Efficiency of High Flux Dialyzers in Chronic Kidney Disease Management
Received Date: May 17, 2024 Accepted Date: June 17, 2024 Published Date: June 20, 2024
doi: 10.17303/jber.2024.8.103
Citation: Awafung Emmanuel Adie, Awafung veronica iye, Mbu Patrick, Ukam Edodi, Otokpa Daniel (2024) Efficiency of High Flux Dialyzers in Chronic Kidney Disease Management. J Biomed Eng Res 8: 1-9
Abstract
Background: Chronic Kidney Disease (CKD) is a global health concern affecting millions of individuals worldwide. Hemodialysis, one of the primary therapy modalities for end-stage renal disease (ESRD), relies on dialyzers to remove waste products and excess fluid from the bloodstream. Over the years, advancements in dialysis technology have led to the development of high flux dialyzers, which offer superior solute clearance compared to conventional dialyzers. This paper aims to provide a comprehensive review on the studies done on efficiency of high flux dialyzers in CKD management, including their impact on solute clearance, clinical outcomes, and cost-effectiveness. The method is to have a comprehensive study using the previous and recent research, dissertation, reviews and articles from nephrology organizations globally, Scopus publications and global search mediums to have a reach and robust information on the use of the high flux dialyzers and the advantages in care of CKD patients.
In conclusion the use of high flux dialyzers in dialysis patients provides multiple advantages, including enhanced clearance of toxins, improved fluid management, reduced inflammation, better biocompatibility, improved anemia control, and potential long-term benefits in mitigating dialysis-related amyloidosis [1 ] These advantages contribute to a higher quality of life, longevity and improved clinical outcomes for patients undergoing dialysis.
Keywords: Chronic Kidney Disease; High Flux Dialyzer; Management; Efficiency
Nomenclature
CKD: Chronic Kidney Disease; QB: The blood flow rate in the blood compartment [mL/min]; QD: The dialysate flow rate in the dialysate compartment [mL/min]; B: Blood; C: Component; D: Dialysate
Introduction
Chronic kidney disease (CKD) is a condition in which the kidneys are damaged and cannot filter blood as well as they should. Because of this, excess fluid and waste from blood remain in the body and may cause other health problems, such as heart disease and stroke [2].
To remedy this failure of the kidney to do its main function, Hemodialysis is the most commonly used type of therapy in Africa and most parts of the world [1] in this method, blood is transported out of the body through tubes and cleaned in a machine using dialysis fluid. The dialysis is typically carried out three times per week. Each session lasts about four to five hours.
According to Centre for disease control and prevention on chronic kidney disease initiate, your kidneys are each just the size of a computer mouse, it filters all the blood in your body every 30 minutes. They work hard to remove wastes, toxins, and excess fluid. They also help control blood pressure, stimulate production of red blood cells, keep your bones healthy, and regulate blood chemicals that are essential to life [1]. According to Epidemiology of chronic kidney disease update review by Toshifumi Nakamura and Jonatan Barrera-Chimal (2022) for the ISN Chronic kidney disease is a progressive condition that affects >10% of the general population worldwide, amounting to >800 million individuals. Chronic kidney disease is more prevalent in older individuals, women, racial minorities, and in people experiencing diabetes mellitus and hypertension. Chronic kidney disease represents an especially large burden in low- and middle-income countries [1] which are least equipped to deal with its consequences. Chronic kidney disease has emerged as one of the leading causes of mortality worldwide, and it is one of a small number of non-communicable diseases that have shown an increase in associated deaths over the past 2 decades. The high number of affected individuals and the significant adverse impact of chronic kidney disease should prompt enhanced efforts for better prevention and treatment.
Role of Dialyzers in Hemodialysis Treatment
Hemodialysis is a procedure where a dialysis machine and a special filter called an artificial kidney, or a dialyzer, are used to get the patient’s blood in to clean the blood of the patient to the dialyzer; the doctor needs to make an access, or entrance, into the blood vessels. This is known as cannulation. Dialysis therapy cannot be done without the part called the DIALYZER, Dialyzer is made up thin fibrous materials, this fiber forms a semipermeable membrane, which allows smaller particles and liquids to pass through. The dialyzer is encased in a sealed plastic cylinder about a foot long and approximately two to three inches in diameter with two openings at the top and two openings at the bottom, these openings allow effective ultrafiltration to take place during hemodialysis therapy as they allow easy possible and effective flow of blood and dialysate into the dialyzer and out of the dialyzer as well as the waste from the dialyzer.
Types of Dialyzers
Dialyzers are divided into tube type, flat type, and hollow fiber type at present, the commonly used dialyzers are hollow fiber type, and the flat-type and tube-type dialyzers used in the early days have been basically eliminated. [1] Dialyzers are described according to the flux, the mass transfer coefficient, hydraulic permeability, and bio-incompatibility.
Dialyzer Flux
According to Davenport A. work on the role of dialyzer membrane flux in bio-incompatibility. Hemodial Int. 2008; Dialyzer flux is defined as β2 macroglobulin clearance with Low flux- < 10 mL/min, mid flux- 10-20 mL/min, High flux >20 mL/min respectively. Rather than hydraulic permeability following reports of improved outcomes from middle molecular weight uremic toxin removal. [1] Low flux, mid flux, and high flux are currently defined as β2 macroglobulin clearance of < 10, 10-20, and >20 mL/min respectively [1] Dialyzer flux is based on molecular weight cut off, molecular weight retention onset, biocompatibility, mass transfer-area coefficient and hydraulic permeability Dialyzer flux were referred to ultrafiltration coefficient.
Dialyzer Design
In the design of a dialyzer certain areas and concentration must be considered which include the improvement of diffusive clearance which deals with the mechanical space, to improve convective clearance which deals with capillary diameter and membrane materials, to improve absorption which also take care of the membrane materials and to prevent clothing which handles the reduction of protein deposit in hemocompatebilty using polymeric biomaterials [1].
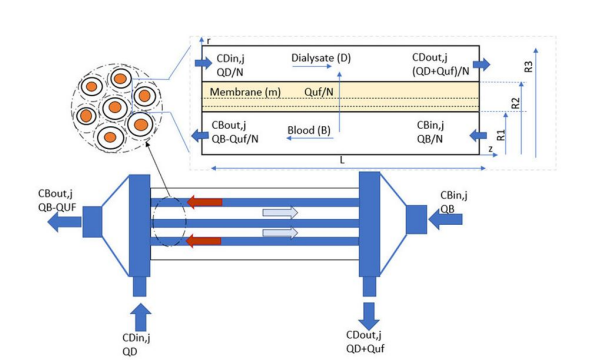
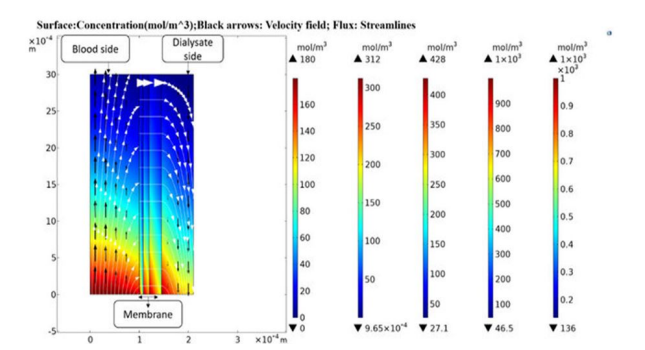
Tuba Yaqoob, Mohammad Ashan and Arshad Hussan work on Computational Fluid Dynamics (CFD) Modeling and Simulation of Flow Regulatory Mechanism in Artificial Kidney Using Finite Element Method (2020) came up with is diagram and analysis.
In this model, a two-dimensional transport of mass and momentum across a three-layer isotropicsemi-permeable membrane with a skin, middle and bulk layer is considered. The velocity profiles onboth the blood and dialysate side are portrayed with the Navier-Stokes equations in Ouseph, R. Hutchison, C.A.; Ward, R.A. Differences in solute removal by two high-flux membranes ofnominally similar synthetic polymers. Nephrol. Dial. Transplant. (2008) [3]Steady-state,isothermal conditions (T = 37 °C) and laminar flow prevail on both blood and dialysate side with highdilution of solutes a review by Bird, R.B. Transport phenomena (2002), and a work by Donato, D. Boschetti-de-Fierro, A.; Zweigart, C.; Kolb, M.(2017) [2]. It is assumed that the viscosity of both blood and dialysate does not changewith applied share. Therefore, these fluids are considered incompressible and Newtonian fluids.
Dr. KfH Nierenzentrum Mu¨nchen-Laim 2011 stated that High-flux hemodialysis utilizes dialyzer membranes with significant porosity to larger molecules (ß2- microglobulin clearance >20 ml/minute) and an ensuing increase in the ultrafiltration coefficient (KUF > 15 ml⁄mmHg per hour) Eknoyan G, Beck GJ 2010 stated. [3] The percentage of end-stage renal disease (ESRD) patients receiving high-flux hemodialysis has increased from 46% in the year 2000 to about two-thirds of patients worldwide in 2009 [4]. This increase was largely driven by the desire to reduce the high morbidity and mortality associated with conventional low-flux dialysis (predominantly cellulosic membranes), although the clinical benefits and risks of high-flux hemodialysis are not well defined. Dr. KfH Nierenzentrum Mu¨nchen-Laim (2011) also concluded that the EDTA-ERA and a large number of national societies of Nephrology in Europe and Japan recommend the exclusive use of ultrapure dialysis fluid for all ESRD patients receiving high-flux hemodialysis. High-flux dialyzers extend not only the range of diffusible molecules but also the potential for convective mass transfer into the patient owing to back filtration of dialysate.
The Potential disadvantages of high-flux dialyzers include loss of albumin into the dialysate when bleach is used for reprocessing [] and back-transfer of dialysate contaminants into the blood [], although some high-flux membranes also adsorb and thus inhibit the back-transfer of endotoxins [3]This unique characteristic makes it very effective in toxin elimination, high volume accumulation and highly efficient in ultrafiltration clearance. Dr. john Agor in his article on high flux or low flux dialyzer (2013) referred High flux dialyzers as 'leakier' dialyzers and that holds true for bi-directional membrane transit. This means that not only can more and larger solutes be removed from the patient—but at least potentially, more water-borne contaminants, e.g. endotoxin, can get into blood through the dialyzer. High flux dialysis is more capable of removing certain inflammatory substances, which may be beneficial for individuals with chronic inflammation related to kidney failure. The choice between high-flux and low-flux dialysis depends on individual patient needs, their medical condition.
Navari et al. study on 60 hemodialysis patients compared two types of hemodialysis buffer (bicarbonate versus acetate) and discovered that spirometry characteristics after dialysis with bicarbonate were higher than acetate in male hemodialysis patients independent of intradialytic weight reduction [4]. Kovacević et al. in a study on 21 hemodialysis patients reported that only forced expiratory flow (FEF50) decreased after five to six years of follow up; however, spirometry findings were similar pri and post dialysis [5]. Similarly, Herrero et al. in 5 years follow up of 43 patients on hemodialysis with bioincompatible membrane showed a significant down slide in pulmonary diffusing capacity possibly due to chronic pulmonary fibrosis [3].
In another study by Alves et al., 61 dialysis patients were evaluated and spirometry was done pre and post dialysis. Improvement of FEV1 and FVC after dialysis was correlated with weight loss of patients [8]. They also concluded that decreased volume overload after dialysis is an important factor in improvement of PFT findings. Conversely, Langs et al. did not find any significant correlation between lung function parameters and intra-dialytic weight loss with cellulose or high flux membrane in 14 hemodialysis patients study done [3].
High-flux membranes seen have high hydraulic permeability and higher solute permeability for middle-- sized solutes than low-flux membrane dialyzers. In 2005, to address the problem of albumin leakage, super high-flux membranes with a large pore size were developed in Japan [3]. In 2008, more than 90% of Japanese patients on hemodialysis were being treated with this type of dialyzer [4]. In africa and most part of the world today, high flux dialyzers have been seen to give more efficiency, furthermore a more recent study done by Masanori Abe, Ikuto Masakane, and co. (2022) dialyzer type, classified by β2MG clearance, was significantly associated with 3-year mortality in this large national cohort study of Japanese dialysis patients. Based on our findings, super high-flux dialyzers might be beneficial for hemodialysis patients.
Conclusion
The use of high flux dialyzers in dialysis patients offers several significant advantages. Firstly, high flux dialyzers have enhanced clearance capabilities, allowing for efficient removal of larger molecular weight toxins and middle molecules. This leads to improved solute removal, resulting in better overall clearance of waste products and toxins from the bloodstream.
Secondly, high flux dialysis membranes have a higher permeability to water, which promotes ultrafiltration and aids in the removal of excess fluid in patients with fluid overload. By effectively managing fluid balance, high flux dialysis can help prevent complications associated with volume overload such as hypertension, heart failure, and pulmonary edema.
Moreover, high flux dialyzers have been shown to reduce the levels of pro-inflammatory cytokines and other uremic toxins. This anti-inflammatory effect can contribute to the overall well-being of dialysis patients, as chronic inflammation is a common finding in end-stage renal disease and is associated with various complications, including car diovascular disease.
Additionally, high flux dialysis membranes offer improved biocompatibility compared to low flux membranes. This reduces the activation of the complement system and platelets, minimizing the risk of clotting, vascular access dysfunction, and subsequent infections. The enhanced biocompatibility also leads to a reduction in the need for systemic anticoagulation during dialysis sessions.
Furthermore, the use of high flux dialyzers has been associated with better control of anemia in dialysis patients. The improved clearance of middle molecules, including inflammatory mediators, may contribute to the preservation of erythropoietin-producing cells in the kidney and consequently improve the response to erythropoietin-stimulating agents.
Lastly, high flux dialysis allows for more efficient removal of certain uremic toxins, such as beta-2 macroglobulin, which is associated with dialysis-related amyloidosis, a condition characterized by the deposition of amyloid fibrils in various tissues. By effectively reducing the levels of beta-2 macroglobulin and other uremic toxins, high flux dialysis can potentially slow the progression of dialysis-related amyloidosis and its associated complications.
In summary, the use of high flux dialyzers in dialysis patients provides multiple advantages, including enhanced clearance of toxins, improved fluid management, reduced inflammation, better biocompatibility, improved anemia control, and potential long-term benefits in mitigating dialysis-related amyloidosis. These advantages contribute to a higher quality of life and improved clinical outcomes for patients undergoing dialysis.
- Hall YN, Larive B, Painter P, et al. (2012) Effects of six versus three times per week hemodialysis on physical performance, health, and functioning: Frequent Hemodialysis Network (FHN) randomized trials. Clinical Journal of the American Society of Nephrology, 7: 782-94
- Chen TK, Knicely DH, Grams ME (2019) Chronic Kidney Disease Diagnosis and Management: A Review. JAMA. 322: 1294-304.
- Song J, Chen Y, Chen Y, Qiu M, Xiang W, Ke B, Fang X (2024) Wnt/β-catenin Pathway Aggravates Renal Fibrosis by Activating PUM2 Transcription to Repress YME1L-mediated Mitochondrial Homeostasis. Biochem Genet.
- Eknoyan G, Beck GJ, Cheung AK, et al. (2002) Effect of dialysis dose and membrane flux in maintenance hemodialysis. New Engl J Med. 347: 2010-19.
- Leypoldt JK, Cheung AK, Carroll CE, et al. (1999) Effect of dialysis membranes and middle molecule removal on chronic hemodialysis patient survival. Am J Kidney Dis. 33: 349-55.
- Stanifer JW, Maro V, Egger J, Karia F, Thielman N, et al. (2015) The epidemiology of chronic kidney disease in Northern Tanzania: a populationbased survey. PloS one.10: e0124506.
- Carrero, Juan Jesus, et al. (2023) "Defining measures of kidney function in observational studies using routine health care data: methodological and reporting considerations." Kidney international, 103: 53-69.
- Davenport A (2008) The role of dialyzer membrane flux in bio-incompatibility. Hemodial Int. 12: S29-33.
- Depner TA, Greene T, Daugirdas JT, Cheung AK, Gotch FA, Leypoldt JK (2004) HEMO Study Group: Dialyzer performance in the HEMO Study: In vivo KoA and true blood flow determined from a model of cross-dialyzer urea extraction. ASAIO J.
- MacLeod AM, Campbell MK, Cody JD, et al. (2005) Cellulose, modified cellulose and synthetic membranes in the haemodialysis of patients with end-stage renal disease. Cochrane Database Syst Rev. CD003234.
- Hornberger JC, Chernew M, Petersen J, Garber AM (1992) A multivariate analysis of mortality and hospital admissions with high-flux dialysis. J Am Soc Nephrol, 3: 1227-37
- United States Renal Data System: USRDS 2002 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health.
- Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, et al. (2002) HEMO Study Group: Effect of dialysis dose and membrane flux on mortality and morbidity in maintenance hemodialysis patients: Primary results of the HEMO study. N Engl J Med. 347: 2010-19.
- Hutchison CA, Heyne N, Airia P, et al. (2012) Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol Dial Transplant. 27: 3823-8.
- Premru V, Kovač J, Buturović-Ponikvar J, Ponikvar R (2013) Some kinetic considerations in high cutoff Hemodiafiltration for acute Myoglobinuric renal failure. Ther Apher Dial. 17: 396-401.
- Kneis C, Beck W, Boenisch O, et al. (2013) Elimination of middle-sized uremic solutes with high-flux and high-- cut-off membranes: A randomized in vivo study. Blood Purif. 36: 287-94.
- Navari K, Farshidi H, Pour-Reza-Gholi F, Nafar M, Zand S, Sohrab Pour H, et al. (2008) Spirometry parameters in patients undergoing hemodialysis with bicarbonate and acetate dialysates. Iran J Kidney Dis. 2: 149-53.
- Kovacević P, Stanetic M, Rajkovaca Z, Meyer FJ, Vukoja M (2011) Changes in spirometry over time in uremic patients receiving long-term hemodialysis therapy. Pneumologia. 60: 36-9
- Herrero JA, Alvarez-Sala JL, Coronel F, Moratilla C, Gámez C, Sánchez-Alarcos JM, et al. (2002) Pulmonary diffusing capacity in chronic dialysis patients. Respir Med. 96: 487-92.
- Lang SM, Becker A, Fischer R, Huber RM, Schiffl H (2006) Acute effects of hemodialysis on lung function in patients with end-stage renal disease. Wien Klin Wochenschr, 118: 108-13.
- Alves J, Hespanhol V, Fernandes J, Marques EJ (1989) Spirometric alterations caused by hemodialysis. Their relation to changes in the parameters commonly used to measure hemodialysis efficiency. Acta Med Port. 2: 195-8.
- Tsuchida K, Minakuchi J (2011) Albumin loss under the use of the high-performance membram Contrib Nephrol, 173: 76-82.
- Abe M, Hamano Wada A et al. (2017) Effect of dialyzer membrane materials on survival in chronic hemodialysis patients: results from the annual survey of the Japanese Nationwide Dialysis Registry PLoS One, 12: e018442.
- Hutchison CA, Heyne N, Airia P, et al. (2012) Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol Dial Transplant, 27: 3823-8.
- Premru V, Kovač J, Buturović-Ponikvar J, Ponikvar R (2013) Some kinetic considerations in high cut-off Hemodiafiltration for acute Myoglobinuric renal failure. Ther Apher Dial. 17: 396-401.
- Baillie J, Lankshear A (2015) Patient and family perspectives on peritoneal dialysis at home: findings from an ethnographic study. J Clin Nurs. 24: 222-34.
- Casey JR, Hanson CS, Winkelmayer WC, Craig JC, Palmer S, Strippoli GF et al. (2014) Patients' perspectives on hemodialysis vascular access: a systematic review of qualitative studies. Am J Kidney Dis. 64: 937-53.
- Foote C, Kotwal S, Gallagher M, Cass A, Brown M, Jardine M (2016) Survival outcomes of supportive care versus dialysis therapies for elderly patients with end-stage kidney disease: A systematic review and meta-analysis. Nephrology (Carlton) 21: 241-53.
- Footman K, Mitrio S, Zanon D, Glonti K, Risso-Gill I, McKee M et al. (2015) Dialysis services for tourists to the Veneto Region: a qualitative study. J Ren Care, 41: 19-27.
- Geberth S, Nowack R (2014) Praxis der Dialyse. Berlin: Springer.
- Harwood L, Clark AM (2013) Understanding pre-- dialysis modality decision-making: A meta-synthesis of qualitative studies. Int J Nurs Stud, 50: 109-20.
- Medical Netcare (MNC) (2017) im Auftrag des Gemeinsamen Bundesausschusses (G-BA). Jahresbericht 2016 zur Qualität in der Dialyse.
- Nistor I, Palmer SC, Craig JC, Saglimbene V, Vecchio M, Covic A et al. (2015) Haemodiafiltration haemofiltration and haemodialysis for end-stage kidney disease. Cochrane Database Syst Rev. 5: CD006258.
- Palmer SC, Palmer AR, Craig JC, Johnson DW, Stroumza P, Frantzen L et al. (2014) Home versus in-centre haemodialysis for end-stage kidney disease. Cochrane Database Syst Rev. 11: CD009535.
- Reid C, Seymour J, Jones C (2016) A Thematic Synthesis of the Experiences of Adults Living with Hemodialysis. Clin J Am Soc Nephrol. 11: 1206-18.
- Smart NA, Dieberg G, Ladhani M, Titus T (2014) Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 6: CD007333.
- Vale L, Cody JD, Wallace SA, Daly C, Campbell MK, Grant AM et al. (2004) Continuous ambulatory peritoneal dialysis (CAPD) versus hospital or home haemodialysis for end-stage renal disease in adults. Cochrane Database Syst Rev. 4: CD003963.
- Wang AY, Ninomiya T, Al-Kahwa A, Perkovic V, Gallagher MP, Hawley C et al. (2014) Effect of hemodiafiltration or hemofiltration compared with hemodialysis on mortality and cardiovascular disease in chronic kidney failure: a systematic review and meta-analysis of randomized trials. Am J Kidney Dis. 63: 968-78.
- Wongrakpanich S, Susantitaphong P, Isaranuwatchai S, Chenbhanich J, Eiam-Ong S, Jaber BL (2017) Dialysis Therapy and Conservative Management of Advanced Chronic Kidney Disease in the Elderly: A Systematic Review.
- Chu CD, McCulloch CE, Banerjee T, Pavkov ME, Burrows NR, et al. (2020) Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. CKD awareness among US adults by future risk of kidney failure. Am J Kidney Dis. 76: 174-83.
- Dharmarajan SH, Bragg-Gresham JL, Morgenstern H, et al. (2017) Centers for Disease Control and Prevention CKD Surveillance System. State-level awareness of chronic kidney disease in the U.S. Am J Prev Med. 53: 300-7.
- Vanholder R, De Smet R, Glorieux G, et al. (2003) Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 63: 1934-43.
- Vanholder R, Van Laecke S, Glorieux G (2008) What is new in uremic toxicity? Pediatr Nephrol. 23: 1211-21.
- Kirsch AH, Lyko R, Nilsson LG, et al. (2017) Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant. 32: 165-72.
- Zickler D, Schindler R, Willy K, et al. (2017) Medium cut-off (MCO) membranes reduce inflammation in chronic dialysis patients—A randomized controlled clinical trial. PloS One. 12: e0169024.
- Leypoldt JK, Cheung AK, Carroll CE, et al. (1999) Effect of dialysis membranes and middle molecule removal on chronic hemodialysis patient survival. Am J Kidney Dis. 33: 349-55.
- Davenport A (2008) The role of dialyzer membrane flux in bio-incompatibility. Hemodial Int. 12: S29-333.
- Eknoyan G, Beck GJ, Cheung AK, et al. (2002) Effect of dialysis dose and membrane flux in maintenance hemodialysis. New Engl J Med. 347: 2010-9.
- Woods HF, Nandakumar M (2000) Improved outcome for haemodialysis patients treated with high-flux membranes. Nephrol Dial Transplant. 15: 36-42.
- Locatelli F, Martin-Malo A, Hannedouche T, et al. (2009) Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol, 20: 645-54.
- Naka T, Haase M, Bellomo R (2010) 'Super high- -flux'or'high cut-off'hemofiltration and hemodialysis. Contrib Nephrol. 166: 181-9.
- Buus NH, Rantanen JM, Krag SP, Andersen NF, Jensen JD (2015) Hemodialysis using high cut off filters in light chain cast nephropathy. Blood Purif. 40: 223-31.
- Hutchison CA, Heyne N, Airia P, et al. (2012) Immunoglobulin free light chain levels and recovery from myeloma kidney on treatment with chemotherapy and high cut-off haemodialysis. Nephrol Dial Transplant. 27: 3823-8.
- Premru V, Kovač J, Buturović-Ponikvar J, Ponikvar R (2013) Some kinetic considerations in high cut-off Hemodiafiltration for acute Myoglobinuric renal failure. Ther Apher Dial. 17: 396-401.
- Kneis C, Beck W, Boenisch O, et al. (2013) Elimination of middle-sized uremic solutes with high-flux and high-- cut-off membranes: A randomized in vivo study. Blood Purif. 36: 287-94.
- Girndt M, Fiedler R, Martus P, et al. (2015) High cut-off dialysis in chronic haemodialysis patients. Eur J Clin Invest. 45: 1333-40.
- Klinkmann H, Wolf H, Schmitt E (1984) Definition of biocompatibility. Contrib Nephrol. 37: 70-7.

Figures at a glance