Diagnostic Pitfalls in Diffuse Interstitial Lung Damage, Presentation of Clinical Cases
Received Date: December 18, 2022 Accepted Date: January 18, 2023 Published Date: January 21, 2023
doi: 10.17303/croa.2023.8.101
Citation: Simona-Ştefania Dobre, Petre Iacob Calistru, Mihaly Enyedi (2023) Diagnostic Pitfalls in Diffuse Interstitial Lung Damage, Presentation of Clinical Cases. Case Reports: Open Access 8: 1-12
Abstract
Diffuse interstitial lesions of the lung parenchyma pose complex problems of positive and differential diagnosis, the etiological spectrum including:
Bacterial (pulmonary tuberculosis-miliary form, brucellosis), fungal (aspergillosis, histoplasmosis), parasitic (pneumocystosis, toxoplasmosis) or viral (SARS-CoV2, HIV) infections
Non-infectious diseases (pneumoconiosis, collagenosis/vasculitis, carcinomatosis, hypersensitivity pneumonitis, etc.).
We present three clinical cases with similar radiological images, namely diffuse interstitial lung damage, but with different diagnosis and etiologies: sarcoidosis stage III of the disease, pulmonary fibrosis after SARS-CoV2 infection, and carcinomatous lymphangitis. In all three cases, the chest computer tomography was the basic investigation in the diagnosis of the lung disease, but the diagnosis of certainty required the performance of other investigative methods.
Keywords: Diffuse interstitial pneumopathy; Sarcoidosis; Pulmonary fibrosis; SARS-CoV2 viral infection
Introduction
Interstitial lung lesions pose complex problems of positive and differential diagnosis, the etiological spectrum including:
1.Infections:
- Bacterial: tuberculosis and nontuberculous mycobacteria, brucellosis
- Fungal: aspergillosis, histoplasmosis, cryptococcosis
- Parasitic: Pneumocystis jiroveci echonococcosis,toxoplasmosis
- Viral :HIV, SARS-CoV2 infections
2. Exposures/ Toxins :
- Drug induced hypersensitivity: metotrexate,infliximab, humira, amiodarona
- Hypersensitivity pneumonitis
- Pneumoconioses (aluminum, cobalt, talc,titanium)
Vasculitis: Churg-Strauss syndrome
- Wegener granulomatosis
Malignancy:
- Lymphoma
- Lymfomatoid granulomatosis
Inflamations:
- Eosinophilic granulomtosis (pulmonaryLangerhans cell histiocytosis)
- Lymphocytic interstitial pneumonitis
- Bronchocentric granulomatosis (Usually associated with asthma and allergic bronchopulmonary aspergillosis)
In this article we will present 3 cases with different diagnoses but with similar radiological appearance. Our aim is to demonstrate that for the differential diagnosis of interstitial lung lesions, numerous additional procedures of greater accuracy are needed (bronchoscopy with bronchoalveolar lavage, transbronchial biopsy, transthoracic biopsy, serological and immunological analyses, microbiological investigations), which along with conventional radiography, computed tomography with contrast substance, to establish the diagnosis of certainty.
Case report
Patient 1
The first patient is a 36-year-old white female, presented on our department with a four- month history of anterior-sided chest pain with associated shortness of breath on exertion. She described the pain as a dull ache that occurred at rest and was not worsened by exertion. She also remarks a chronic dry cough that she had for the prior several years that was not associated with illness or exercise and a 5-pound weight loss over the previous month.
Upon arrival, hers vital signs were all within normal limits, and the pulmonary clinical examination revealed sibilant and subcrepitant bronchial rales bilaterally,predominantly basal, SaO2= 91-94% in atmospheric air.Also, there were no visible skin or subcutaneous lesions.
The electrocardiogram showed a normal sinus rhythm of 82 beats per minute without any sign of ischemia.Laboratory tests including complete blood count, completemetabolic profile, and acute ischemia tests (Troponin, CK,CK-MB) were all negative.
Pulmonary function exploration (spirometry) revealed restrictive ventilatory dysfunction with a reduction in FEV1 by 42% and FVC by 33%, IT = 76.
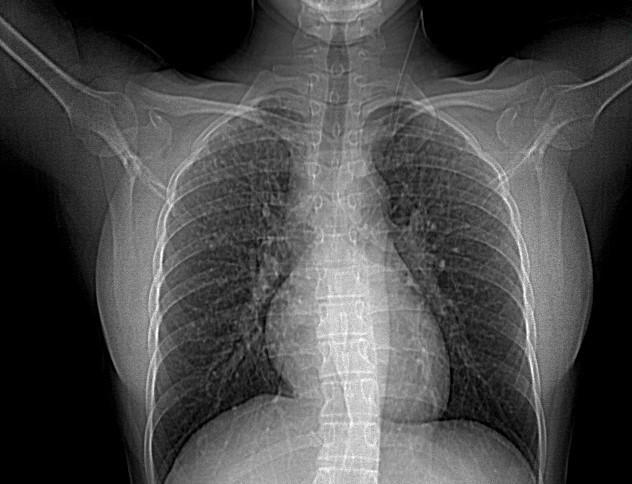
Intravenous contrast computed tomography of the chest revealed middle and upper lung nodularity with a perilymphatic distribution involving the central peribronchial vascular regions as well as the subpleural and fissural surfaces. (Figure 2).
Based on the clinical and imaging data with diffuse interstitial damage and bilateral hilar adenopathy, pulmonary sarcoidosis was suspected and the patient underwent bronchoscopy with bronchioloalveolar lavage and transbronchial biopsies. Bronchoalveolar lavage was negative for fungal infections, acid-fast bacilli, and malignant cells and showed lymphocyte proliferation. Transbronchial lung biopsy showed numerous well-formed, non- caseating granulomas embedded in dense hyaline sclerosis.
According to the histopathological examination with the presence of non-caseating granulomas, we diagnosed her as: Pulmonary sarcoidosis stage 2, and he started taking daily prednisone and sulfamethoxazole/ trimethoprim three times a week for eight weeks. After the treatment, the symptoms decreased significantly and a chest CT showed a clear reduction in lung field shadows and an impression of reducted mediastinal lymph node enlargement.
Patient 2
The second patient, 58-year-old patient, female, diagnosed with an ovarian neoplasm, operated on in 2017 and polychemotherapy with numerous therapeutic lines, presents with a dry, irritating cough, sometimes with frequent attacks in the supine position, associated with mixed dyspnea at low exertion and lying down, fatigue, weight loss significant (9 kg in 2 months).
The onset is recent, 2 months ago, with cough, to which progressive dyspnea and general asthenia were associated in the last 2 weeks.
On admission, the patient's condition is serious,Pulmonary stetacustic we find the reduction of the vesicular murmur, with subcrepitant bronchial rales bilaterally basal.
The biological samples show anemia with hypochromia and microcytosis (Hb 8.9 g/dl), thrombocytosis 459 mil/mm3, inflammatory syndrome (CRP= 9.9mg/dL,Ferritin > 2000 μg/L).
The antero-posterior chest X-ray highlights the accentuation of the lung pattern due to the vascular and interstitial components, minimal right basal pleural effusion (Figure 3).
The diagnostic assumptions included: bilateral pulmonary disseminated process: pulmonary metastases (carcinomatous lymphangitis), tuberculosis.
A computer tomography of the chest was performed with the contrast substance, which found:
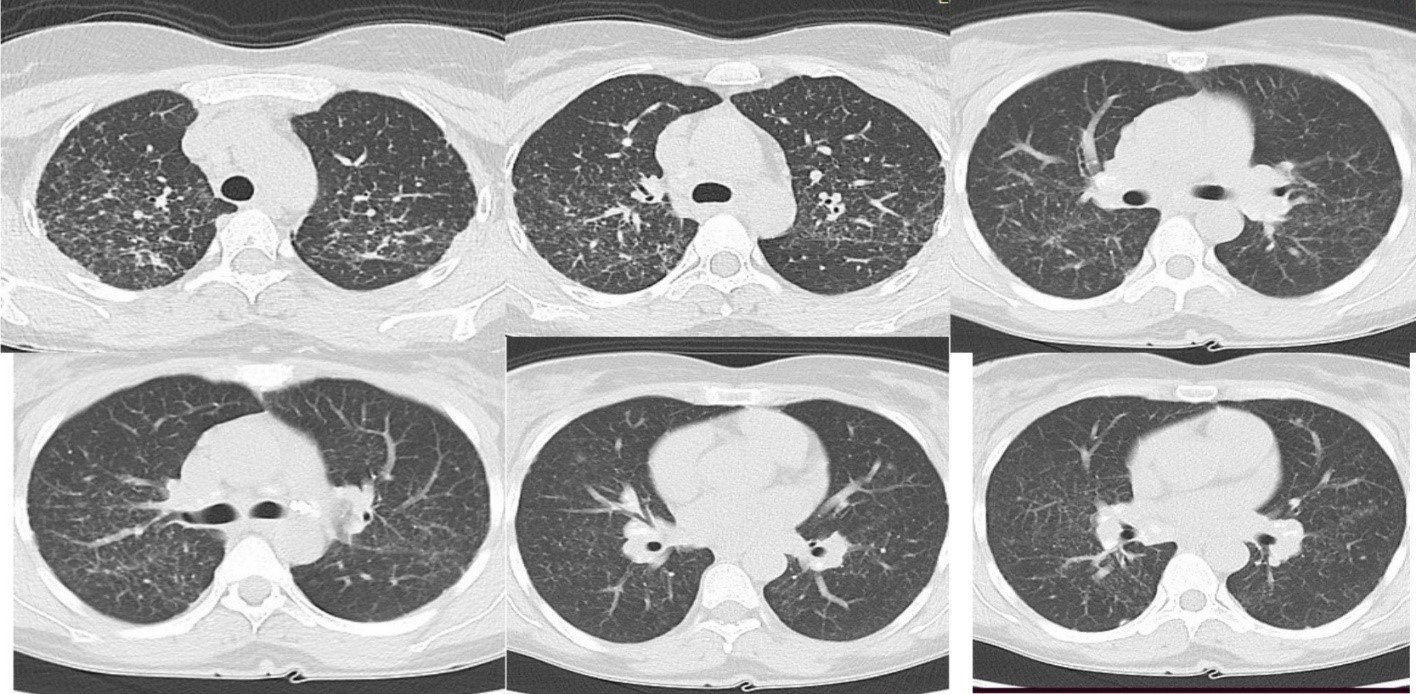
At the level of the chest, the presence of nodular thickenings of the interlobular septa and bronchovascular bundles (small centrilobular nodules), septal lines and polygons, bead-like thickening of the interlobular fissures, free fluid in the right pleural cavity 7 mm and hilar lymphadenopathy (Figure 4)
A lung biopsy was discussed, but based on the oncological antecedents, the medical history and the tomographic investigation, the diagnosis of pulmonarycarcinomatous lymphangitis with an ovarian starting point was established (Ovarian neoplasm with peritoneal carcinomatosis, with secondary hepatic, pancreatic, ureteral,pulmonary, lung biopsy is no longer necessary)
During the hospitalization, the general condition without dynamics is maintained, the excruciating dry cough and low fever, the general asthenia progresses, the patient was taken over by the oncology service for specialized treatment.
Patient 3
The third case is of a 75-year-old man who presented to our clinic for fever, cough and dyspnea that started 5 days ago and with progressive worsening of dyspnea and cough.
The patient has a medical history of hypertension,dyslipidemia, grade 2 obesities and OSA with CPAP.
The patient has not been vaccinated against SARSCoV-2.
At presentation, body temperature was 38.5˚C,blood pressure was 140/80 mmHg, heart rate was 90 beats per minute, respiratory rate was 28 breaths per minute, and SpO2 was 87% on room air. Chest examination revealed crackles on auscultation in all lung fields.
Laboratory findings included an increased WBC count (13.560 /μl reference range, 4000- 10000 /μl) with neutrophilia (84.8%; reference range, 50-70%) and lymphopenia (8,7%; reference range, 25-40%), elevated CRP levels (39 mg/l; reference range, 0-0,3 mg/l), elevated LDH levels (500 U/l; reference range, 135-225 U/l) and elevated ferritin levels (816 ng/ml; reference range, 13-150 μg/ml).
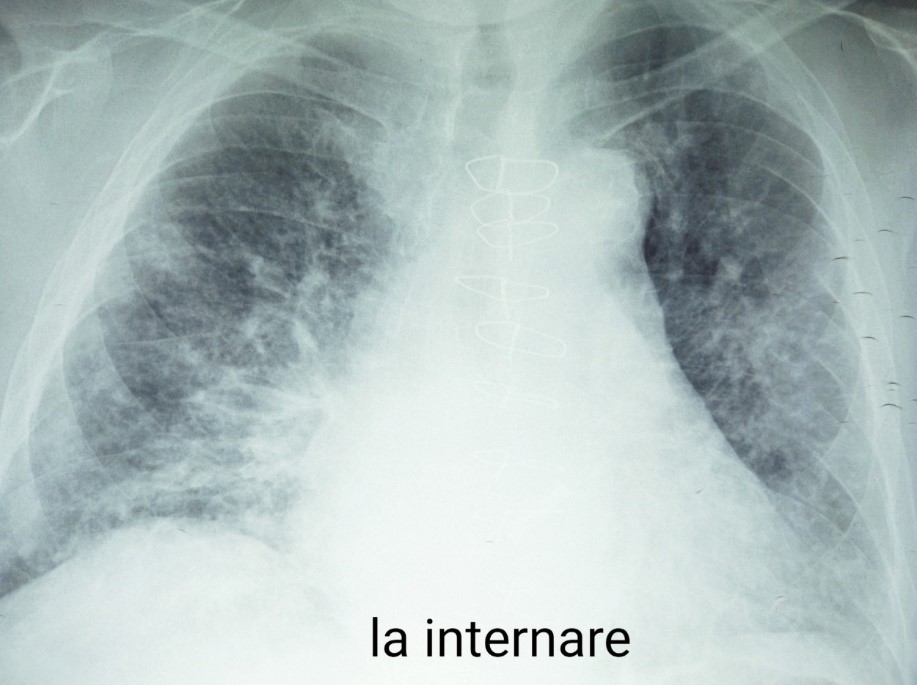
Chest radiography revealed diffuse infiltrates in both lung fields with predominantly subpleural and basal JScholar Publishers Case Reports: Open Access 2023 | Vol 8: 101 disposition, with consolidations in the right middle lung field and left lower lung field - with the appearance of viral interstitial pneumonia (Figure 5).
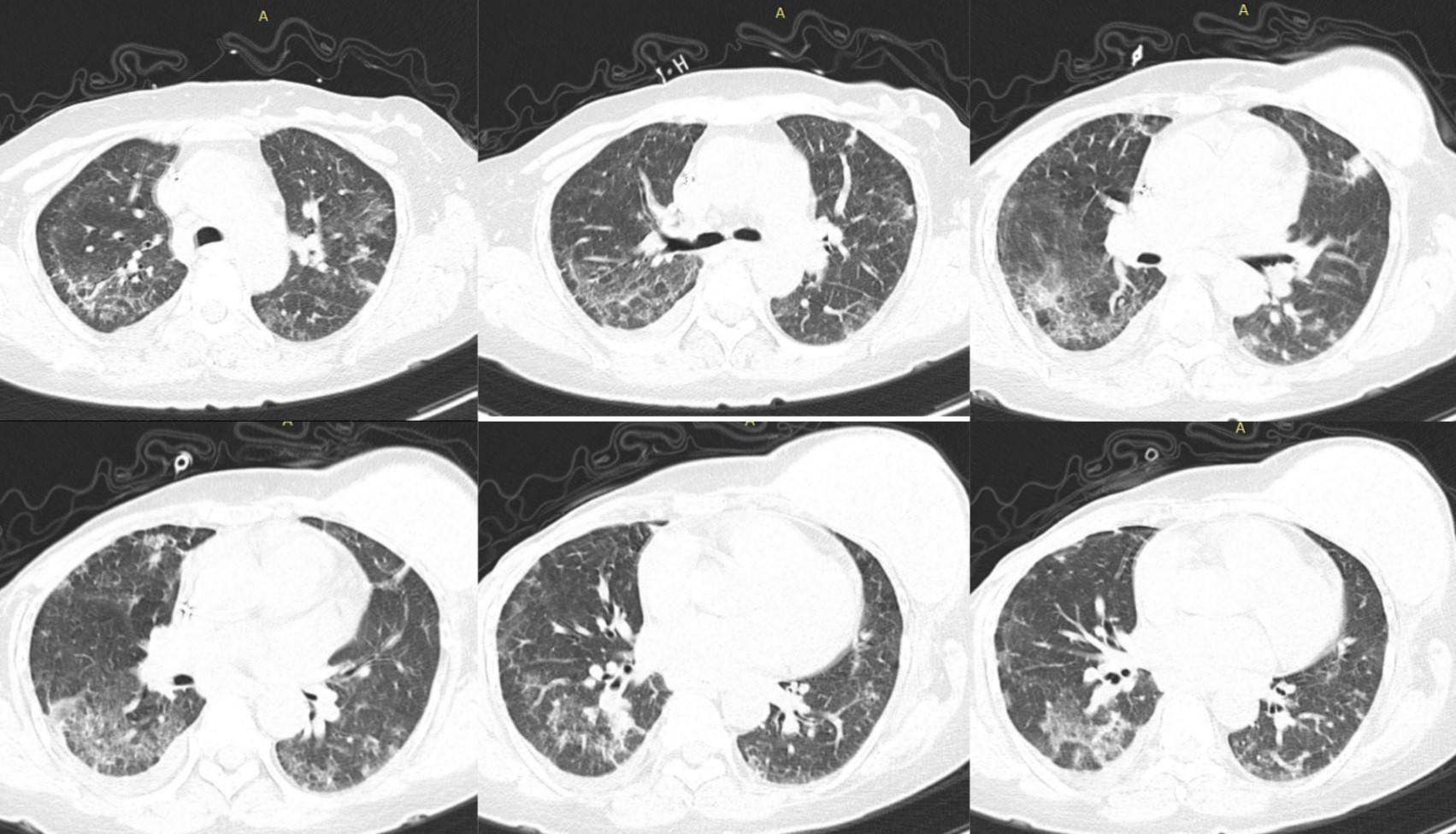
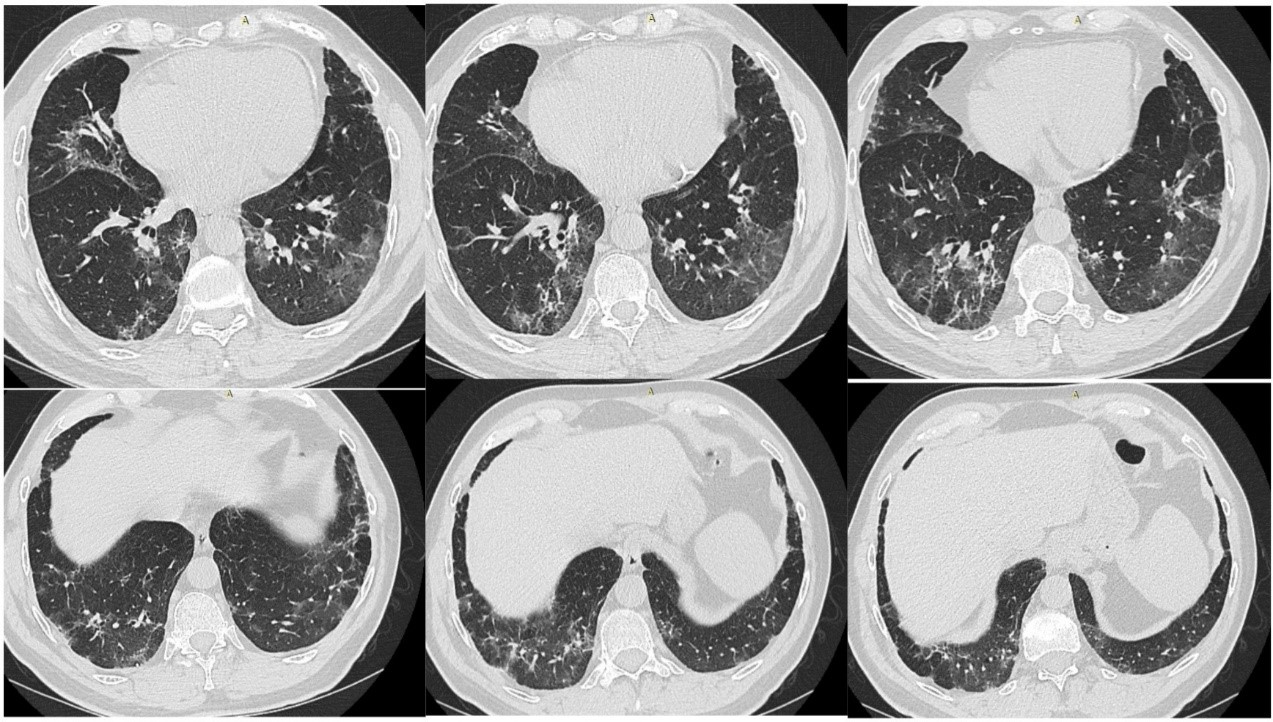
Thoracic computed tomography reveals areas of pulmonary condensation with a "matted glass" appearance, associating the presence of small densifications in the band with a fibrotic appearance, with a relatively symmetrical arrangement, more importantly in the lower lobes, with a predominantly subpleural topography. (Figure 6)
Based on clinical and radiological data, COVID18 infection with interstitial lung involvement was suspected.
The patient was tested by RT-PCR testing of a nasopharyngeal swab sample for SARS-CoV- 2, with positive RT-PCR result and diagnosed as organized pneumonia associated with COVID infection19.
The patient was hospitalized for 14 days, received specific antiviral treatment, systemic corticosteroid therapy, oxygen therapy, with slowly favorable evolution of symptoms, with considerable improvement of oxygenation levels and improvement of biological constants.
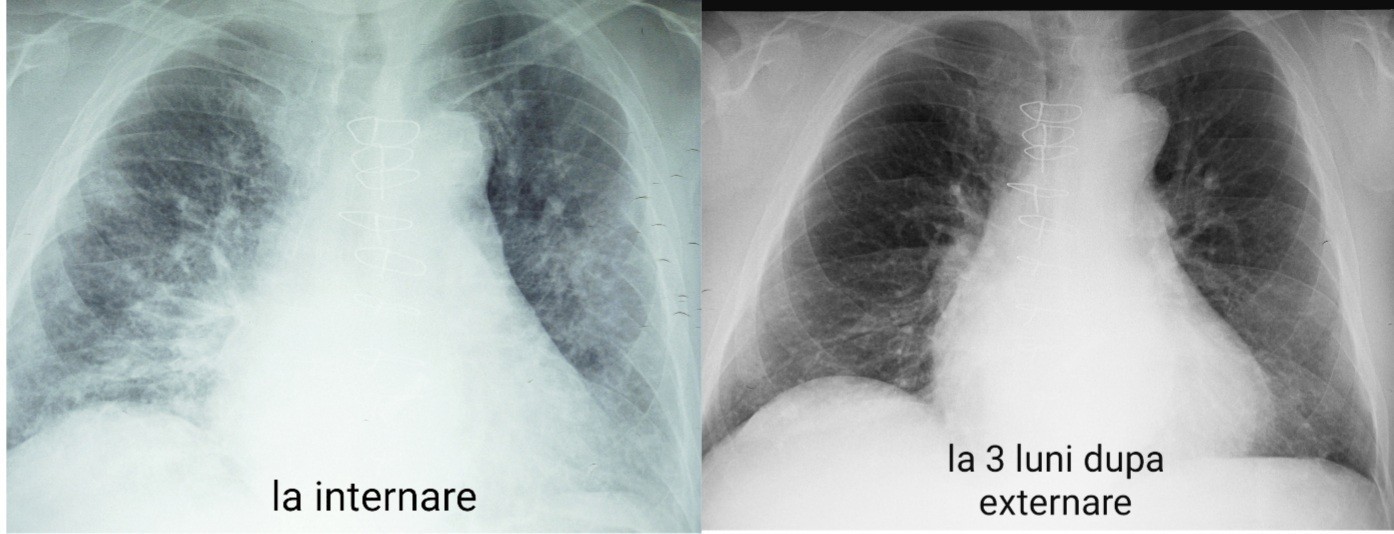
A chest X-ray and a CT scan at discharge showed a marked improvement of the pulmonary infiltrates previously observed.
The patient returned for evaluation 3 months after discharge with no respiratory symptoms, and the follow-up chest radiograph illustrated a significant improvement in pulmonary infiltrates at the 3-month follow-up (Figure 7).
Dicussion
The presented cases arouse interest through the issue of establishing the etiological diagnosis; early diagnosis is of great practical importance, because it allows the timely establishment of treatment that can stop the evolution of the pathological process.
Sarcoidosis is a multisystem inflammatory disease of unknown etiology that predominantly affects the lungs and intrathoracic lymph nodes. Sarcoidosis is manifested by the presence of noncaseating granulomas (NCG) in the tissues of the affected organs. (1)
The incidence varies from 5 to 40 cases per 100,000 inhabitants, in Romania the prevalence of the disease is approximately 4 cases per 100,000 inhabitants, with a higher frequency in the female sex and the 20-40 age group.
Chest X-ray is essential for evaluation (2). Approximately 60-70% of patients with sarcoidosis have characteristic radiological images; sarcoidosis may present with equivocal radiological aspects for this diagnosis, depending on the stage of the disease.
CT scanning is more sensitive than radiography in detecting lymphadenopathy and early parenchymal disease, and high-resolution CT (HRCT) can identify alveolitis or active fibrosis, the results being well correlated with the yield of lung biopsy (3,4,5).
However, in most cases, the diagnosis of certainty requires bronchial biopsy with histological evidence of noncaseating granulomas, with negative special tests for mycobacteria and fungi (6,7).
Prognostic markers of the disease include advanced changes on chest X-ray and the presence of extrapulmonary involvement (predominantly cardiac and neurological) and evidence of pulmonary hypertension (8)
Several studies have demonstrated that the most important marker for prognosis is the initial stage of the chest X-ray, and in stage II of the disease, as in the case of our patient, remission under oral corticosteroid therapy is in 40-70% of cases. (9,10)
In conditions of known malignancy, the presence of the symptoms of excruciating dry cough, sometimes with hemoptysis and progressive dyspnea up to respiratory failure, requires the presence of carcinomatous lymphangitis as a differential diagnosis.
The location of the primary tumor is very varied, the most common being breast cancer 33%, gastric 29%, lung 17%, ovarian adenocarcinoma 6%, pancreas 4%, prostate 3% and, more rarely, in colon cancer, larynx, melanomas , seminomes (11,12,13).
Chest X-ray shows reticulonodular, confluent, bilaterally disseminated, symmetrical opacities, septal thickenings, Kerley A and B lines, areas of subpleural edema (similar manifestations to interstitial edema), hilar and mediastinal adenopathies (20-40% of cases), pleural fluid collections (30-50% of cases). (14)
Pulmonary HRCT, which has a clearly superior sensitivity compared to chest radiography, can highlight: nodular thickening of the interlobular and peribrochovascular septa, associated with alveolar opacities, with peripheral or central distribution, often asymmetric. (15,16)
In the presented patient, ovarian adenocarcinoma was the source of pulmonary metastasis. The respiratory symptomatology given by carcinomatous lymphangitis was the cause that required medical consultation, with the diagnosis of secondary determinations in an advanced stage with reserved prognosis and low chances of survival.
Lung CT was highly suggestive for establishing the diagnosis of carcinomatous lymphangitis, showing the association of peripheral and central accentuated peribronchovascular pattern with images of asymmetric alveolar opacities.
COVID-19 became a global pandemic after the first case of severe acute respiratory syndrome (SARS) coronavirus-2 (SARS-CoV-2) was reported in Wuhan, China in December 2019. While cases continue to rise, questions about the clinical course and long-term implications of the infection remain unanswered.
In the presence of symptoms of acute respiratory infection associated with a picture suggestive of interstitial lung damage, in the current epidemiological context it should be considered as a differential diagnosis with pneumonia associated with the viral infection COVID19.
Although the rRT-PCR test remains the gold standard for the diagnosis of COVID-19, the high false-negative rate limits the prompt diagnosis of this disease. [17–19). Therefore, CT scanning plays a key role in the diagnosis and management of this infectious disease. The imaging changes in COVID-19 pneumonia are diverse: 78-80% "ground glass" opacities, 50% consolidation, interlobulation, septal thickening with fibrotic changes. (20, 21, 22).
As in the case of patient 3 presented, although the clinical symptoms of COVID-19 pneumonia are the same as those of common upper respiratory tract infection, the chest CT examination had a key role in the diagnosis and management of COVID-19 pneumonia.
As medical evidence, CT diagnosis was written in “Diagnosis and Treatment of Pneumonia Infected by 2019-nCoV (implementation process 5th edition)” published by the National Health Commission of the People's Republic of China (23).
However, it is difficult to distinguish COVID-19 pneumonia from other viral pneumonias based on CT results alone, a positive RT-PCR test on a pharyngeal swab, confirming the diagnosis of COVID-19 pneumonia.
The chest CT examination is also of great importance in monitoring the evolution of lung lesions at a distance, studies so far have shown a resolution of lesions of at least 82% in mild and moderate forms of bola and at least 76% for severe forms, at an interval up to 1 month
post-infection. (24, 25) And in the presented case, the radiological evolution of the patient was significantly favorable, with a remission of the lesions of more than 75%, compared to the initial extension.
Despite the highly suggestive radiological Figure of interstitial lung damage that appears in numerous pathological entities, currently there are still limits regarding the definite establishment of a positive diagnosis, sometimes requiring laborious and expensive investigations.
Conclusion
The common feature of all the presented cases is the monomorphic radiological appearance which contrasts with the etiological polymorphism. The differential diagnosis of interstitial lung lesions is often laborious, involving numerous procedures: imaging (conventional radiography, computed tomography with contrast material), bronchoscopy with bronchoalveolar lavage, transbronchial biopsy, transthoracic biopsy, serological and immunological tests, microbiological investigations. Conventional lung radiography remains one of the basic methods in the diagnosis of lung diseases, but sometimes requires the addition of other investigative methods with higher accuracy, aimed at guiding the diagnosis with certainty.
- Ten Berge B, Kleinjan A, Muskens F, Hammad H, Hoogsteden HC, Hendriks RW, et al. (2012) Evidence for local dendritic cell activation in pulmonary sarcoidosis. Respir Res. 13:33.
- Stanton KM, Ganigara M, Corte P, et al. (2017) The Utility of Cardiac Magnetic Resonance Imaging in the Diagnosis of Cardiac Sarcoidosis. Heart Lung Circ. 26: 1191-9.
- Teirstein AS, Machac J, Almeida O, Lu P, Padilla ML, Iannuzzi MC (2007) Results of 188 whole- body fluorodeoxyglucose positron emission tomography scans in 137 patients with sarcoidosis. Chest. 132:1949-53.
- Ahmadian A, Pawar S, Govender P, Berman J, Ruberg FL, Miller EJ (2017) The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J Nucl Cardiol. 24: 413-24.
- Mostard RL, Voo S, van Kroonenburgh MJ, Verschakelen JA, Wijnen PA, Nelemans PJ, et al. (2011) Inflammatory activity assessment by F18 FDG-PET/CT in persistent symptomatic sarcoidosis. Respir Med. 105: 1917-24.
- Trisolini R, Tinelli C, Cancellieri A, Paioli D, Alifano M, Boaron M, et al. (2008) Transbronchial needle aspiration in sarcoidosis: yield and predictors of a positive aspirate. J Thorac Cardiovasc Surg. 135: 837-42.
- Oki M, Saka H, Kitagawa C, Kogure Y, Murata N, Ichihara S, et al. (2012) Prospective study of endobronchial ultrasound-guided transbronchial needle aspiration of lymph nodes versus transbronchial lung biopsy of lung tissue for diagnosis of sarcoidosis. J Thorac Cardiovasc Surg. 143: 1324-9.
- Nardi A, Brillet PY, Letoumelin P, Girard F, Brauner M, Uzunhan Y, et al. (2011) Stage IV sarcoidosis: comparison of survival with the general population and causes of death. Eur Respir J. 38: 1368-73.
- McKinzie BP, Bullington WM, Mazur JE, Judson MA (2010) Efficacy of short-course, low-dose corticosteroid therapy for acute pulmonary sarcoidosis exacerbations. Am J Med Sci. 339: 1-4.
- . Pietinalho A, Tukiainen P, Haahtela T, Persson T, Selroos O (2002) Early treatment of stage II sarcoidosis improves 5-year pulmonary function. Chest. 121: 24-31.
- Anish Thomas, MD and Robert Lenox, MD (2008) Pulmonary lymphangitic carcinomatosis as a primary manifestation of colon cancer in a young adult. CMAJ. 179: 338-340.
- Khachekian A, Shargh S, Arabian S (2015) Pulmonary Lymphangitic Carcinomatosis from Metastatic Gastric Adenocarcinoma: Case Report. J Am Osteopath Assoc. 115: 332-7.
- Deepak Chandra, John Dickinson, Pulmonary Lymphangitic Carcinomatosis in Metastatic Breast Cancer (2017) American Journal of Respiratory and Critical Care Medicine. 195: A5881
- Prakash P, Kalra MK, Sharma A, Shepard JA, Digumarthy SR (2010) FDG PET/CT in assessment of pulmonary lymphangitic carcinomatosis. AJR Am J Roentgenol. 194: 231-6.
- . Stein DL, Freeman LM (2005) Lymphangitic spread of breast carcinoma: scintigraphic pattern with chest x-ray and computed tomography correlation. Clin Nucl Msed. 30: 615- 6.
- Padley S.P.G., Hansell D.M, Jennings P (1991) Comparative accuracy of high resolution CT and chest radiography in the diagnosis of chronic diffuse infiltrative lung disease // Clin. Radiol. 44: 222-6.
- Ai T, Yang ZL, Hou HY, et al. (2020) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology.
- . Xie XZ, Zhong Z, Zhao W, et al. (2020) Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology.
- Yang W, Sirajuddin A, Zhang XC, et al. (2020) The role of imaging in 2019 novel coronavirus pneumonia (COVID-19). Eur Radiol.
- C Liu, J Zhou, L Xia, X Cheng, D Lu (2020) 18F-FDG PET/CT and serial chest CT findings in a COVID-19 patient with dynamic clinical characteristics in different period- Clinical Nuclear Medicine.
- Lei J, Li J, Li X, Qi X (2020) CT Imaging of the 2019 Novel Coronavirus (2019-nCoV) Pneumonia. Radiology. 2020.
- Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, et al. (2020) Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. J Eur Radiol.
- National Health Commission of the People’s Republic of China (2020) Diagnosis and Treatment of Pneumonia Infected by 2019-nCoV. (trial implementation 5th Edition). Chin J Integr Tradit West Med.
- Wanshu Zhang, et al.(2020) The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Int. J. Infection Diseases.
- C Zheng T Ye, L Li, L Li, D Liu, J Wang, Chest CT (2022) patterns from diagnosis to 1 year of follow-up in patients with COVID-19 RL Hesketh, - Radiology

Figures at a glance