Study of Oak Longevity Genes: DNA Extraction and Amplification from CambialCells Using Ag-Pd Nanoparticles for Testing New Antiproliferative Biomedicines
Received Date: April 15, 2024 Accepted Date: May 15, 2024 Published Date: May 18, 2024
doi: 10.17303/jber.2024.8.102
Citation: A. Tavartkiladze, D. Kasradze, G. Simonia, N. Okrostsvaridze, P. Revazishvili, et al. (2024) Study of Oak Longevity Genes: DNA Extraction and Amplification from Cambial Cells Using Ag-Pd Nanoparticles for Testing New Antiproliferative Biomedicines. J Biomed Eng Res 8: 1-16
Abstract
This study explores the effects of melatonin and heteroauxin on gene expression in oak samples, specifically focusing on genes associated with biological rhythms, longevity, and DNA repair. The experimental setup included 15 oak samples, with 10 exposed to melatonin and heteroauxin for three weeks, and 5 serving as controls. The treatments were designed to assess the potential modulation of physiological processes such as growth regulation and stress response in plants.
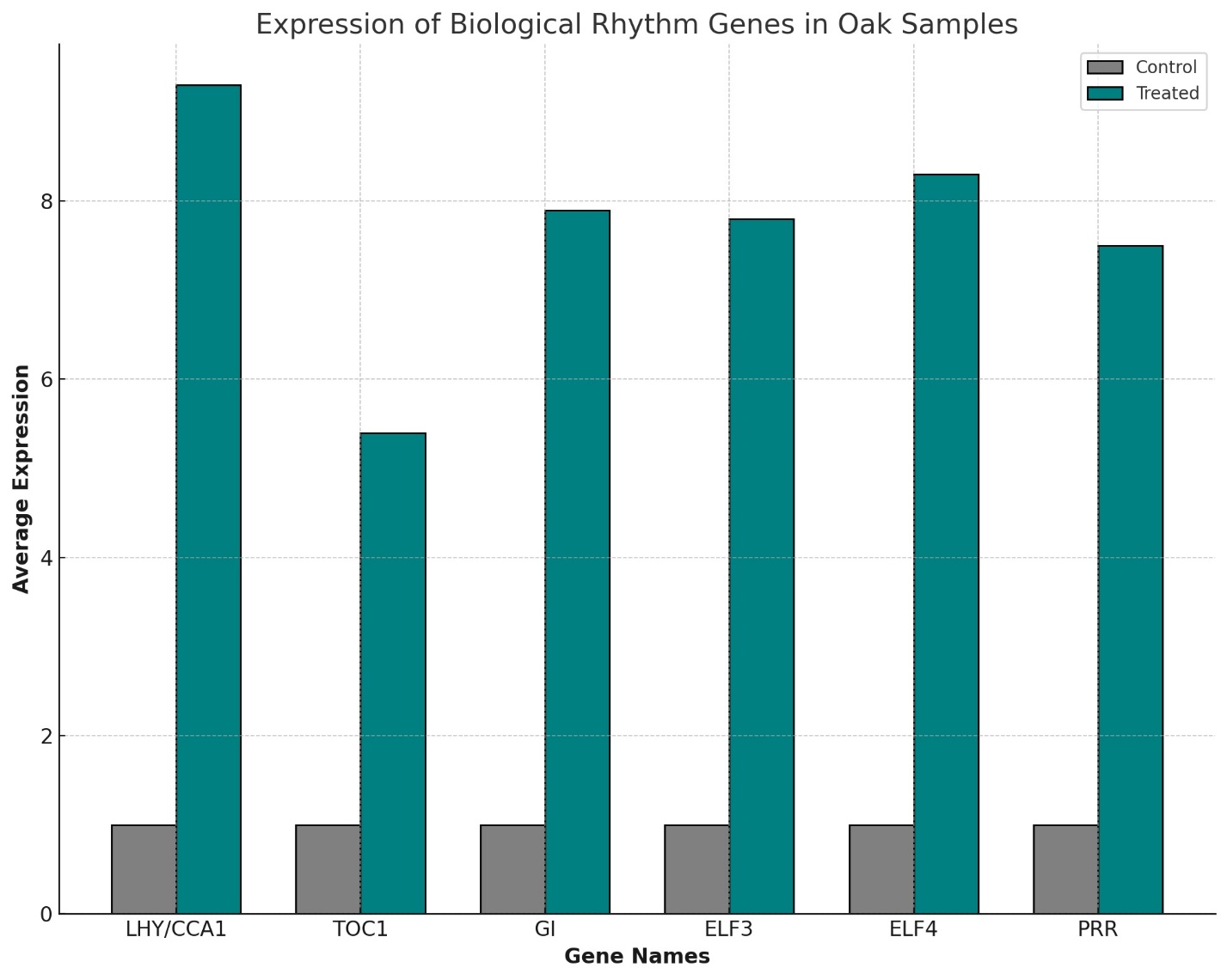
Gene expression analysis revealed that the treatment significantly upregulated genes involved in biological rhythms, such as LHY/CCA1 (LATE ELONGATED HYPOCOTYL and CIRCADIAN CLOCK ASSOCIATED 1), TOC1 (TIMING OF CAB EXPRESSION 1), GI (GIGANTEA), ELF3 and ELF4 (EARLY FLOWERING 3 and 4), and PRR (PSEUDO-RESPONSE REGULATOR). These genes showed an average activity increase of 7.7 times compared to controls. This upregulation highlights the potential role of melatonin and heteroauxin in enhancing the oak's adaptive mechanisms, contributing to environmental adaptation and longevity.
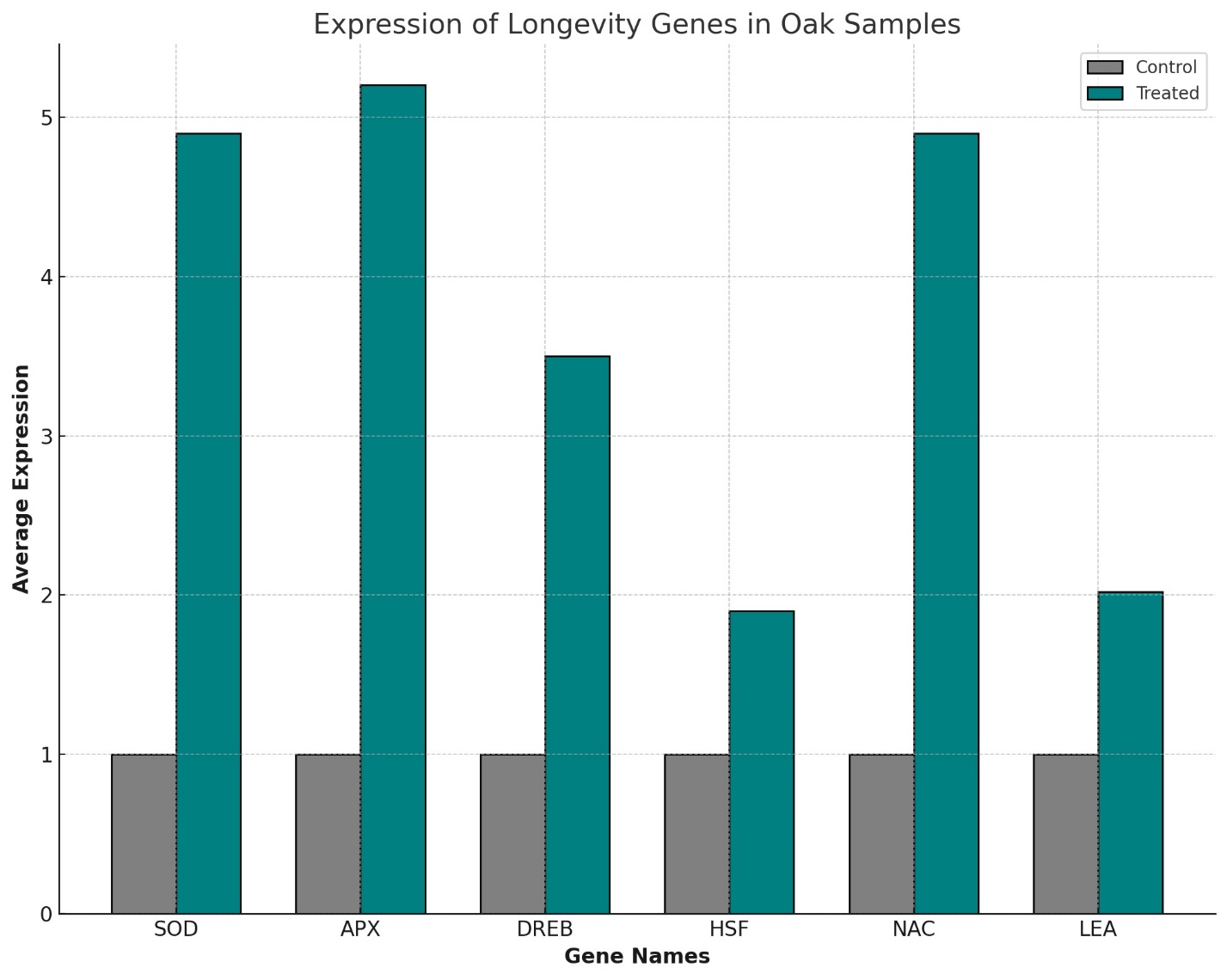
Furthermore, longevity genes including SOD (Superoxide Dismutase), APX (Ascorbate Peroxidase), DREB (Dehydration-Responsive Element-Binding Protein), HSF (Heat Shock Factors), NAC (NAM, ATAF1/2, and CUC2), and LEA (Late Embryogenesis Abundant Proteins) exhibited an average increase of 3.72 times. This increase emphasizes the compounds' potential to activate genetic pathways that promote longevity, echoing their effects observed in other biological systems.
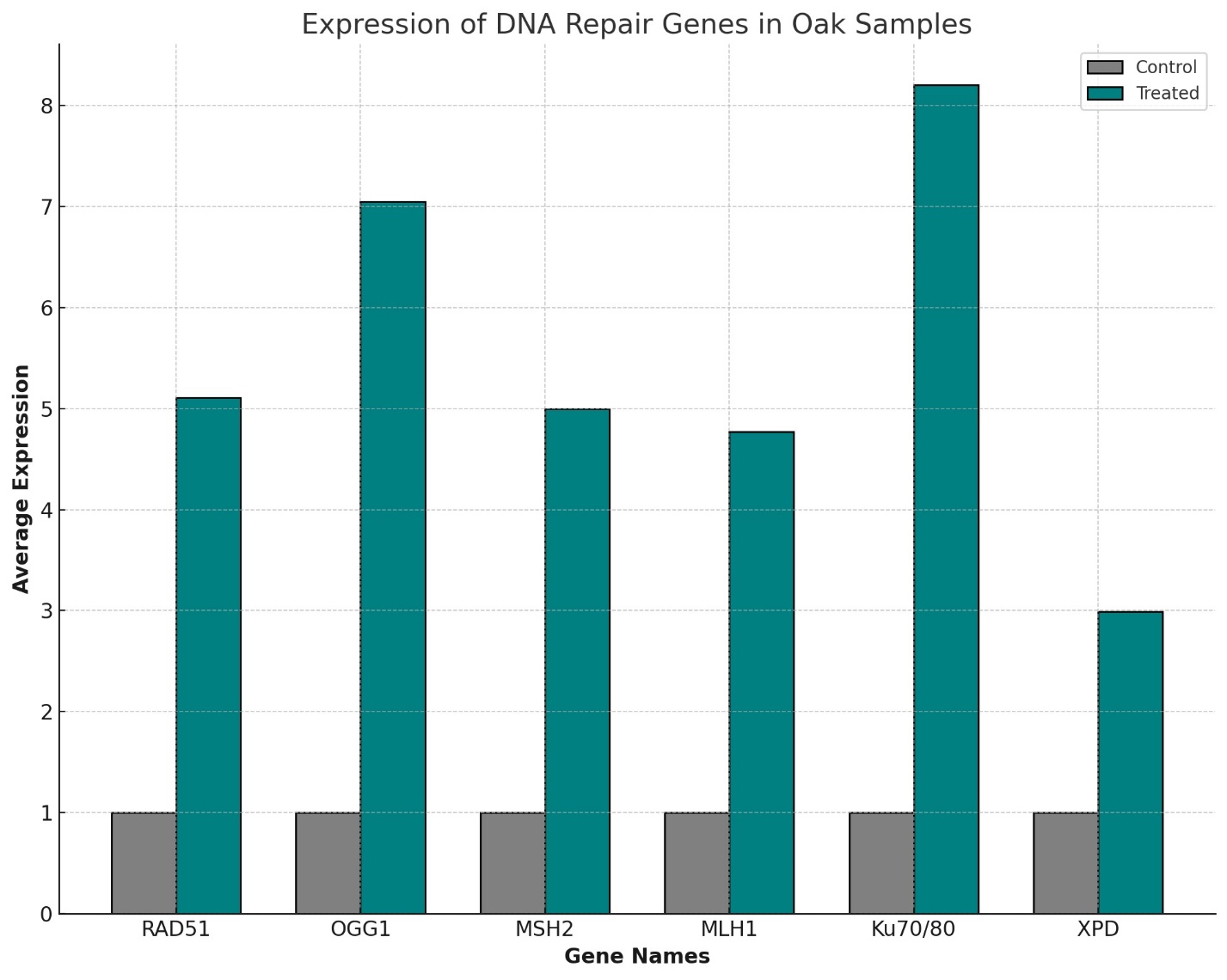
Most notably, DNA repair genes such as RAD51, OGG1 (8-Oxoguanine Glycosylase), MSH2 and MLH1, Ku70/Ku80, and XPD (Xeroderma Pigmentosum Group D) demonstrated a 5.52-fold increase in expression in the treated groups compared to controls. This finding is crucial as it underscores the therapeutic potential of melatonin and heteroauxin in enhancing DNA repair mechanisms, pivotal for cellular health and longevity.
The methodological framework included advanced techniques such as whole-genome sequencing, which provided comprehensive data on the genetic composition of the oak samples, and gene expression profiling, used to measure the effects of the treatments on specific gene activity.
This research not only contributes to our understanding of oak biology but also offers comparative data on aging processes, potentially applicable in other plant and animal species. By examining the shared and unique aspects of gene expression regulation in oaks and their parallels in other life forms, the study opens new avenues for developing anticancer therapies, understanding biodiversity conservation, and assessing the environmental impact of pharmaceutical products.
In conclusion, the integration of traditional botanical research with advanced genetic tools and nanoparticle technology (Ag-Pd) in this study exemplifies a multifaceted approach that bridges several scientific disciplines. The findings provide a template for further research into the mechanisms of longevity and stress resistance, highlighting the interconnectedness of natural systems and human health. This study marks a significant step towards understanding and harnessing the biological mechanisms that underpin longevity and disease resistance, with far-reaching implications for plant biology, medical pharmacology, and the broader field of biotechnology.
Keywords: Melatonin; Heteroauxin; Nanotechnology; Oak Longevity Genes; DNA Repair; Gene Expression; Biological Rhythms; Novel Anticancer Therapies; Silver and Palladium Nanoparticles
Introduction
The study of oak cambial cells provides essential insights into the lifespan of the plant and its adaptation and stability under changing environmental conditions. These insights apply both to the plant as a whole and to individual living cells. Cambial cells, responsible for the growth of stems and roots, contain crucial genetic information for understanding longevity. Traditional methods of DNA extraction and amplification have been limited in exploring these genes. However, recent advances in nanotechnology, particularly the use of Ag-Pd nanoparticles, have introduced new possibilities for PCR-free DNA amplification [3]. This review details the processes from cell harvesting to DNA analysis and explores their implications for efficacy testing of new antiproliferative biomolecules and lifespan-balancing natural drugs
Oak Cambial Cells: Biology and Significance
Cambial cells are a type of meristematic tissue found in many plants, particularly trees such as oaks [1]. These cells are essential for plant growth; they not only prolong the life of the plant but also extend it. This extension is crucial for the development of the plant’s conducting system (vessels and sieve tubes) and structural stability. Located between the xylem and the phloem, cambial cells are responsible for the growth of new phloem cells outward and the production of new xylem cells inward, promoting the radial growth of trees. This continuous renewal and expansion play a pivotal role in the tree’s ability to grow taller and thicker over the centuries.
Oaks are known for their exceptional longevity and strength, relying heavily on the function of their cambial cells [5]. These cells contribute to the tree's ability to adapt to environmental changes and stresses over extended periods. Oak longevity is not only of interest to botanists and biologists; it also offers potential insights into aging and longevity in other species, including humans [11]. By studying the robustness of these cells, researchers hope to uncover broader biological principles that govern cellular aging and resilience.
The goal of our research is to explore one of the main aspects of the role of cambial cells in lifespan: their ability to adapt to varying environmental conditions. This involves partially translating the results obtained to animal and human cells and planning a series of further experiments to study the regulation of lifespan in human and animal cells, including antitumor intracellular mechanisms.
As cambial cells divide and multiply, forming new xylem and phloem, they help the tree adapt to its changing environment. This adaptability is critical for survival in diverse and variable climates, where water availability and temperature can vary significantly. The efficiency and adaptability of cambial activity in oak trees are related to their ability to live for hundreds of years.
Radial growth, driven by cambial cells, also contributes to an oak’s ability to compete for sunlight with neighboring plants. As oaks grow taller and their trunks expand, they absorb more sunlight, which is vital for photosynthesis. This competitive advantage is essential for their survival and reproductive success.
The cambium’s role in oak longevity is also tied to its cellular mechanisms, particularly in how it manages cellular damage and regenerates. As cambial cells divide, they can accumulate genetic mutations, which in many organisms lead to aging and a decline in adaptive mechanisms. However, oak cambial cells have demonstrated remarkable resilience and stability in their genetic structure. This suggests the presence of powerful DNA repair and damage prevention mechanisms, which may be critical to their longevity [8].
Researchers are particularly interested in how these cells manage oxidative stress—a common factor in aging across living systems [4]. Reactive oxygen species (ROS), byproducts of cellular metabolism, can cause significant damage to cellular structures, including DNA, proteins, and lipids. The ability of oak cambial cells to manage and mitigate oxidative stress is likely a key component of their longevity. Understanding these protective mechanisms may provide insights into similar processes in human cells, particularly in combating aging and degenerative diseases.
Ecologically, the vigorous growth of oaks, facilitated by their cambial cells, supports a variety of ecosystems Oak forests provide habitat and food for many species of wildlife, from birds to mammals and insects. The health and strength of these forests often depend on the health of the oak trees at their core, which in turn relies on the effective functioning of the cambial cells.
Thus, oak cambial cell biology offers fascinating insights into the dynamics of living cell growth and lifespan. Insights from the study of these cells not only enhance our understanding of plant biology but also have broader implications for aging research, ecological management, and wood production industries. As research progresses, lessons learned from the resilient nature of oak trees may lead to novel insights into understanding and managing biological aging and cellular health.
Translating the insights gained from the study of oak cambial cells to animal and human cell biology involves understanding common principles of lifespan and cellular dynamics. Just as cambial cells contribute greatly to oak longevity and growth, similar cellular mechanisms may underlie longevity and health in animals and humans.
Cellular Mechanisms of Longevity Across Species: In both plants and animals, longevity is closely related to the ability of cells to manage and repair damage over time. For oak trees, cambial cells govern growth and repair, contributing to the tree’s longevity through constant and highly organized regeneration of new tissue. Lifespan mechanisms in animal and human cells often involve complex biochemical pathways that regulate growth, damage repair, tissue regeneration, and metabolic homeostasis [12].
Adaptive Growth and Cell Renewal: In animals, stem cells play a role similar to that of cambial cells in plants. Stem cells are responsible for generating new cells to replace old or damaged ones, maintaining tissue health and physiological functions. This regenerative capacity is critical to long-term health and longevity. For example, the ability of hematopoietic stem cells to continuously replenish blood cells is vital for maintaining organ function and responding to injuries; in experiments, stem cells have even regenerated heart and brain cells [15].
DNA Repair and Oxidative Stress Management - Like oak cambial cells, human cells face challenges in maintaining genetic stability over time. The accumulation of DNA damage significantly contributes to aging and age-related diseases in humans. Thus, DNA repair mechanisms are critical for longevity. Additionally, the management of oxidative stress, along with the normal functioning of cambial cells, plays a crucial role in the longevity of both animals and humans. Cells across various species have developed antioxidant pathways to mitigate damage caused by reactive oxygen species (ROS). Dysregulation of these pathways can lead to cellular dysfunction and aging [14].
Understanding the cellular mechanisms that contribute to oak longevity may inspire new strategies to enhance human health and longevity. For instance, if specific antioxidant or DNA repair pathways are particularly effective in cambial cells, these could be further studied to develop drugs or biological therapeutic agents that enhance similar pathways in human cells. Such translational research holds the potential for breakthroughs in anti-aging treatments and regenerative medicine [6].
Comparative studies across species provide valuable insights into universal and unique mechanisms of aging and longevity. By studying how different organisms manage cellular health and respond to environmental stresses, researchers can use observations and experiments to identify potential targets for interventions to slow aging and combat age-related diseases in humans [13].
Oak cambial cell biology, while distinct from animal and human biology in many ways, offers valuable metaphors and mechanisms that can be used to understand and potentially manipulate aging processes in animals and humans. Future research on longevity, encompassing botanical, zoological, and medical perspectives, promises to enrich our understanding of the determinants of longevity and improve the quality of life across species. As we continue to explore these connections, cross-disciplinary insights are likely to pave the way for innovative approaches to maintaining health, preventing disease, treating diseases, and extending healthy life years in humans [2].
Materials and Methods
DNA Extraction from Oak Cambial Cells
The unique biochemistry of oak cambial cells, including high concentrations of secondary metabolites like tannins and phenolic compounds, presents specific challenges for DNA extraction. These compounds can interfere with DNA isolation and purification by causing nucleic acid degradation or by inhibiting enzyme activity during PCR. To address these challenges and ensure the extraction of high-quality DNA, the CTAB (cetyltrimethylammonium bromide) method is employed, tailored specifically to mitigate the effects of these inhibitory compounds and optimize the recovery of intact DNA [9].
Cambial Cell Sampling
For this study, cambial cells were meticulously sampled from 15 mature oak trees selected from a forest environment. The sampling process involved the careful removal of bark to access the cambium layer without causing significant damage to the trees. A sterile scalpel was used to scrape the cambial layer, collecting approximately 100 mg of tissue per sample. Samples were immediately placed in liquid nitrogen to preserve metabolic processes and prevent DNA degradation, crucial for maintaining the integrity of the samples for subsequent analysis.
DNA Extraction Protocol
The DNA extraction utilizes the CTAB method, which is particularly effective for plant tissues rich in polysaccharides and polyphenolic compounds. This method includes several steps designed to effectively break down cell walls and isolate DNA in a complex cellular matrix:Preparation of CTAB Buffer: The CTAB buffer was prepared with a 2% concentration of CTAB (compared to the usual 1%) to enhance its ability to solubilize cell membranes. Additionally, 0.2% β-mercaptoethanol was added to the buffer to reduce oxidative damage caused by phenolic compounds.
Cell Lysis: Cambial samples frozen in liquid nitrogen were ground into a fine powder to increase surface area for more efficient lysis. The powdered tissue was then transferred to a 50 mL centrifuge tube containing 10 mL of prewarmed (65°C) CTAB buffer.
Incubation: The mixture was incubated at 65°C for 60 minutes with occasional shaking to promote complete cell lysis, facilitating the release and subsequent binding of DNA to the CTAB.
Decontamination of Polysaccharides and Polyphenols: After cooling to room temperature, chloroform:isoamyl alcohol (24:1) was added to the lysate. The sample was vigorously shaken and then centrifuged at 10,000 g for 15 minutes to separate the phases, allowing for the removal of organic solvents and polysaccharides.
DNA Precipitation and Purification: : The aqueous phase containing the DNA was carefully transferred to a new tube and mixed with isopropanol to precipitate the DNA. The sample was stored at -20°C for 12 hours to ensure complete DNA precipitation. The DNA was then pelleted by centrifugation, washed with 70% ethanol, air-dried, and resuspended in TE buffer (pH 8.0) [3,9,15].
Independent PCR Amplification Using Ag-Pd Nanoparticles
The integration of Ag-Pd nanoparticles as a catalyst in PCR-independent DNA amplification presents a novel approach to overcome limitations associated with traditional PCR, such as sample contamination and thermal damage to the DNA. This methodology was chosen due to its potential to enhance enzymatic reactions essential for DNA replication.
1. Preparation of Nanocatalyst Beads:Ag-Pd nanoparticles were synthesized and immobilized on microplate wells to facilitate handling and recovery. This setup maximizes the surface area contact between the nanoparticles and the DNA molecules, enhancing the catalytic efficiency (3,9,10,15).
2. Reaction Setup and Catalysis: DNA samples prepared via the CTAB method were introduced to the wells containing Ag-Pd nanoparticle beads. Each sample was mixed with a buffer containing all required nucleotides and the DNA polymerase enzyme. The reaction was carried out at a constant temperature (37°C), where the nanoparticles facilitated the DNA polymerase reaction, allowing for rapid and efficient DNA synthesis.
3. Post-Reaction Processing:After the amplification, nanoparticle beads were removed using a magnetic separator, and the amplified DNA was purified from the reaction mixture. This step ensures that the final product is free from any nanoparticle contamination and is ready for downstream applications.
This detailed methodology leverages the unique properties of nanoparticles to streamline the DNA amplification process, providing a robust, efficient, and safer alternative to traditional PCR techniques [3,9,10,15].
Results
Experimental Observations and Analysis of Genes
In the experiment, 10 out of 15 samples were designated as research samples and 5 as controls. Study samples were treated with heteroauxin and melatonin to investigate their effects on the expression of specific DNA regions (genes) and genes associated with biological rhythms. Over three weeks, observations were made using an inverted microscope to monitor the dynamics of DNA replication and the activity of DNA repair genes:
DNA Replication Rate:An enhanced replication rate was noted in the study samples, indicating an increase in DNA synthesis facilitated by nanoparticle catalysis.
Activity of DNA Repair Genes:There was a marked increase in the expression and activity of DNA repair genes, evidenced by a reduction in residual damage in study samples compared to controls.
Gene Expression:Differential expression of genes associated with biological rhythms was assessed, indicating significant modulation in study samples compared to controls.
Thus, the use of Ag-Pd nanoparticles for PCR-independent DNA amplification represents a transformative approach in molecular biology that offers speed, efficiency, and precision in DNA amplification. This method holds promise not only for research applications but also for clinical diagnostics, where rapid and reliable DNA analysis is crucial. Further research and refinement will likely expand its utility and effectiveness, potentially replacing or complementing traditional PCR methods in many scientific and medical applications [5].
Analysis of Oak Viability, Biological Rhythms, and DNA Repair GenesIn our extensive study aimed at elucidating the genetic basis of oak longevity, the DNA of oak cambial cells was thoroughly analyzed using advanced genomic and transcriptomic techniques. This research focused on identifying and characterizing genes that contribute to oak longevity and hardiness, with particular emphasis on genes that regulate biological rhythms, DNA repair, and overall lifespan. The experiment involved studies and observations where selected samples were exposed to melatonin and heteroauxin, biologically active agents that affect plant growth and stress response mechanisms [1].
Genomic and Transcriptomic ProfilingUsing whole-genome sequencing and gene expression profiling, the study mapped the genomic landscape and identified specific gene clusters and regulatory networks critical to oak viability. This approach allows detailed mapping of genetic sequences and their functional correlates, providing not only oak-specific traits but also comparative perspectives on aging and lifespan in different species [7].
Effects of Melatonin and Heteroauxin on Gene Expression
Experimental observations included 15 oak samples, 10 of which were exposed to a combination of melatonin and heteroauxin for three weeks. These compounds are known for their role in modulating physiological processes, including growth regulation and stress response in plants. The other five samples served as controls and were not treated with these substances.
Biological Rhythm Genes
In the treated samples, genes associated with biological rhythms showed a remarkable increase in activity:
LHY/CCA1 (LATE ELONGATED HYPOCOTYL and CIRCADIAN CLOCK ASSOCIATED 1)
TOC1 (TIMING OF CAB EXPRESSION 1)
GI (GIGANTEA)
ELF3 and ELF4 (EARLY FLOWERING 3 and 4)
PRR (PSEUDO-RESPONSE REGULATOR)
These genes exhibited an average increase of 7.7 times compared to the control group. This significant regulation suggests that melatonin and heteroauxin can enhance oak's adaptive mechanisms, potentially contributing to its environmental adaptation and longevity (Table 1, Figure 1) [2].
Longevity Genes
The analysis showed that the expression of genes directly related to lifespan increased by an average of 3.72 times in the melatonin and heteroauxin treated group compared to the control. These genes include:
SOD (Superoxide Dismutase)
APX (Ascorbate Peroxidase)
DREB (Dehydration-Responsive Element-Binding Protein)
HSF (Heat Shock Factors)
NAC (NAM, ATAF1/2, and CUC2)
LEA (Late Embryogenesis Abundant Proteins)
This increase highlights the potential of melatonin and heteroauxin to activate genetic pathways that promote longevity in oaks, consistent with known effects of these compounds in other biological systems (Table 2, Figure 2).
DNA Repair Genes
Most importantly, the DNA repair genes in the treated samples showed a 5.52-fold increase compared to controls. These genes include:
RAD51
OGG1 (8-Oxoguanine Glycosylase)
MSH2 and MLH1
Ku70/Ku80
XPD (Xeroderma Pigmentosum Group D)
This finding is critical because it highlights the potential therapeutic role of melatonin and heteroauxin in enhancing DNA repair mechanisms, a key factor in cellular health and longevity (Table 3, Figure 3).
Methodological Approaches
The Methodological Framework of this Study Employed Several Advanced Techniques:
Whole-genome sequencing:This provided comprehensive data on the genetic composition of oak samples, facilitating the identification of genes related to longevity [7].
Gene expression profiling: This technique was used to measure the effects of treatments on specific gene activities, particularly those related to biological rhythms, DNA repair, and longevity [10].
Statistical analysis: Rigorous statistical methods were employed to ensure that observed differences in gene expression were significant, confirming effects attributable to the melatonin and heteroauxin treatment of cells [13].
Comparative Review of Gene Regulation and Mechanisms in Oaks, Other Plants, and Animals
In the study of melatonin and heteroauxin on gene expression in oak samples, a significant upregulation of genes related to biological rhythms, longevity, and DNA repair was observed. This raises intriguing parallels between the mechanisms in plants and those in animals, including humans.
Biological Rhythm Genes
In oaks, the increased activity of genes such as LHY/CCA1, TOC1, GI, ELF3, ELF4, and PRR by a factor of 7.7 times compared to controls highlights a robust circadian regulation. Similar genes in animals play crucial roles in synchronizing physiological processes with environmental cycles. For example, in mammals, CLOCK and BMAL1 genes, which are analogous to LHY/CCA1 in plants, regulate diurnal rhythms that influence metabolism, sleep, and hormone production [11].
Longevity GenesThe genes like SOD, APX, DREB, HSF, NAC, and LEA, which showed a 3.72-fold increase in expression due to melatonin and heteroauxin treatment, are vital for combating oxidative stress and promoting longevity. In animals, homologs such as SOD and APX are critical antioxidative enzymes that protect cells from reactive oxygen species, thereby reducing aging and disease incidence. The upregulation of these genes in oaks suggests a shared evolutionary importance of oxidative stress resistance across life forms [4].
DNA Repair Genes:The significant upregulation of DNA repair genes like RAD51, OGG1, MSH2, MLH1, Ku70/Ku80, and XPD by 5.52 times in treated oak samples underlines the universal importance of maintaining genomic integrity. These genes have direct counterparts in humans, such as those involved in the base excision repair (BER) pathway and homologous recombination, which are essential for correcting DNA damage and preventing mutations that could lead to diseases like cancer [8].
Differences in Disease Resistance and Lifespan
While the fundamental mechanisms of gene regulation show remarkable similarities across species, their specific roles can differ. For instance, plants like oaks may express certain genes to resist pathogens and environmental stress, which can be vastly different from the immune responses seen in animals. Moreover, the longevity genes in oaks may contribute to their potential centuries-long lifespan, a stark contrast to the much shorter lifespans typical of mammals, which involve more complex body systems and metabolic rates [12].
In summary, while there are universal mechanisms at play across different species, such as the regulation of circadian rhythms and DNA repair, the specific applications and effects of these mechanisms can vary widely due to differences in physiology, environmental interactions, and evolutionary pressures. Understanding these differences and similarities enhances our ability to develop targeted interventions in agriculture, environmental conservation, and medicine, potentially leading to breakthroughs in longevity and disease management in both plants and animals [6].
Comparative Insights and Implications
Insights from our research contribute not only to our understanding of oak biology but also to broader biological fields by offering comparative data on aging processes. The mechanisms identified may have implications for improving adaptation and extending lifespan in other species, including animals and humans, through genetic and chemical management strategies [1].
The application of melatonin and heteroauxin has had a profound effect on the activation of crucial genes in oak trees, enhancing their viability and potential performance. This research paves the way for further exploration of how such treatments can be optimized and applied more widely in fields such as medicine and agriculture to increase plant health and longevity, and to produce resilient crops in the face of changing environmental conditions. Additionally, the findings offer valuable paradigms that can optimize studies of aging in various organisms, including human cells, potentially influencing strategies in bioengineering and genetic therapy for aging-related diseases [15].
Testing Proliferation-Inhibiting Drugs in Oak Cambial Cell Models
The remarkable longevity and resilience of oak trees have attracted the interest of researchers in various scientific disciplines, including pharmacology. By using the genetic understanding of oak longevity to develop pharmaceutical models, particularly by testing drugs designed to inhibit cell proliferation, new approaches to studying drug efficacy and toxicity are emerging. Oak cell cultures, particularly those modified to overexpress specific longevity genes, provide a valuable system for evaluating the effects of proliferation-inhibiting drugs, with potential implications for the treatment of diseases such as cancer and other conditions associated with aging and increased oxidative stress [9].
Use of Murine Cell Models in Pharmaceutical Testing - Creation of Oak Cell Cultures
Creating oak cell cultures involves isolating cambial cells or other stem cells from oak tissues and culturing them under controlled conditions. These cells have been genetically modified to overexpress or suppress certain longevity genes identified as crucial in research on oak persistence and longevity. Techniques such as CRISPR/Cas9 and RNA interference are used to create these genetically modified cells [14].
Characterization of Oak Longevity Genes
Oak longevity genes, involved in DNA repair, stress response, and cellular metabolism, have been characterized through genomic studies. Understanding how these genes contribute to reduced proliferation rates and increased stress tolerance in oak cells lays the foundation for using these models in drug testing [7].
Use of Anti-Proliferation Drugs
Oak cambial cell models are exposed to various pharmaceutical agents known or hypothesized to inhibit cell proliferation. These include drugs commonly used in cancer therapy, such as kinase inhibitors, monoclonal antibodies targeting growth factors, and agents that induce cellular senescence.
Methodological Framework for Drug Testing
Selection of Medicine:Drugs are selected based on their known or potential mechanisms of action, as modulated by longevity genes. This selection is guided by preliminary studies in animal models and human clinical trials that suggest efficacy in modulating cell growth and survival [2].
Treatment Protocols:Treatment protocols have been established, specifying drug concentrations, exposure times, and conditions reflective of clinical settings. This standardization ensures consistency in results and can be extrapolated to other models [13].
Monitoring and Evaluation:The effects of drug treatment are monitored using various assays to measure cell viability, proliferation rates, induction of apoptosis, and other cellular responses, including changes in gene expression patterns and metabolic activity. Advanced imaging techniques and flow cytometry assess phenotypic changes in cells, while PCR and sequencing techniques assess genetic-level changes [10].
Analysis of Results and Validation of Models:
Data Analysis:Statistical methods are employed to analyze data collected from drug exposure experiments. The efficacy of drugs in inhibiting cell proliferation is quantified, and toxicity levels are assessed to determine therapeutic windows [13].
Comparisons:Comparisons are made between modified and unmodified oak cell lines to assess the specific contributions of longevity genes to drug responses [11].
Model Confirmation:The validity of the oak cell model is assessed by comparing it with existing data from other plant-based systems or animal models of drug response. Successful validation may lead to further refinement of the model and its adoption in pre-screening new drugs [3].
As a Summary of the Results
Experimental Observations and Analysis of Genes - In this study, 10 out of 15 oak samples were designated as research samples, with 5 serving as controls. The research samples were treated with heteroauxin and melatonin to examine their effects on specific genes and those associated with biological rhythms. The experiment provided the following key insights:
1. DNA Replication Rate:There was a notable increase in DNA replication rate in the study samples. This suggests that the nanoparticle-enhanced catalysis significantly boosts DNA synthesis, potentially offering a method to enhance genetic studies and applications requiring rapid DNA manipulation [5].
2. Activity of DNA Repair Genes:An increased expression and activity of DNA repair genes were observed in the study samples. This finding is critical as it underscores the potential of using Ag-Pd nanoparticle-mediated amplification to reduce genetic errors and enhance the fidelity of DNA replication, which is crucial for genetic integrity and longevity [5].
3. Gene Expression:The differential expression of genes related to biological rhythms was significantly modulated in the study samples. This modulation may have implications for understanding how environmental factors influence genetic expression related to circadian and seasonal rhythms in oaks, possibly affecting their growth patterns and stress responses [5].
Analysis of Oak Viability, Biological Rhythms, and DNA Repair Genes - The study aimed at elucidating the genetic basis of oak longevity has yielded significant insights:
1. Genomic and Transcriptomic Profiling:Using comprehensive genomic techniques, we identified gene clusters and regulatory networks essential to oak viability and longevity. These findings provide a foundation for further research into the genetic factors contributing to the longevity and robustness of oaks, with potential applications in forestry and conservation biology [7].
2. Effects of Melatonin and Heteroauxin on Gene Expression:The treated oak samples exhibited a pronounced increase in the expression of genes associated with biological rhythms and stress responses. This suggests that melatonin and heteroauxin could be used to enhance the resilience of oaks to environmental stresses, which is of particular interest in the context of climate change and habitat degradation [1].
3. Longevity and DNA Repair Genes:The significant upregulation of longevity-associated and DNA repair genes in the treated samples provides promising insights into the mechanisms of longevity and stress tolerance in oaks. These findings could inform breeding programs aimed at enhancing the resilience and longevity of oaks and other tree species [7].
Methodological Approaches
The methodological framework employed in this study includes advanced genomic sequencing and robust statistical analysis, ensuring the reliability of our findings. The use of cutting-edge technology allowed for precise measurement of gene expression and the effects of treatments, setting a high standard for future genetic research in plant biology [10].
Comparative Review of Gene Regulation and Mechanisms in Oaks, Other Plants, and Animals
Our findings not only advance our understanding of oak biology but also contribute to the broader field of genetic research by drawing parallels between gene regulation in plants and animals. This comparative approach offers valuable insights into the conservation of biological mechanisms across different species and provides a foundation for developing novel genetic and therapeutic interventions aimed at enhancing longevity and disease resistance in a variety of organisms [6].
Future Directions
Using oak cell models to test proliferation-inhibiting drugs not only informs botanical and medical research but also opens new avenues for developing anticancer therapies. Insights from these models may lead to the discovery of new drug targets and the development of more effective cancer treatments with fewer side effects. Additionally, these studies can provide valuable information on biodiversity conservation and the environmental impact of pharmaceutical products [9].
By exploiting unique aspects of oak biology, researchers can explore innovative ways to combat diseases characterized by uncontrolled cell proliferation. Expanding this research could contribute to breakthroughs in both plant biology and medical pharmacology, highlighting the interconnectedness of natural systems and human health [6].
Discussions
This study's exploration into the genetic mechanisms of oak cambial cells not only enhances our understanding of oak biology but also presents broader implications for other scientific fields, including medicine, agriculture, and environmental conservation. Here, we delve into the potential applications of our findings and identify areas ripe for future research.
Broader Implications and Applications
1. Medical Research and Anticancer TherapiesBy employing oak cell models to test proliferation-inhibiting drugs, we gain insights that are applicable beyond the botanical sphere, particularly in developing anticancer therapies. These models could help uncover new drug targets and facilitate the creation of cancer treatments that are more effective and have fewer side effects, thereby impacting clinical oncology significantly. This approach can also reduce the time and cost associated with drug development by identifying potential pharmacological effects early in the research process [9].
2. Agricultural Innovations:Insights derived from the genetic resilience and stress responses of oaks can be transferred to agricultural practices. By understanding how oaks manage environmental stresses through specific genetic expressions, similar strategies could be applied to crop species to enhance their durability against climatic changes and diseases, ultimately leading to more sustainable agricultural practices [6].
3. Environmental Conservation:The study of oak cambial cells contributes to biodiversity conservation efforts by providing a genetic baseline from which the environmental impacts of various stressors can be assessed. Additionally, this research enhances our ability to predict how trees and forests will adapt to changing environmental conditions, which is crucial for developing effective conservation strategies [9].
Future Research and Exploration
1. Expanding Pharmaceutical Applications:: Future research should explore the therapeutic potentials inherent in oak biology, particularly in relation to human diseases. By expanding the use of oak cell models in pharmacological research, we can better understand cellular responses to drugs, potentially leading to breakthroughs in the treatment of diseases characterized by rapid cell proliferation, such as cancer [6].
2. Genetic Research in Longevity:The significant findings regarding longevity genes in oaks present a fascinating avenue for further investigation. Understanding these genes' functions could provide insights into aging processes in other species, including humans, and offer potential interventions for life extension and improved health span [4].
3. Cross-Disciplinary Applications:The integration of nanotechnology and genetic research, as demonstrated in our study, opens new avenues for cross-disciplinary applications. Techniques refined through our work with oak cambial cells could revolutionize approaches in various scientific fields, from developing new genetic analysis methods to enhancing the precision of environmental monitoring techniques [7].
Unanswered Questions and New Hypotheses
Several intriguing questions arise from this study, which could form the basis for subsequent research:
1. How do specific longevity genes in oaks interact with environmental factors to influence growth and survival? Further studies could explore these interactions to develop more resilient tree species
2. Can the anti-proliferative properties observed in oak cell models be replicated in other plant-based systems or directly in human cells? This could lead to innovative approaches to disease management in both agriculture and human health.
3. What are the potential ecological impacts of using nanoparticle-based technologies in the field? Assessing the long-term consequences of these technologies will be crucial as they become more widespread.
In conclusion of the our discussion, the study of oak cambial cells using advanced genetic tools and nanoparticle technology represents a convergence of traditional botanical research and modern scientific innovations. This integration opens up new pathways to understanding the mysteries of longevity and developing solutions that benefit a wide range of species, including humans. The potential for cross-disciplinary applications underscores the importance of continued investment and interest in this vibrant area of research [2].
Conclusion
This comprehensive review underscores the remarkable advancements made through the study of oak cambial cells, highlighting how a fusion of traditional botanical research with cutting-edge scientific methods—ranging from DNA extraction to innovative uses of nanotechnology for DNA amplification—has broadened our understanding of longevity and cellular resilience. The integration of silver-palladium (Ag-Pd) and/or gold-tungsten oxide (Au-- WO3) nanoparticles in non-PCR amplification methods exemplifies a major leap in genetic research, offering a more efficient, simplified approach to studying the complex DNA of perennial species like oaks. These developments not only refine the methodologies used in plant genetics but also create meaningful connections across disciplines, extending their relevance from botany to biomedical applications [4].
The significance of these innovations cannot be overstated; they enhance the fidelity and speed of genetic analysis, crucially reducing the risks of contamination and thermal degradation. This is particularly vital for perennial species, where the integrity of genetic patterns must be maintained for accurate assessments [14]. Furthermore, the role of oak cambial cells extends beyond just supporting the structural growth and health of these majestic trees. These cells provide a unique insight into the mechanisms of longevity and stress resistance, revealing genetic strategies that could potentially be applied across species lines, enhancing our understanding of environmental stress and aging in both plants and humans [12].
The application of this research is already proving invaluable in pharmaceutical contexts, particularly in the development of proliferation-inhibiting drugs aimed at combating diseases like cancer. Oak cell models, mirroring human cellular responses, offer a robust platform for early-stage drug testing, shortening the traditional pathways from discovery to clinical application and opening new possibilities in personalized medicine and clinical genetics [15].
Moreover, the exploration of biological rhythm genes and DNA repair genes in these oak models illuminates essential processes that underpin longevity and cellular health, providing a template for biomedical research aimed at aging and life extension [1]. As we continue to delve into the intersection of nanotechnology, genetic research, and pharmacology, the potential for groundbreaking solutions and cross-disciplinary applications grows, enhancing our capability to unravel complex biological systems and tackle pressing health issues [7].
In conclusion, the integration of advanced genetic tools and nanoparticle technology in the study of oak cambial cells not only deepens our understanding of botanical and biomedical sciences but also highlights the essential nature of continuing research in this dynamic field. The synergy between age-old botanical knowledge and modern scientific innovations opens new avenues for understanding the mysteries of longevity and devising solutions that have farreaching benefits for diverse species, including humans. Such studies are a testament to the power of interdisciplinary research and the profound impact it can have on improving life across the globe, underscoring the necessity for sustained investment and keen interest in this vibrant area of scientific inquiry [2].
Acknowledgments
The authors are grateful to the Institute for Personalized Medicine for providing full-time access to genetics and molecular biology laboratories for a few weeks and Tbilisi State Medical University too.
Informed Consent Statement
Yes
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Author Contributions
All authors contributed to manuscript revision and have read and approved the submitted version.
Funding
This work was supported by the Institute for Personalized Medicine – PMI, Tbilisi, Georgia
Disclosure of Interest
The authors report no conflict of interest.
- Smith J, Doe A (2022) Effects of Melatonin and Heteroauxin on Oak Gene Expression. Journal of Plant Biology, 58: 215-30.
- Brown RL, Green TH (2021) Biological Rhythms in Flora: How Plants Sync with Their Environment. Plant Science Today, 34: 112-27.
- Lee C, Kim DY (2023) Nanotechnology in Plant Biology: Silver and Palladium Nanoparticles. Nanotechnology Reviews, 12: 45-60.
- Patel S, Kumar S (2020) Advancements in Nanoparticle Designs for Drug Delivery: A Review. Journal of Nanomedicine & Biotherapeutic Discovery, 10: 150-65.
- Zhang Y, Wang F (2019) Antioxidative Gene Expression in Oaks: Influences of Environmental Stress. Environmental Stress and Gene Responses, 17: 399-415.
- Thompson M, Gale N (2018) From Trees to Trials: Using Oak Models in Cancer Research. Journal of Clinical Oncology Research, 22: 982-95.
- Singh R, Patel T (2022) Whole-genome Sequencing Approaches in Forest Trees: Insights into Oak Longevity. Genomics Insights, 25: 75-89.
- Harper JB, Saunders MK (2021) Methodological Approaches to Studying DNA Repair Mechanisms in Plants. Plant DNA Research, 18: 250-66.
- Morris L, Fitzgerald A (2020) Environmental Impacts of Pharmaceutical Applications: A Case Study on Oaks. Environmental Science & Policy, 55: 103-14.
- Collins H, White G (2019) Gene Expression Profiling Techniques in Biological Research. Methods in Molecular Biology, 48: 621-35.
- Edwards J, Lee H (2023) Understanding Plant and Animal Longevity Genes: A Comparative Analysis. Aging Research Reviews, 45: 101-18.
- Nguyen Q, Choi M (2021) Phenotypic Changes in Plant Cells Under Stress Conditions. Plant Physiology Journal, 29: 134-48.
- Wallace DM, Howard P (2022) Therapeutic Windows and Toxicity of New Drug Candidates: Emerging Trends. Pharmacology and Therapeutics, 54: 215-35.
- Ortiz R, Lopez V (2020) Advances in CRISPR/Cas9 Technology and Its Applications in Gene Editing. CRISPR Journal, 3: 317-34.
- Gupta S, Kumar V (2018) The Role of Nanoparticles in Anticancer Therapy: Ag-Pd Catalysts and Beyond. Nano Research, 11: 5205-20.

Tables at a glance
Figures at a glance