Hum Retroviruses. 29: 435-40.
- Kumar S and K Samaras, (2018) the Impact of Weight Gain During HIV Treatment on Risk of Pre-diabetes, Diabetes Mellitus, Cardiovascular Disease, and Mortality. Front Endocrinol (Lausanne). 9: 705.
- McCann K, et al., (2021) Implications of weight gain with newer anti-retrovirals: 10-year predictions of cardiovascular disease and diabetes. AIDS, 2021. 35: 1657-65.
- Bannister WP, et al., (2022) Changes in body mass index and clinical outcomes after initiation of contemporary antiretroviral regimens. AIDS. 36: 2107-19.
- O'Halloran, JA, et al., (2022) Integrase Strand Transfer Inhibitors Are Associated With Incident Diabetes Mellitus in People with Human Immunodeficiency Virus. Clin Infect Dis. 75: 2060-65.
- Scarsi, KK, et al., (2020) HIV-1 Integrase Inhibitors: A Comparative Review of Efficacy and Safety. Drugs. 80: 1649-76.
- Panel on Clinical Practices for Treatment of, H.I.V.I., (1998) Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. Afr J Med Pract. 5: 79-104.
- Saag, M.S., et al., (2018) Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. JAMA. 320: 379-96.
- Eckard, A.R. and G.A. McComsey, (2020) Weight gain and integrase inhibitors. Curr Opin Infect Dis. 33: 10-19.
- Dupont, E. and J.C. Yombi, (2023) antiretroviral therapy and weight gain in antiretroviral treatment-experienced HIV patients: A review. AIDS Rev. 25: 54-64.
- Calza L, et al., (2020) Weight gain in antiretroviral therapy-naive HIV-1-infected patients starting a regimen including an integrase strand transfer inhibitor or darunavir/ritonavir. Infection. 48: 213-21.
- Bourgi K, et al., (2020) Greater Weight Gain in Treatment-naive Persons Starting Dolutegravir-based Antiretroviral Therapy. Clin Infect Dis. 70: 1267-74.
- Ewetola R, et al., (2021) Disparities in HIV Clinical Stages Progression of Patients at Outpatient Clinics in Democratic Republic of Congo. Int J Environ Res Public Health. 18.
- Chen X, et al., (2020) the association between BMI and health-related physical fitness among Chinese college students: a cross-sectional study. BMC Public Health. 20: 444.
- Guo F, et al., (2021) longitudinal change in bone mineral density among Chinese individuals with HIV after initiation of antiretroviral therapy. Osteoporos Int. 32: 321-32.
- Bailin SS, et al., (2020) Obesity and Weight Gain in Persons with HIV. Curr HIV/AIDS Rep. 17: 138-150.
- Markakis K, et al., (2024) Weight Gain in HIV Adults Receiving Antiretroviral Treatment: Current Knowledge and Future Perspectives. Life (Basel). 14(11).
- Bakal D.R, et al., (2018) Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. J Antimicrob Chemother. 73: 2177-85.
- Hasse B, et al., (2014) Obesity Trends and Body Mass Index Changes after Starting Antiretroviral Treatment: The Swiss HIV Cohort Study. Open Forum Infect Dis. 1: ofu040.
- Lam JO, et al., (2024) Changes in Body Mass Index over Time in People with and Without HIV Infection. Open Forum Infect Dis. 11: ofad611.
- McKellar MS, et al., (2024) Rapid viral suppression using integrase inhibitors during acute HIV-1 infection. J Antimicrob Chemother.
- Wohl DA, et al., (2019) Bictegravir combined with emtricitabine and tenofovir alafenamide versus dolutegravir, abacavir, and lamivudine for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 6: e355-63.
- Stellbrink HJ, et al., (2019) Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV. 6: e364-72.
- Wei Y, et al., (2023) Efficacy and safety profiles of dolutegravir plus lamivudine vs. bictegravir/emtricitabine/tenofovir alafenamide in therapy-naïve adults with HIV-1. Chin Med J (Engl). 136: 2677-85.
- Hester EK, S. Greenlee and SH. Durham, (2022) Weight Changes With Integrase Strand Transfer Inhibitor Therapy in the Management of HIV Infection: A Systematic Review. Ann Pharmacother 2022: 10600280211073321.
- Bhagwat P, et al., (2018) Changes in Waist Circumference in HIV-Infected Individuals Initiating a Raltegravir or Protease Inhibitor Regimen: Effects of Sex and Race. Open Forum Infect Dis. 5: ofy201.
- Pantazis N, et al., (2024) Changes in bodyweight after initiating antiretroviral therapy close to HIV-1 seroconversion: an international cohort collaboration. Lancet HIV. 11: e660-9.
- Mulligan K, VW Tai and M Schambelan, (1997) Energy expenditure in human immunodeficiency virus infection. N Engl J Med. 336: 70-1.
- Hileman CO, et al., (2015) Differential Reduction in Monocyte Activation and Vascular Inflammation with Integrase Inhibitor-Based Initial Antiretroviral Therapy Among HIV-Infected Individuals. J Infect Dis. 212: 345-54.
- Lake JE, et al., (2014) Switch to raltegravir decreases soluble CD14 in virologically suppressed overweight women: the Women, Integrase and Fat Accumulation Trial. HIV Med. 15: 431-41.
- El Kamari V, et al., (2019) Lower Pretreatment Gut Integrity Is Independently Associated With Fat Gain on Antiretroviral Therapy. Clin Infect Dis. 68: 1394-1401.
- Ngono Ayissi K, et al., (2022) Inhibition of Adipose Tissue Beiging by HIV Integrase Inhibitors, Dolutegravir and Bictegravir, Is Associated with Adipocyte Hypertrophy, Hypoxia, Elevated Fibrosis, and Insulin Resistance in Simian Adipose Tissue and Human Adipocytes. Cells. 11.
- Montoya JL, et al., (2019) Evidence-informed practical recommendations for increasing physical activity among persons living with HIV. AIDS. 33: 931-39.
- Lake JE, et al., (2017) Practical Review of Recognition and Management of Obesity and Lipohypertrophy in Human Immunodeficiency Virus Infection. Clin Infect Dis. 64: 1422-29.
- Drechsler H, et al., (2024) Choice of antiretroviral therapy has low impact on weight gain. AIDS. 38: p. 1731-9.
- Maman O, et al., (2024) The effect of a treatment switch to integrase Strand transfer inhibitor-based regimens on weight gain and other metabolic syndrome-related conditions. BMC Infect Dis. 24: 221.
- Lake, J.E., et al., (2020) Risk Factors for Weight Gain Following Switch to Integrase Inhibitor-Based Antiretroviral Therapy. Clin Infect Dis. 71: e471-7.
- Hwang YJ, et al., (2024) Association between switching to integrase strand transfer inhibitors and incident diabetes in people with HIV. AIDS. 38: 1696-1702.
- Kousari AE, et al., (2024) Weight change with antiretroviral switch from integrase inhibitor or tenofovir alafenamide-based to Doravirine-Based regimens in people with HIV. HIV Res Clin Pract. 25: 2339576.
- Venter WDF, et al., (2021) Weight gain stopping/switch rules for antiretroviral clinical trials. Aids. 35: S183-8.



Tables at a glance
Figures at a glance