Removal of Phenolic Compound from Pharmaceutical Waste Water by the Application of Carbonized Rice Husk as an Efficient Adsorbent
Received Date: October 17, 2024 Accepted Date: November 17, 2024 Published Date: November 20, 2024
doi: 10.17303/jcec.2024.3.103
Citation: Igbonekwu LI, Uzoh CF, Abonyi MN, Ezeike CC, Ezechukwu CM et al. (2024) Removal of Phenolic Compound from Pharmaceutical Waste Water by the Application of Carbonized Rice Husk as an Efficient Adsorbent. J Chem Eng Catal 3: 1-17
Abstract
The current study focuses on using carbonized rice husk, an agricultural waste, as an efficient adsorbent to remove phenolic chemicals from pharmaceutical wastewater using batch adsorption method. The effect of time, dosage, temperature, concentration and the pH of the effluent and how they affect the adsorption rate was studied. Adsorption kinetic, thermodynamics and isotherm studies were performed for better understanding of the behaviour and sorption potentials of the carbonized rice husk. The applicability of the kinetic model was best described by the pseudo-second-order kinetic model. Adsorption of phenol on adsorbents was found to increase by decreasing phenol effluent concentration, increasing pH up to 5, and increasing effluent temperature up to 35oC. The adsorption behavior of the Phenol was studied using Freundlich, Temkin, and Langmuir adsorption isotherm models. From the study, the Langmuir isotherm with the correlation coefficient value of 0.987 was the highest amongst the entire isotherm considered which showed that the adsorption of phenol onto RH was better described by the Langmuir model. The present findings suggest that Rice Husk (RH) is environmentally friendly, efficient, and low-cost biosorbent that is useful for the removal of Phenol from wastewater.
Keywords: Rice Husk; Adsorption; Phenol; Kinetics Models; Isotherms Models
Introduction
Industries frequently release their waste into the environment without properly treating it, which poses serious environmental risks in many developing nations. According to [1], these untreated industrial pollutants have a negative effect on water quality, disrupt ecosystems, and have complex impacts on flowing water systems. Pharmaceutical waste disposal seems to be mostly unregulated; some waste is thrown directly into sewers or lagoons without any treatment, eventually infiltrating into surface and ground waters [1]. Organic pollutant-filled wastewater has a high suspended solids content, which prevents photosynthetic organisms from getting light. The riverbed is altered when these materials settle, rendering it an undesirable home for a variety of invertebrates [2].
According to Polat et al. (2006), phenol is a major contaminant present in wastewater from a variety of industries, including pulp and paper, gas and coke production, tanning, textiles, plastics, rubber, medicines, metals, and petroleum refineries. Serious environmental and health problems have been brought about by the uncontrolled release of phenol into the environment [3]. The United States Environmental Protection Agency (USEPA) states that less than 1.0 µg/l of phenol is permitted in surface water [3]. Due to its irritating, poisonous, and carcinogenic qualities, phenol is dangerous even at low doses. It can impair the eyes, skin, respiratory, and gastrointestinal systems and, in certain situations, cause gene alterations (Damjanovi et al., 2010). According to USEPA, phenol is the 11th of the 126 priority pollutants (Caturla et al., 1988). Thus, it is critical to treat phenol in industrial wastewater, especially from pharmaceutical sources, before discharging it into water bodies. A variety of techniques, such as coagulation, filtration combined with coagulation, precipitation, ozonation, ion exchange, reverse osmosis, and advanced oxidation processes, have been developed to remove phenolic chemicals from pharmaceutical effluents (Girish et al., 2012). These techniques, nevertheless, can be costly and intricate. Adsorption with activated carbon is the most popular and successful treatment solution for phenolic wastewater among those that are available. Adsorption is the process by which atoms, ions, or molecules from a gas, liquid, or dissolved solid accumulate on the surface of a material, forming a layer known as the adsorbate. This phenomenon is purely surface-based, meaning that the adsorbate adheres only to the surface of the adsorbent and does not infiltrate the material's interior. Unlike absorption, where the substance is taken up into the bulk of the material, adsorption involves the attraction of particles to the surface without any significant penetration into the bulk structure [4]. Similar to surface tension, adsorption arises from surface energy. In a bulk material, the atoms are surrounded by other atoms, satisfying all their bonding requirements—whether ionic, covalent, or metallic. However, surface atoms of the adsorbent are not fully surrounded, leaving them available to interact with adsorbates. The type of bonding that occurs during adsorption depends on the specific nature of the interacting species, but it is generally classified into two types: physisorption, which involves weak van der Waals forces, and chemisorption, which involves stronger covalent bonding. Adsorption can also result from electrostatic attraction. The nature of the adsorption process can influence the structure of the adsorbed species [5]. For instance, polymer physisorption from solution can cause the polymer to adopt flattened or compressed structures on the surface [6]. Any carbon-rich substance can be used to make activated carbon, and the final product's characteristics will depend on the initial material used. Numerous substances, including zeolites, silica gel, and activated alumina, have been investigated as possible adsorbents for the removal of phenol in wastewater treatment. But these materials are less commercially viable because they are expensive and non-renewable [7]. As a result of this, there has become an increase in research on the production of activated carbon for wastewater treatment applications using locally accessible agricultural products (Girish et al., 2013). Mohd Din et al. (2009) assert that biomass derived from these agricultural resources is widely available, inexpensive, and renewable. Banana peel [8], palm seed coat [9], oil palm empty fruit bunch [10], black stone cherries [11], vetiver roots [12], sugarcane bagasse (Karunarathne et al., 2013), and Luffa cylindrical [13] are just a few of the agricultural materials that have been tested as adsorbents for removing phenolic compounds from wastewater. These materials provide an agricultural waste management option and an alternative to conventional sources. (Mohd Din et al., 2009).
Rice husk (RH) is one of the primary agricultural wastes produced in rice-cultivating countries. Despite its numerous applications, its economic benefits and contributions have not been sufficiently highlighted [14,15]. Global production of RH is very significant and falls in the range of tens of millions of tonnes per annum [16]. Typically, about 50% of the husk produced in a rice mill is burnt onsite to produce steam for driving mechanical milling machineries [17]. However, the combustion of these solid wastes emits suspended solid which often exceed the permissible limits stipulated by United State environmental protection agency (USEPA). Apart from its partly uses in silica-based industries, building materials or as fertilizer, much has not been explored on its application as a potential adsorbent for phenol removal.
In this study, rice husk was subjected to chemical and thermal processes to generate an adsorbent. We looked at these modified materials' ability to extract phenol from pharmaceutical effluent. Batch experiments were used to investigate the impacts of these factors, including pH, adsorbent dosage, initial phenol concentration and adsorption temperature. Optimal experimental conditions were identified, and thermodynamic studies were conducted to understand the nature of the adsorption process. Three potential mechanisms have been identified in the literature for phenol adsorption: electron donor-acceptor complex formation, π-π dispersion interaction, and hydrogen bonding (Moreno-Castilla, 2004). Different kinetic models (pseudo-first-order, pseudo-second-order, and Elovich) and adsorption isotherms (Langmuir, Temkin, and Freundlich) were assessed to discover which models best explained the experimental results to comprehend these processes and the interactions between the adsorbent and the adsorbate.
Materials and Method
The effluent samples used for this study were collected from the wastewater unit of the pharmaceutical industry in Awka, Anambra State Nigeria. The pH value was determined on site and the wastewater was preserved in a refrigerator maintained at 40C, at this temperature, bacteria are inactive and biodegradation is inhibited [18]. The othor phosphoric acid and sodium hydroxide used in this research work was of analytical grade and was purchased at Head bridge Onitsha, Anambra, Nigeria.
Adsorbent
The rice husk (RH) utilized in this study was collected as waste from Achalla in the Anambra State, Nigeria, local government area. It was then sieved using a mechanical shaker to get rid of extraneous items and dirt. After being oven-dried for an hour at 105°C in order to eliminate excess remaining moisture, it was packed away in an airtight container for subsequent use, this process was in agreement with [13] in their research work titled “Characterization of adsorbent developed from rice husk: Effect of surface functional group on phenol adsorption”. The process of washing and drying of the rice husk was carried out at Chemical Engineering Laboratory Nnamdi Azikiwe University Awka Nigeria.
Thermal Activation of RH
The dried rice husk measuring 200g was carbonized at a temperature of 4000C in a muffle furnace. This carbonization process was in agreement with [19] in their research work titled “Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements”. The thermal activation process was done in order to open pore spaces for effective adsorption process. And it was carried out at Scientific Equipment Development Institute (SEDI) Enugu, Nigeria.
Chemical Activation of RH
According to [34], 200g of carbonized rice husk (RH) was combined in a 1:1 volumetric ratio with 3 molar concentrations of othor-phosphoric acid (3M H3PO4) to chemically activate the adsorbent. It was thoroughly combined with the chemical overnight, and when the slurry was produced, sufficient distilled water was used to wash it until the sample's pH was 7. After being dried in an oven for six hours at 105°C to eliminate extra moisture, it was stored pending additional research. The processes involved in the Chemical Activation of RH were carried out at the Chemical Engineering Laboratory of Nnamdi Azikiwe University Awka.
Batch Adsorption Studies Chemical
The effects of adsorbent dose, contact time, pH, pharmaceutical effluent temperature, and initial phenol concentration on phenol uptake were examined in a number of batch tests. Wastewater with different concentrations of phenol was generated from the stock solution for these investigations, labeled, and stored in separate glass-stoppered conical flasks. Subsequently, suitable doses of adsorbent were added to the wastewater which had a consistent particle size of 150μm. Using a magnetic stirrer, the system was stirred at a constant rate at various temperatures and contact times while it is placed on top of hot plate. After allowing the suspension to cool, Whatman No. 1 filter paper was used to filter it. The filtrate was analyzed to evaluate the concentration of phenols in the treated wastewater by using a UV spectrophotometer. The absorbance of the supernatants was determined by UV-VIS spectrophotometer at 270nm wavelength. The amount of phenol adsorbed at equilibrium (qe) and at a time, t (qt) was calculated using Eqs. 1 and 2 respectively. The percentage removal of phenol from the pharmaceutical effluent was calculated using Eq. 3. (Akpomie et al., 2012).
where qe is the equilibrium adsorption capacity per gram of dry weight of the adsorbent, (mg/g): qt is the adsorption capacity at time, t (mg/g): C0 is the initial concentration of phenol in the solution (mg/l); Ce is the final or equilibrium concentration of phenol in the solution (mg/l); Ct is the amount of phenol adsorbed at time, t (mg/l); V is the volume of the solution (1); and W is the dry weight of adsorbent (g).
Results and Discussion
The results obtained from the above studies have been discussed with credible explanations as follows:
Scanning Electron Microscopy Result
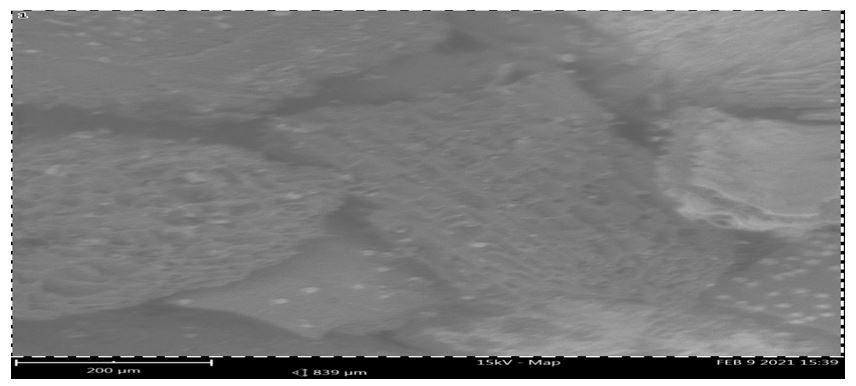
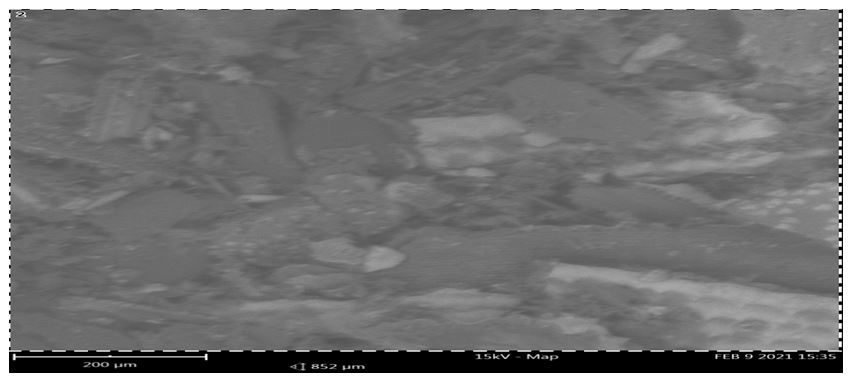
Plates 1-2 display the SEM micrographs for the absorbent. Scanning electron microscopy (SEM) was utilized to determine the surface morphologies of the used and unused RH. The unused RH's SEM pictures showed uneven, hexagon-shaped edges, well-formed flakes, and coarsely packed structures. Before adsorption, the surface of the RH had large, well-developed pores. This could be because of the impact of impregnation with the orthophosphoric acid during the adsorbents' chemical activation. It was also important for the adsorbents' pores to form during the thermal and chemical activation processes, as this increased their surface area and pore volume. However, the well-developed pores on the prepared chemically activated RH adsorbent could be the main factor that led to the high phenol uptake in this study.
Additionally, there was a significant decrease in the interstitial spaces in plate 2's used RH. This might be due to some of the empty areas in the unused adsorbents being filled with adsorbed phenol. It is evident from the SEM images of plates 1 and 2 that the adsorbents' porosity increased by both chemical-based and heat activation. Chemical activation produces a structural defect in the adsorbent as a result of reactions that expand the pore spaces within the adsorbents. This increased pore volumes and surface area of the adsorbent and positively impact the adsorption rates.
Fourier Transform Infrared Result
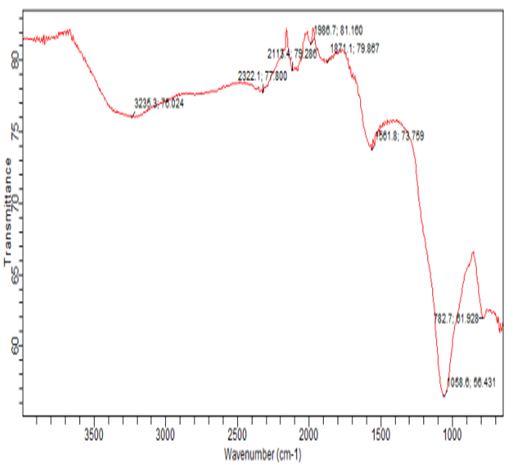
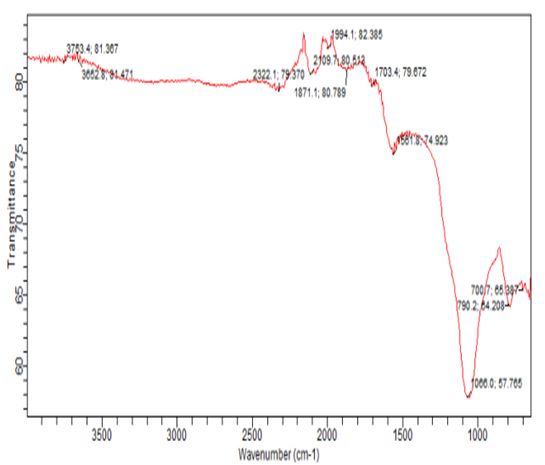
The FTIR technique is a useful tool for determining the distinctive functional groups that are essential to the adsorption of aromatic compounds [13]. The vibrational frequency variations in the functional groups of the adsorbents were ascertained by analyzing the FTIR spectra of RH before and after phenol sorption. Figures 1 and 2 depict the FTIR spectroscopic analysis of the RH adsorbent both before and after adsorption. Four main adsorption bands were evident in the RH picture at 2900–3500 cm-1, 1700–2400 cm-1, 1300–1750 cm-1, and 500–1000 cm-1. For the unused RH, a broad band with two maximum peaks could be seen at 3235.3 and 2322.1. The band was observed to move to 3753.4 and 3652.8cm-1 upon phenol adsorption. It should be mentioned that the hydrogen-bonded OH group of alcohols and phenols has been identified as the source of the bands in the 3000–3650 cm-1 range. (Lua and Yang 2003). Si-O or C-O stretching in alcohol, ether, or hydroxyl groups may be the cause of the sharp adsorption band at 1148.0 cm-1 [20].
At 2900-3500cm-1, 1700-2400cm-1, 1300-1750, and 500-1000cm-1, there were four main adsorption bands visible in the RH image. For the unused RH, a broad band with two maximum peaks at 3235.3 and 2322.1 was observed. Following phenol adsorption, the band was observed to move to 3753.4 and 3652.8 cm-1. Note that the bands in the 3000–3650 cm-1 range have been identified as belonging to the OH group of alcohols and phenols that are hydrogen bonded (Yang and Lua 2003). The sharp adsorption band at 1148.0cm-1 may be ascribed to either Si-O or C-O stretching in alcohol, ether or hydroxyl groups [20].
Influence of Adsorbent Dosage and Contact Time
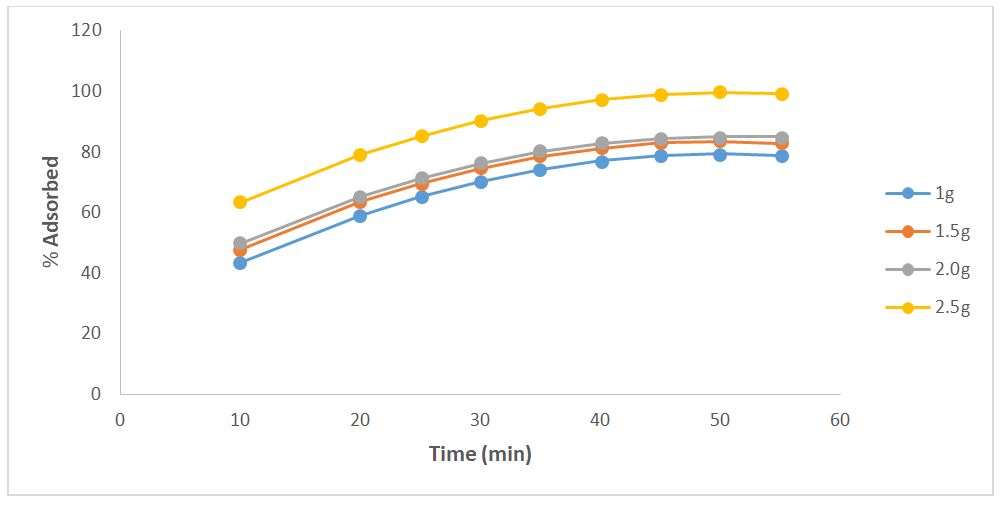
The influence of adsorbent dosage on the adsorptive uptake of phenol onto RH adsorbent was studied at the optimum conditions of 35oC, 35mg/l, and pH of 5 for effluent temperature, concentration, and pH, respectively. The result was presented in Figure 1, which showed that the removal of phenol increased with an increase in the adsorbent dosage; from Figure 1, the maximum percentage removals of phenol was 84.99%. Generally, an increase in percentage removal efficiency with an increase in dosage was expected because as the adsorbent dose increased, the number of active sites for binding phenol molecules on the adsorbent increased and more phenol was attached to the adsorbent surface [21]. However, after a certain adsorbent dosage, the adsorption capacity remains constant. This constant is generally due to the presence of a large number of accessible surfaces–active groups compared to the adsorbate amount. This is in agreement with [21].
Additionally, the results of many batch experiments demonstrated that, for all adsorbent combinations, a longer contact duration increases the percentage of phenol removed. At first, RH quickly absorbed the phenol. However, following the initial 35 minutes, the phenol removal efficiency was shown to hold steady for the next 55 minutes (Figure 1). There may have been more adsorption sites available early in the adsorption process, which would explain the first fast phase. Similar results have been identified by [22] in earlier investigations.
Influence of Effluent Temperature on Phenol Adsorptive Rate
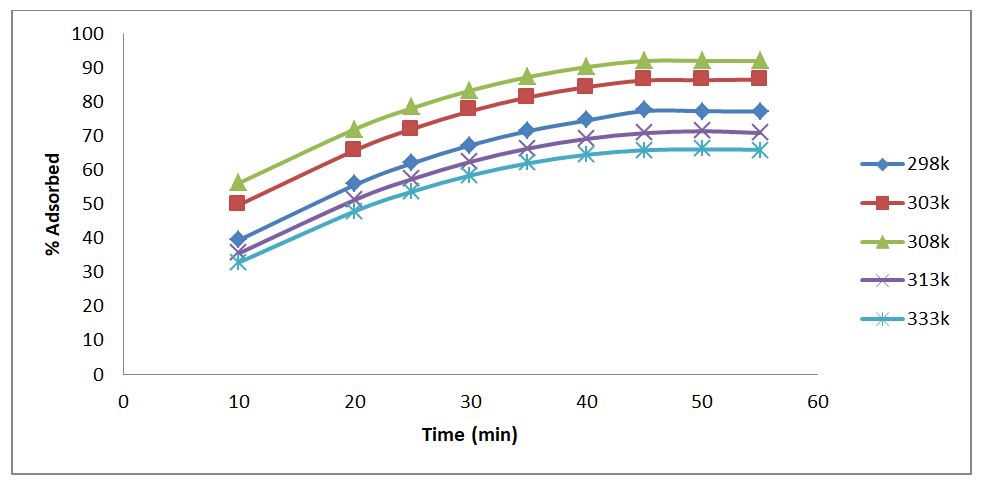
The effect of temperature on the adsorptive uptake of phenol onto RH adsorbent was examined while maintaining the other influencing parameters at an effluent pH of 5, an adsorbent dosage of 1g, and an effluent concentration of 35 mg/l. Figure 2 illustrates that the percentage of phenol adsorbed dropped at higher effluent temperatures, with the maximum adsorption rate occurring at an effluent temperature of 35oC. This decline in adsorption at higher temperatures may be partially attributed to the weakening of adsorptive forces between the active sites of the adsorbent and phenol, and partly due to the enhancement of the thermal energies of the adsorbate, thus making the attractive forces between the adsorbent and phenol insufficient to retain the adsorbed molecules at the binding site. [23]. An increase in phenol's adsorption capacity as temperature drops indicates that the process is exothermic; in this instance, the adsorbate and adsorbent have significantly lower favorable intermolecular interactions as temperature increases than the adsorbate and solvent. Similar findings were reported by [19], who found that phenol uptake was an exothermic process that was favored at lower temperatures.
The forces keeping the phenol to the surface of the adsorbents were physical, or physicosorption, because the adsorption process was exothermic. The van der Waals forces between the non-reacting molecules were the cause of this. At reduced temperatures, the phenol stabilization reached its peak because the molecules were more firmly attached to the adsorbent's surface [22].
Influence of pharmaceutical effluent pH on Phenol Adsorptive Rate
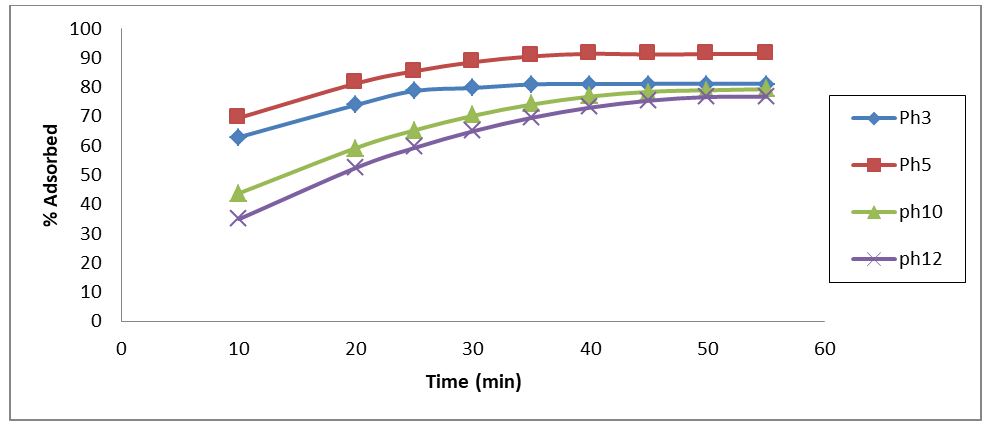
Under ideal circumstances, effluent temperature of 35°C, adsorbent dose of 1 g for all adsorbents, and effluent concentration of 35 mg/l—the impact of effluent pH on the adsorptive uptake of phenol onto RH adsorbent was investigated. the outcome is given in Figure 3. The plot demonstrated that phenol adsorption increased in tandem with a solution's pH rise from 3 to 5, but that uptake steadily declined with a subsequent pH increase from 6 to 10.
At a pH of 3, RH's phenol adsorption efficiency was 81% during the ideal 35-minute contact time (Figure 3). At pH 5, the uptake efficiency increased to 91%; however, at pH 6 and pH 10, it fell to 74.16% and 69.63%, respectively. The ionic state of these functional groups, as well as the phenol chemistry in the solution, can all have an impact on how pH-dependent phenol adsorption is. Consequently, the increased solubility of phenol in the aqueous solution and the availability of OH- ions on the adsorbent, which restricts the diffusion of phenolate ions, were the causes of the reduction in phenol adsorption at higher pH values. When phenol is adsorbed at a pH greater than 5, it indicates that the positively charged phenol ions bind through electrostatic attraction to negatively charged functional groups on the surface of the RH. This is because at a pH of 5 more functional groups carrying negative charges would be exposed at the carbon surface [24].
Influence of Effluent Concentration on Phenol Adsorptive Rate
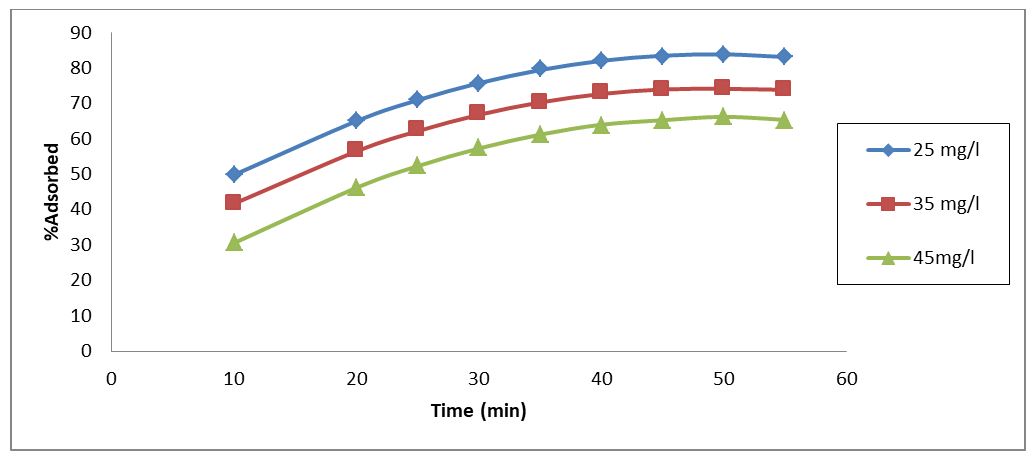
The impact of effluent phenol concentration on a fixed amount of adsorbent dosage was studied under ideal conditions, which included an effluent pH of 5, an effluent temperature of 35 °C, an adsorbent dosage of 1.0 g, and an optimal contact time of 35 minutes. The result is shown in Figure 4. The plot in Figure 4 for RH demonstrated that an increase in effluent phenol concentration from 25 mg/l to 45 mg/l resulted in a corresponding decline in phenol uptake efficiency. This is because there are not enough active sites available as effluent phenol concentration rises.
Equilibrium Studies
There are several isotherm equations available for analyzing experimental sorption equilibrium parameters, the most common being the Langmuir and Freundlich models.
The Langmuir Isotherm
Where Ce is the concentration of adsorbate at equilibrium (mgg−1), KL is Langmuir constant related to adsorption capacity (mgg−1), which can be correlated with the variation of the suitable area and porosity of the adsorbent which implies that large surface area and pore volume will result in higher adsorption capacity, qm is the maximum adsorption capacity for a complete monolayer coverage.
The Freundlich Isotherm
The Freundlich is applicable to adsorption processes that occur on heterogenous surfaces [27]. It also gives an expression which defines the surface heterogeneity and the exponential distribution of active sites and their energies [27]. The linear and non lineear form of the Freundlich isotherm are as follows;
Where KF is adsorption capacity (l/mg) and 1/n is adsorption intensity; it also indicates the relative distribution of the energy and the heterogeneity of the adsorbate sites. The values of n in the range of 2-10 represent good adsorption, 1-2 moderate adsorptions and less than 1 represents poor adsorption characteristics [28].
Temkin Isotherm
One of the earliest isotherms to be described, the Temkin isotherm suggests that the interactions between the adsorbent and adsorbate causes the heat of adsorption to decrease linearly with increasing coverage. According to [29], the nonlinear and linearized forms of the Temkin isotherm have typically been utilized in the forms shown in Eqs. 8 and 9, respectively.
Where: KT (lg−1) is the Temkin isotherm constant, bT (Jmol−1) is a constant related to the heat of sorption and R (8.314Jmol−1K−1) is the gas constant.
For Langmuir isotherm, the constant K is related to the affinity between the adsorbent and adsorbate (vijayaraphavan et al., 2005). A low value of kL indicates favourable adsorption. The low values of kL found for the adsorbent, as shown in Table 2, suggest that the phenol adsorption on RH was favorable. The Langmuir isotherm gave a satisfactory fit to the experimental data, according to the analysis of the result based on the R2 value. An important property of the Langmuir isotherm can be expressed in terms of a dimensionless constant equilibrium parameter, RL. The isotherm's shape is shown by the RL values. Table 2 illustrates the value of the RL achieved at various RH dosages. The value, which ranges from 0 to 1, indicates that the adsorption procedure was effective. The strength of adsorption during the adsorption process is determined by the value of 1/n for the Freundlich isotherm. It is a parameter for heterogeneity. The anticipated heterogeneity increases with decreasing value of 1/n. Furthermore, the adsorption can be classified as linear (n = 1), chemical (n < l) based on the value of the parameter n. Table 2 demonstrates that the value of n for RH is tiny and less than 1, suggesting that the adsorption process was chemical in nature. Additionally, 239.56 is the value of the Freundlich constant (KF), which is used as a proxy for adsorption capacity. The correlation coefficient (R2) values were used to assess the applicability of the isotherm equations to describe the adsorption process. The Langmuir isotherm gave the highest correlation coefficient value among all the isotherms considered in this study (Table 2). This indicated that the Langmuir model was better and able to describe the adsorption of phenol onto RH than the other two models. Consequently, this suggested that adsorption occurred as the monolayer phenol adsorbed onto the homogenous adsorbent surface.
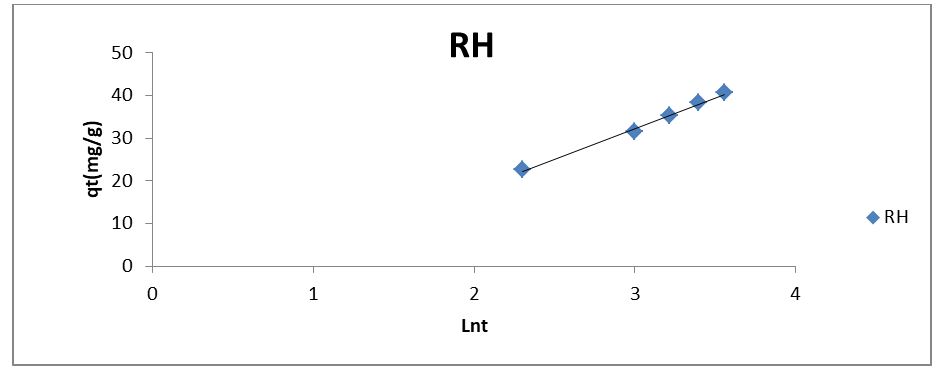
Kinetic Studies
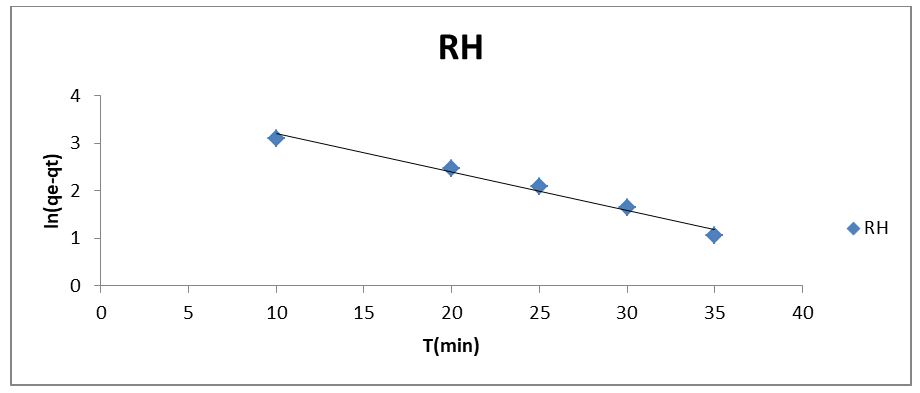
Adsorption mechanisms are evaluated by applying the major adsorption kinetic models. In this research work, three kinetic models (pseudo-first order, pseudo-second order, and Elovich model) were applied to the experimental data to investigate the kinetics of sorption of phenol on RH. First and second order assumed the adsorption process to be pseudo-chemical in nature, while Elovich model is applied to explain whether adsorption is physical adsorption or chemisorption [9]. The linear form of Lagergren's first-order rate equation is as follows (Lagergren, 1898).
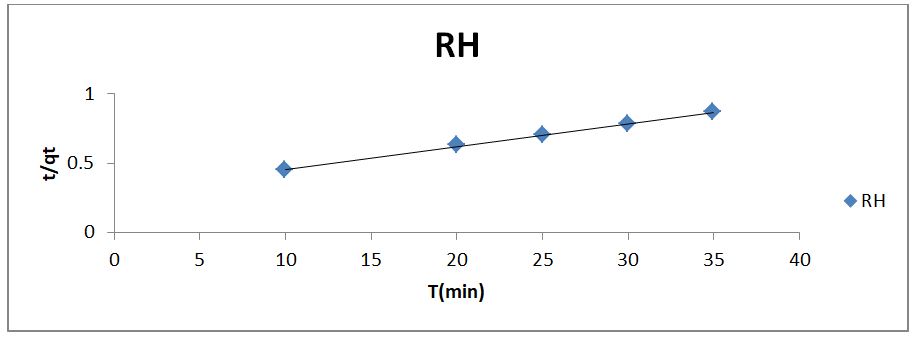
where qe is the amount of phenol adsorbed onto the adsorbent at equilibrium (mg/g), qt is the amount of phenol adsorbed onto the adsorbent at any time t (mg/g), and Kp1 (min-1) is the rate constant of the pseudo-first-order adsorption which can be calculated from the slope of the linear plot of ln(qe − qt) vs. t The linearized form of the pseudo-second-order model is given as
where the slope and intercept of the plot of t/qt versus t can be used to experimentally calculate the equilibrium adsorption capacity (qe) and the second order constants k2 (g/mg h). The constants and R2 values derived from the linear plots for the pseudo-first order, pseudo-second order, and Elovich kinetic models, respectively, ompilwere ced in Table 3 after taking into account linear kinetic models based on the R2 analysis. In comparison to the pseudo-first-order and Elovich kinetic models, it was discovered that the R2 value for the pseudo-second-order kinetic model was comparatively high. The high R2 value for the pseudo-second-order model indicated that the kinetic model may be used to explain the phenol uptake adsorption process on the RH adsorbent.
Thermodynamic Studies
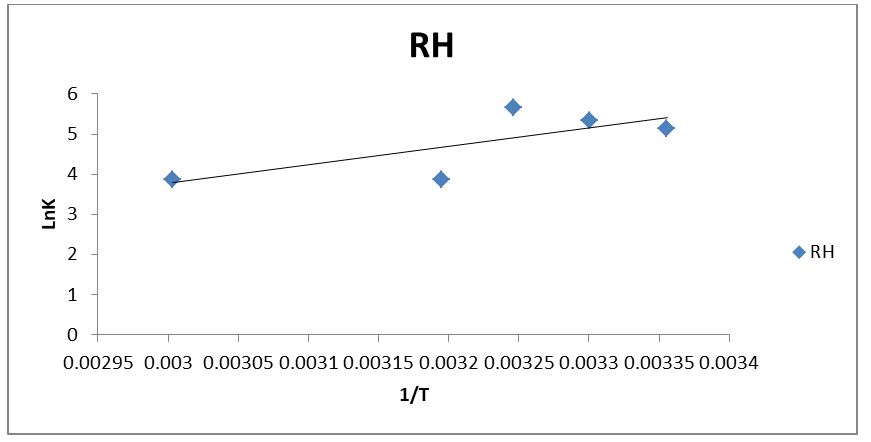
The thermodynamic parameters related to changes in adsorption standard free energy (∆G), the change in enthalpy of reaction (∆H), and the standard change in entropy of adsorbate–adsorbent reaction (∆S), can be calculated by using the Van’t Hoff’s equations as stated below (Yao et al., 2010)
Where R is the gas constant (8.314J/mol K), Kb is the Langmuir isotherm constant at different temperature (L/mg), and T is the absolute temperature in Kelvin. The values of (∆H) and (∆S) can be determined from the slopes and the intercepts of the linear graph of ln(Kb) versus 1/T. From Table 4, the value of the ∆G became increasingly negative with an increase in temperature from 298k to 313k for RH adsorbent but decreased negatively with a further increase in temperature from 323k to 333k. This indicated that the adsorption processes were feasible and spontaneous [30]. The ∆G value for RH adsorbent at a particular temperature was close indicating that such spontaneity was independent of the temperature of adsorption (Okolo, et al., 2000).
For the RH adsorbent, the ∆S value was negative and remained unchanged with temperature. Strong interactions between phenol and the adsorbent (RH) are suggested by a negative value of ∆S, which translates to a decrease in the degree of freedom of the adsorbed species. Additionally, a higher order of reaction during the phenol's adsorption onto the adsorbent was suggested by the negative value of ∆S. It also demonstrated the effectiveness of the adsorbent materials bonded with phenol. The exothermic nature of the adsorption process was demonstrated by the negative values of ∆H. According to [31], the value of ∆H was less than 84 kJ mol-1, indicating the physical character of the sorption. The entire process of adsorption was exothermic. Previous studies with comparable findings included [30,32,33].
Conclusion
The following conclusions were drawn from the research work;
Recommendation
- The RH adsorbent was found to be potent adsorbents for the removal of phenol from pharmaceutical effluent.
- The amount of phenol adsorbed was found to be dependent on dosage, pharmaceutical effluent pH, contact time and effluent temperature. Percentage removal of phenol was found to increase with increase in adsorbent dose, contact time but decreases with increase in effluent pH, and pharmaceutical effluent temperature.
- The Langmuir isotherm best described the adsorption process for linear equilibrium adsorption studies of RH adsorbent while the linear kinetic data were best fitted to pseudo second order kinetic model for the adsorbent.
- The thermodynamic studies showed that the adsorption process was physical, spontaneous and exothermic in nature.
Since this research work has established that RH is potent and low cost adsorbent for the removal of phenol from pharmaceutical effluent, it is recommended that a trial scale up be conducted for the industrial removal and recovery of phenol from pharmaceutical effluent containing phenol. Also consideration should also be given to other waste materials that may likely be present in the effluent and how RH will be able to reduce their quantity or remove them entirely.
- Ethan H, Sanders R, Johnson T (2003) Regulation and management of pharmaceutical waste. Journal of Environmental Management, 77: 145-56.
- Smith J, Brown L (2018) Impacts of suspended solids on aquatic ecosystems. Environmental Science Publications.
- Chung KT, Wong TY, Chou MW (2003) Health and environmental effects of phenol. Environmental Science & Technology, 37: 2735-40.
- Atkins PW, De Paula J, Keeler J (2023) Atkins' physical chemistry. Oxford university press.
- Ferrari L, Kaufmann J, Winnefeld F, Plank J (2010) Interaction of cement model systems with superplasticizers investigated by atomic force microscopy, zeta potential, and adsorption measurements. Journal of colloid and interface science, 347: 15-24.
- Carroll GT, Jongejan MG, Pijper D, Feringa BL (2010) Spontaneous generation and patterning of chiral polymeric surface toroids. Chemical Science, 1: 469-72.
- Lan J, Wang B, Bo C, Gong B, Ou J (2023) Progress on fabrication and application of activated carbon sphere in recent decade. Journal of Industrial and Engineering Chemistry, 120: 47-72.
- Achak M, Raji M, Barka N (2009) Removal of phenolic compounds from wastewater using banana peel. Journal of Hazardous Materials, 165: 285-92.
- Rengaraj S, Ghafoor K, Shankar K (2002) Removal of phenolic compounds from wastewater using palm seed coat. Journal of Hazardous Materials, 93: 119-32.
- Alam MZ, Gupta VK, Ahamad K (2009) Adsorption of phenolic compounds onto activated carbon. Journal of Environmental Science and Health, Part A, 35: 779-98.
- Arana R, González M, Ibarra I (2010) Adsorption of phenol on activated carbon: Comparison of batch and column studies. Journal of Hazardous Materials, 176: 182-90.
- Altenor S, Djelal H, El-Hamdaoui A (2013) Removal of phenol from aqueous solution by adsorption onto activated carbon prepared from olive stones. Journal of Environmental Management, 120: 184-90.
- Abdelwahab O, Amin N (2013) Characterization of adsorbent developed from rice husk: Effect of surface functional group on phenol adsorption. Journal of Applied Sciences, 10: 1060-7.
- Prabhakaran P, Ranganathan R, Kumar VM, Rajasekar R, Devakumar L, Pal SK (2017) Review on parameters influencing the rice breakage and rubber roll wear in sheller. Arch Metall Mater, 62: 1875-80.
- Bodie AR, Micciche AC, Atungulu GG, Rothrock MJ, Ricke SC (2019) Current trends of rice milling byproducts for agricultural applications and alternative food production systems. Front Sustain Food Syst, 3: 47.
- Abbas A, Ansumali S (2010) Global potential of rice husk as a renewable feedstock for ethanol biofuel production. BioEnergy Research, 3: 328-34.
- Sookkumnerd C, Ito N, Kito K (2005) Financial viability of huskfuel steam engine as energy saving technology in Thai rice mill. Appl Energy, 82: 64-80
- Ademoroti CMA (1996) Standard methods for water and wastewater analysis. Foludex Press.
- Lekan TP, Adeyinka SY, Olusola AA, Mayowa A (2019) Brilliant Green dye sorption onto snail shell–rice husk: statistical and error function models as Parametric Isotherm predictors. Journal of Environmental science and Technology, 12: 65-80.
- Park SJ, McClain T, Tian Y, Suib SL, Karwacki CJ (1997) Adsorption and photocatalytic oxidation of phenol and substituted phenols using granular activated carbon and porous solid photocatalysts. Chemosphere, 35: 1397-411.
- Malihe Z, Hassan M (2020) Effect of adsorbent dose and pH on the removal of phenol using activated carbon from agricultural waste. Environmental Technology & Innovation, 19: 100856.
- Hameed BH, Rahman AA (2008) Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. Journal of Hazardous Materials, 160: 576-81.
- Mota JPB, Lyubchik SI (2008) Adsorption of phenol on activated carbon: Adsorption equilibrium. Journal of Hazardous Materials, 160: 576-81.
- Terzyk AP (2003) Further insights into the role of carbon surface functionalities in the mechanism of phenol adsorption. *Journal of Colloid and Interface
- Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. Journal of the American Chemical Society, 40: 1361-403.
- Elmorsi TM (2011) Equilibrium isotherms and kinetic studies of removal of methylene blue dye by adsorption onto miswak leaves as a natural adsorbent. Journal of Environmental Protection, 2: 817-27.
- Ayawei N, Ebelegi AN, Wankasi D (2015) Modelling and interpretation of adsorption isotherms. Journal of Chemistry, 1-1.
- Hamdaoui O, Naffrechoux E (2007) Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon. Journal of Hazardous Materials, 147: 381-94.
- Temkin MI, Pyzhev V (1939) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physicochimica URSS, 12: 327-56.
- Fu J, Chen Z, Wang M, Liu S, Zhang J, Zhang J, Xu Q (2009) Adsorption of phenol from aqueous solution by four kinds of modified resin adsorbents. Journal of Hazardous Materials, 166: 133-7.
- Bazrafshan E, Mahvi AH, Zazouli MA, Balarak D (2015) Equilibrium and kinetics studies on adsorption of fluoride from aqueous solution using cationic surfactant modified bentonite. Journal of Environmental Health Science and Engineering, 13: 1-0.
- Bhatnagar A, Hogland W, Marques M, Sillanpää M (2014) An overview of the modification methods of activated carbon for its water treatment applications. Chemical Engineering Journal, 219: 499-511.
- Cañizares P, Lobato J, Paz R, Rodrigo MA, Sáez C (2006) Advanced oxidation processes for the treatment of olive oil mills wastewater. Chemosphere, 67: 832-8.
- Misra RD, Tyagi VK, Singh R, Misra KK (2005) Fourier transform infrared spectroscopic study of carboxy terminated butadiene-acrylonitrile copolymer and its hydrogenated product. Polymer Bulletin, 55: 243-51
- Bansal RC, Goyal M, Singh N (2006) Activated carbon adsorption. CRC Press.
- Daffalla SB, Mukhtar H, Shaharun MS (2010) Characterization of adsorbent developed from rice husk: Effect of surface functional group on phenol adsorption. Journal of Applied Sciences, 10: 1060-7.
- Hajjaji M, Kacim S, Alami A, El-Bouadili A, Mountassir Y (2001) Influence of calcination temperature on the adsorption of phenol on hydroxylated minerals. Applied Clay Science, 20: 1-8.
- Mohan D, Pittman CU (2006) Activated carbons and low-cost adsorbents for remediation of contaminated soils, sediments, and water. Critical Reviews in Environmental Science and Technology, 36: 433-67.
- Sahu JN, Das SK (2012) Adsorption of phenol from aqueous solution by using activated carbon prepared from agricultural waste. Journal of Environmental Chemical Engineering, 2: 1383-91.
- Siddique MN, Choudhury N (2009) Optimization of adsorbent dose and contact time for removal of phenol using activated carbon. International Journal of Environmental Science and Technology, 6: 245-54.

Tables at a glance
Figures at a glance