Consequence of Retarding Admixtures on the Setting Time of Gypsum in Hot Weather and its Impact on Porosity
Received Date: December 18, 2024 Accepted Date: January 18, 2025 Published Date: January 21, 2025
Citation: Fauzia Anjum Chattha, Khalid Javed, Fatima Naeem, Soha Ahmad (2025) Consequence of Retarding Admixtures on the Setting Time of Gypsum in Hot Weather and its Impact on Porosity. J Chem Eng Catal 4: 1-10
Abstract
The objective of this research is to investigate the influence of commonly available chemicals on the setting time of plaster of Paris (POP). Set-time retardants were incorporated at three different concentrations under hot curing conditions (high temperature and low humidity). The test results revealed that under normal curing conditions (25°C and 60% relative humidity), the efficiency of retardants in increasing the setting time improves with higher retardant content, up to a certain limit. However, beyond this optimal content, the excessive retardant addition adversely affects the material's properties by increasing its porosity. This, in turn, results in instability and reduced strength. At significantly higher concentrations, the material becomes highly brittle and difficult to handle. Furthermore, the study found that retardants are most effective when mixed with water immediately after combining water and plaster of Paris, without any delay.
Keywords: Setting Time; Curing; Retardants; Effect on Porosity; Effect on Density
Introduction
Dry plaster powder, primarily composed of calcium sulfate, transforms into gypsum when mixed with water. Gypsum has been widely used as a building material since ancient times [1]. In Pakistan, high-quality gypsum reserves are found in the Suleman Range, Muzaffarabad, and the Kohat region [2-4]. A large gypsum deposit at Montmartre in Paris gave rise to the name "plaster of Paris" for this material. Plaster of Paris (POP) is a fine powder that forms a paste when mixed with water [5]. After a short time, the paste thickens and hardens, making it a versatile material for various construction and artistic applications [6].
The setting time start approximately with in one minute after adding the plaster to water (and paste preparation in lab mixer). The setting of unmodified plaster starts about 10 minutes after mixing [7] and completes in about 45 minutes; but not fully set for 72 hours [8]. In particular, with regard to the setting time, the binding materials must vary from the period of a few minutes up to several hours. In order to satisfy these requirements, the use of set regulating admixtures is necessary. When plaster or gypsum is heated, again re-form as gypsum if mixed with water [9].
Pouring of plaster gradually in to the water while shaking makes homogenous slurry. The proper quantity of gypsum is right enough to peep out over the surface of the water that is, about equal volumes of each ingredient. Hot weather of countries like Pakistan that used to be 44-48°C in summer rapidly causes evaporation of water from the surface of the fresh paste of gypsum [10]. Consequently, the paste sets rapidly than its normal setting and shortens the length of time for molding operations. For example, it has reported that when the temperature of cement mortar with water/gypsum (w/c) ratio of 0.6 increases from 27°C to 45.5°C, both the initial and final setting times are reduced to half. Other problems such as rapid decrease of slump, formation of cold joints and plastic shrinkage cracking, increased difficulty in air entrainment, enhanced permeability and reduced durability and reduction in ultimate strength may arise due to hot weather [11]. Gypsum plaster or plaster of Paris produces by heating gypsum to about 150°C [12].
When plaster or gypsum is heated above 200°C, anhydrite formed that can be re-formed as gypsum on mixing with water [13]. Plaster of Paris impregnate gauze bandages to make a sculpting material called Modroc [14]. It is like clay, because paste can easily be shaped into a resilient and lightweight structure. This is the material also used to make classic plaster known as orthopedic casts to protect limbs with broken bones.
Experimental
All chemical used were of commercial grade although most of them were household chemicals and were used without further purification.
General Procedure
Set Time Determination
A number of retardants with varying concentrations ranging from 0.1-2.0 percentage (w/w %) were employed to plaster of Paris and water mixture. Their effect on setting time studied under optimized conditions as function of time taken by mixture to show ceased flow but remain as soft as penetrable. Set time calculated from initial and final setting time after adding aqueous mixture of water (same volume of water in all cases) and varying amount of retardants. All experiments completed at room temperature (35-42°C) and compared to tap water as standard.
Vicat needle was used to determine the setting times of gypsums by the penetration of the needle inside the material during the hardening process following UNE-EN 13279-2. Samples took in rubber cone-shape molds of 40 mm of height, inner diameters of 65 and 75 mm, finally noted time. Setting times calculated with Equation (1) where Ti is the setting time of the gypsum in min, t1 is the time in minutes when Vicat needle achieves 22˘2 mm of depth and t0 is the time in minutes when water and gypsum are in contact. This test determines the initial time of hardening process and finalizes when the needle sinks 22˘2 mm of depth, which means that the hardening process has final [15].
Measurement of Porosity
The samples heated in an oven at 105 to 110°C for 4-5 hours and the dry weight, D, in grams to the nearest 0.1 g was determined. The samples placed in water and boiled for 2 h. During the boiling period, samples completely covered with water, in order to avoid contact of the samples with the heated bottom of the container. After the boiling period, the samples cooled to room temperature while still completely merged in water for minimum 24 h. The samples weighed after boiling while suspended in water to determine the suspended weight S, in grams to the nearest 0.1 g. After determining the suspended weight, blot each sample lightly with a moistened smooth cotton cloth to remove all drops of water from the surface and weighed to determine the saturated weight, W, in grams by weighing in air to the nearest 0.1 g [16]. Porosity determined by the following standard equation (2).
Density Measurement
The bulk density calculated in grams per cubic centimeter using dry weight, saturated weight and suspended weights of the samples. Density calculated [14] with the help of equation (3).
Density = Dry weight / (Saturated weight-Suspended weight)
Result and Discussion
When water is added to plaster of Paris (POP), it gradually sets and hardens under normal climatic conditions. However, in some countries, including Pakistan, higher summer temperatures (35–45°C), low relative humidity, and hot winds cause rapid evaporation of water from the surface. This results in an accelerated setting process, leaving insufficient time for proper designing and molding. To ensure adequate working time, particularly when unavoidable delays occur between mixing and placement, the use of setting retarders or retarding admixtures becomes essential. These retardants help counter the adverse effects of extreme climatic conditions, providing sufficient time for operation and preventing other detrimental impacts on the material’s performance.
Plaster of Paris (POP) sets very quickly but may take several hours to fully cure. Additives can be used to either speed up the process (accelerators) or slow it down (retardants). Several factors influence the curing time of POP, including humidity, room temperature, water additives or mineral content, the amount of water used, the mixing method, and the condition of the plaster itself. As a rule, POP parts cured at room temperature take approximately 20 to 30 minutes to harden, though denser and thicker pieces may require longer curing times. In our study, we used commonly available chemicals that are practical for both laboratory and home applications. In hot weather conditions like those in Pakistan, POP mixed with normal tap water set in approximately 15 minutes. However, using cold water delayed the setting time to around 20 minutes (Table 1).
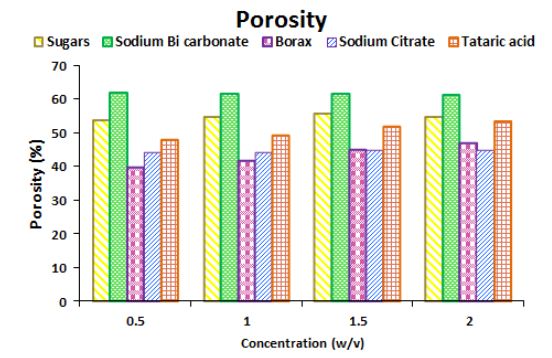
Different solids such as sugar, common salt, sodium bicarbonate, borax, sodium citrate, tartaric acid, citric acid and starch (w/w ratio to plaster of Paris, (Table 1 and Figure 1) were used to study the delay in set time. We observed that citric acid and starch showed delay in set time ample extension than tap water.
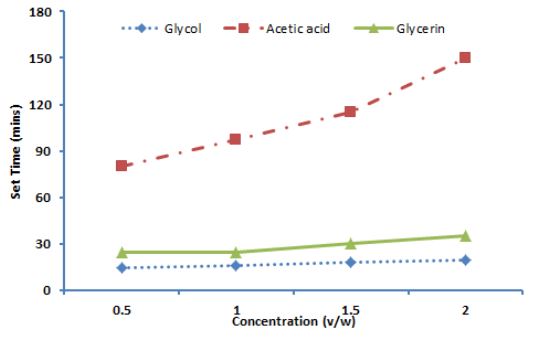
Chemicals used like propylene glycol, glycerin, glue and hydrochloric acid (v/w ratio to plaster of Paris, Table 2 & Figure 2) cast-off and excellent delay in set time observed in all these chemicals. The temperature of the water used to mix plaster of Paris plays a vital role in the length of time it takes for a plaster part to set. Plaster of Paris mixed with cold water takes a significantly longer time to cure than plaster mixed with warm water or tap water. The higher the temperature of the water used, the more rapidly the drying of plaster of Paris parts occurs.
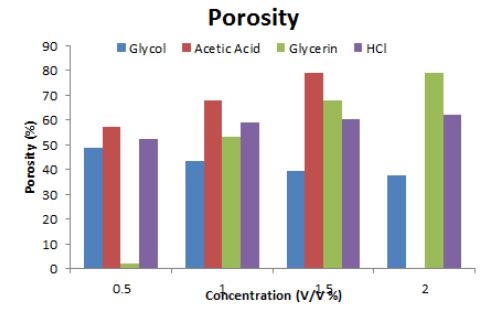
The delay in set time badly affected the porosity and turned it to brittle hence weakened the binding of material or crystals. Porosity is a measure of the void spaces in a material, and is a fraction of the volume of voids over the total volume, presented in percentage (0 and 100%). Strictly speaking, some tests measure the "accessible void", the total amount of void space accessible from the surface (closed-cell foam). Increase in porosity result in loose binding, brittleness and hence difficult to handle. All compound only increased its set time without any effect on its porosity except acetic acid and hydrochloric acid that increased its porosity beyond its acceptable value.
A Retarding Material Set Retardation Mechanisms
- Adsorption of the retardants on the surface of particles may slowdown the hydrolysis and hence forms a protective skin over the surface of plaster of Paris.
- Adsorption of the retarding compound onto nuclei of calcium hydroxide, poisoning their growth, thus disturbing continued hydration. Due to disturbance in continued hydration, thus crystal formation is delayed effectively. It can straight forwardly affect its porosity and set time is delayed [17].
- Formation of complexes with calcium ions increases their solubility and discouraging the formation of the nuclei of calcium hydroxide and therefore is slowing of set time is favored. Therefore, retarding materials like acetic acid, citric acid and tartaric acid forms calcium-acetate, calcium-citrate and calcium-tatarate and hence delays the setting time profoundly.
- Precipitation around particles of insoluble deriva- tives of retarding compounds formed by reaction with the highly alkaline aqueous solution forms a protective skin.
The addition of the retardant chemicals caused marked retardation in setting times. On addition of retardants the rate of hydration gets slow down. Consequently, the necessary amount of the hydration products giving rigidity to the plaster paste required longer time. Thus, plaster pastes containing retardant remains plastic for longer time.
Effect on Porosity
Porosity or void fraction is a measure of the void (i.e. "empty") spaces in a material, and is a fraction of the volume of voids over the total volume, between 0 and 1, or as a percentage between 0 and 100%. Strictly speaking, some tests measure the "accessible void", the total amount of void space accessible from the surface (closed-cell foam). There are many ways to test porosity in a substance or part, such as industrial CT scanning or manual methods. Retarding materials not only affect the rate of hydration but also disturb its porosity (Figure 3 and 4) shows the effect of porosity with an increase in the concentration of the retardants for each sample. In figure 3 there is no significant effect on porosity by increase in concentration. Use of these retardants are considered to be useful as these increase set time by change in its hydration with increase in concentration but no distress on the porosity. On the other hand, some of the chemicals showed remarkable effect on the porosity particularly acetic acid (Figure 4). In general, such chemicals when used directly affect the void places of plaster of Paris. Increase in concentration, results in too many large voids fraction results in brittleness thus possess loose bond between the gypsum particles decreases leading to the lower strength of the sample [18].
Influence on Density
In order to use gypsum or plaster of Paris for different purposes, depending upon the different setting times are required different setting times for the production of reasonable working spans. Therefore, retardants are considerable that can increase set time without any distress on porosity or binding of gypsum. In our experiments, no appreciable bad affect found on the density of Plaster of paris while using retardants.
Conclusion
In extremely hot climates like Pakistan, high summer temperatures, low relative humidity, and hot winds cause rapid evaporation of water from surfaces, leading to a shorter setting time for plaster of Paris (POP). This accelerated setting process leaves insufficient time for proper designing and molding. To address this, additives are used to either accelerate or retard the setting process. In this study, various solid and liquid chemicals were tested for their ability to delay the setting time. Borax and citric acid proved to be highly effective retardants, while sodium bicarbonate and starch caused a moderate delay in the setting time. However, excessive delay in setting often increases porosity, making the material brittle and weakening its crystal binding. Among the tested chemicals, acetic acid caused the maximum delay in setting time with minimal impact on porosity. The use of retardants was found to effectively increase the setting time by altering the hydration process, particularly with increasing concentrations, without significantly affecting the porosity or density of POP. However, some chemicals, including acetic acid, exhibited notable effects on porosity. In our experiments, no appreciable negative impact on the density of POP was observed when using retardants.
- Zubair MA, Chaudhry MA, Khan MA (1984) Gypsum Plaster as Material of construction. Pak J. Sci. Ind. Res. 27: 107-12
- Malkani SM, Alyani MI, Khosa MH, Tariq S, Saeed F, et al. (2016) Mineral Resources of Pakistan-an update. Lasbela, U. J.Sci. Techl. 5: 90-114.
- Khan SA, Chughtai MZ, Khan H, Faisal M, Kanwal F (2004) Gypsum Plaster as Building from Kohat Gypsum J. chem. Soc. Pak. 26: 101-6.
- "Mineral deposits of Pakistan". pakboi.gov.pk. 2012, Retrieved 2013-07-23.
- Anthony, John W, Bideaux, Richard A, Bladh, Kenneth W, Nichols, Monte C. eds. "Gypsum". Handbook of Mineralogy. V (Borates, Carbonates, Sulfates). Chantilly, VA, US: Mineralogical Society of America. ISBN 0962209708.
- Plaster of Paris definition. Webster's New World College Dictionary at Your Dictionary.com. 2003.
- Schmidt VE, Somerset JH, Porter RE (1973) "Mechanical Properties of Orthopeadic Plaster Bandages" Journal of Biomechanics (Elsevier), 6: 173-85
- Foerst W (1957) Of Ullmann Encyklopädie of the technical ones Chemistry. 3-rd ed., Vol. 8, pp. 97-132. Munich: Urbane and Black mountain, in 1957.
- Deer WA, Howie RA, Zussman J (1992) "An Introduction to the Rock Forming Minerals." Pearson Education Limited, England, 2nd Edition, 614.
- Fattuhi NI (1988) The setting of mortar mixes subjected to different temperatures. An Int. J. Cem. Concr. Res, 15: 1-5.
- Mahler DB, Ady AB (1960) An explanation for the hygroscopic setting expansion of dental gypsum products. J Dent Res. 39: 578-89.
- Müller M, Fischer HB (2006) To the mechanical activation from Calciumsulfatdihydrat. Proceedings to 16th ibausil. Bauhaus university of Weimar / Germany, 09/2006. Conference tape 1, pp. 0817-27.
- Ruffer C (1987) The Gipsabbinden: Acceleration and Delay. Ceramic magazine. 39: 13-5.
- Schmidt VE, Somerset JH, Porter RE (1973) "Mechanical Properties of Orthopeadic Plaster Bandages". Journal of Biomechanics. Elsevier. 6: 173-85.
- UNE-EN 13279-2:2006. Gypsum Binders and Gypsum Plasters—Part 2: Test Methods; Asociación Española de Normalización y Certificación (AENOR): Madrid, Spain.
- ASTM C20-00. Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water, ASTM International, West Conshohocken, PA, 2015, www.astm.org.
- Lewry J, Williamson J (1994) The Setting of Gypsum Plaster: Part II, The development of Microstructure and Strength. J. Mat. Sci., 29: 5524-552.


Tables at a glance
Figures at a glance