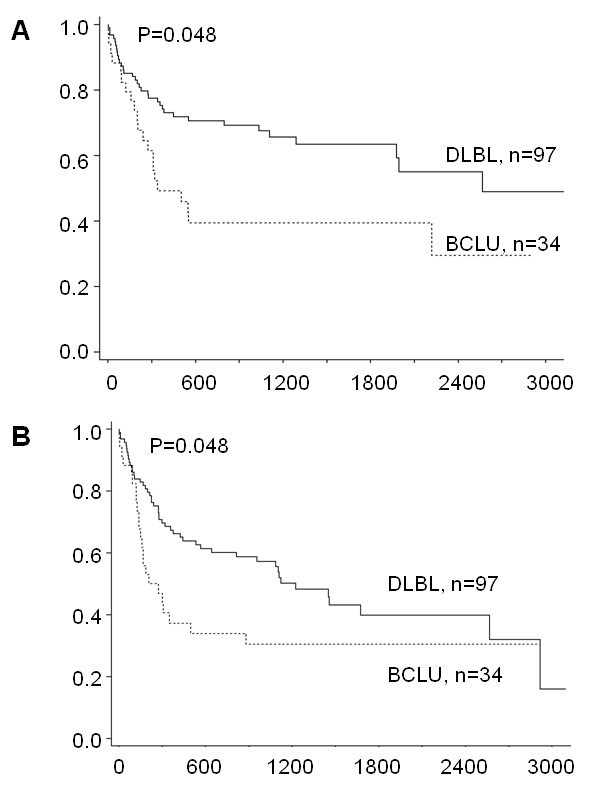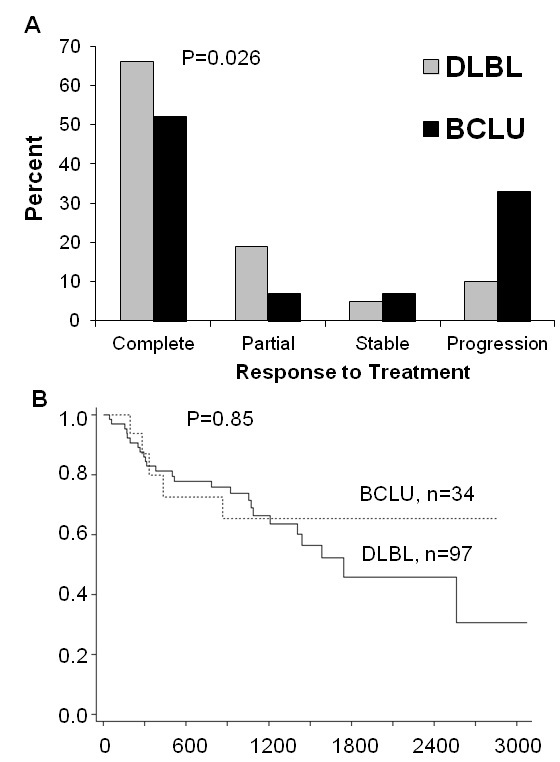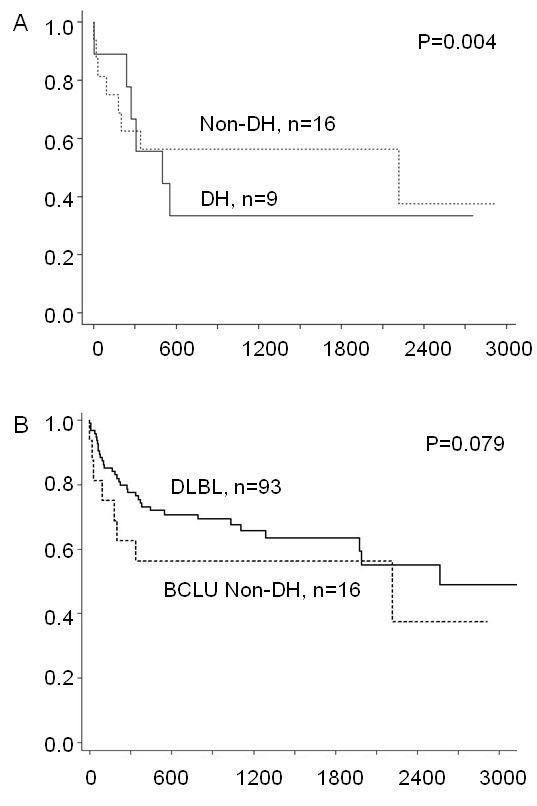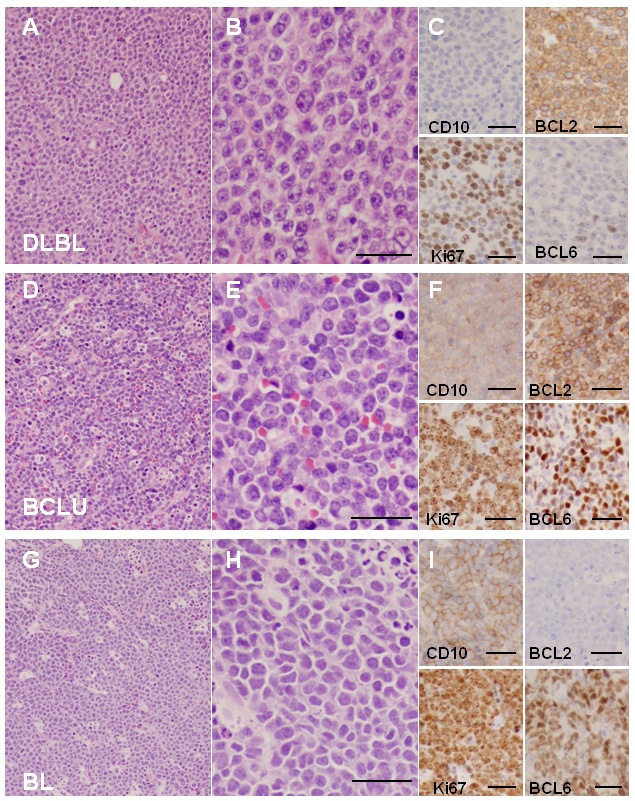
Figure 1 Morphologic and immunohistochemical features characteristic of DLBL, BCLU and BL. A-C: Low power (200x, A) and high power (400x, B) views of DLBL stained with H&E shows characteristic nuclear pleomorphism, prominent nucleoli and abundant cytoplasm. This example of DLBL shows strong immunohistochemical staining for BCL2 (Dakocytomaton) and weak staining for CD10 (Novacastra) and BCL6 (Biocare Medical) and has a Ki67 (Dakocytomaton) proliferation index of approximately 50-60% (C). D-F: Low (D) and high power (E) views of BCLU stained with H&E show intermediate sized cells with abundant tingible-body macrophages giving a low power "starry sky" appearance (D). The cells show some nuclear pleomorphism with only a portion of cells containing prominent nucleoli (E). This case of BCLU shows staining for BCL2, BCL6 and CD10, and a Ki67 proliferation index of approximately 80-90% (F). G-I: Low (G) and high power (H) view of BL stained with H&E shows the classic "starry sky" appearance of BL composed of intermediate sized cells with monotonous nuclei containing finely clumped chromatin and no prominent nucleoli. There is abundant apoptosis and necrosis (H). This case of BL shows strong staining for BCL6 and CD10, negative staining for BCL2 and has a Ki67 proliferation index approaching 100% (I). Low power views were taken at 200x magnification and high power views were taken at 400x magnification. Images were taken using an Olympus BX41 microscope with Olympus 20x/0.40 and 40x/0.65 lenses with an Olympus DP70 digital camera and recorded with scale bars embedded into representative images using Olympus DP Controller Version 3.1.1.267 imaging software. Images cropped from their original size have accompanying scale bars. All scale bars represent 50 µm. DLBL, Diffuse large B-cell lymphoma; BCLU, Bcell lymphoma unclassifiable with features intermediate between DLBL and BL; BL, Burkitt lymphoma; H&E, Hemetoxolin and Eosin.