Jaundice with Massive Abdominal Mass
Received Date: February 28, 2023 Accepted Date: March 29, 2023 Published Date: April 03, 2023
doi: 10.17303/croa.2023.8.105
Citation: Omar Khamag, Sharon Cox, Ashmitha K Rajkumar (2023) Jaundice with Massive Abdominal Mass. Case Reports: Open Access 8: 1-5
Abstract
Stajano, Fitz-Hugh, Curtis Syndrome is an atypical clinical presentation of upper genital infections, characterized by few pelvic symptoms and perihepatitis determining right hypochondrium pain, tenderness, and "violin strings" hepatophrenic adhesions. This infrequent clinical presentation leads to frequent late or misdiagnoses, such as cholecystitis, appendicitis, urolithiasis, or hepatophrenic abscesses.
In this paper we carried out a historical review of knowledge of this particular clinical presentation.
Keywords: Ginecology; Stajano´s Syndrome; Phrenic reaction in gynecology; Fitz-Hugh; Curtis Syndrome; Chlamydia;Gonococcus
Case report
A 6-week-old female infant presented with 3-days of jaundice and abdominal distension. History revealed pale stools and dark urine for 1 week. Examination confirmed fever, jaundice, a massively distended abdomen and dilated abdominal wall veins. The patient was commenced on intravenous antibiotics and investigated.
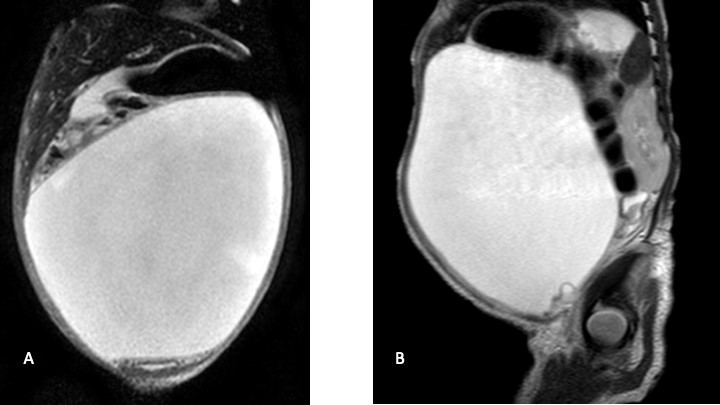
Ultrasound revealed a large, central abdominal cyst with echogenic debris, a collapsed gallbladder and patent IVC and hepatic veins. The portal vein was not visualized. The uterus and bladder were normal. There was no ascites. On MRI (Figure 1) the cyst extended to the porta-hepatis, but no clear biliary cause was found. The differential diagnosis included loculated ascites, and a duplication or mesenteric cyst.
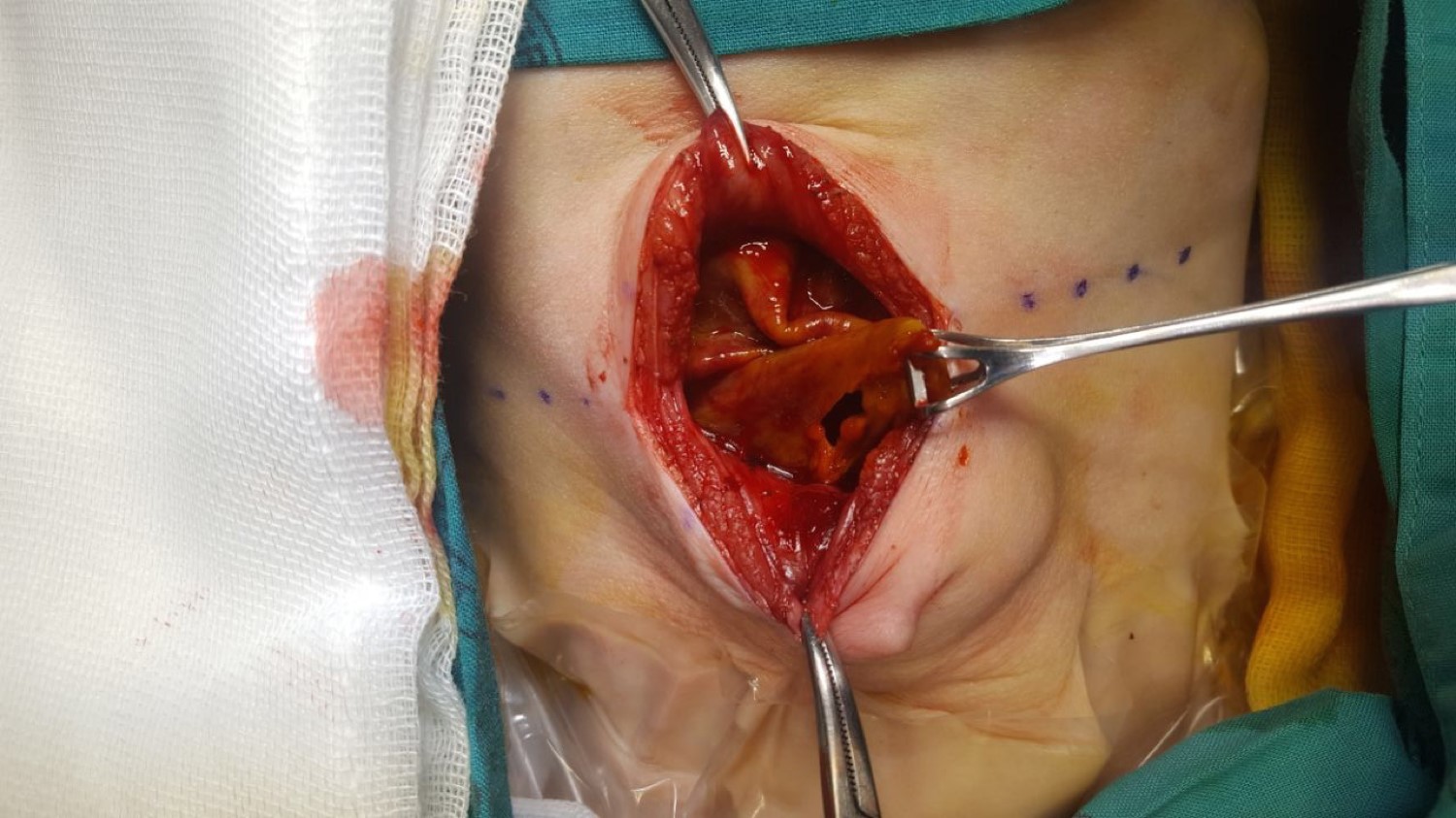
During MRI the patient developed respiratory distress due to abdominal distension - possibly exacerbated by sedation. Resuscitation and emergent surgical exploration followed. Intraoperative findings were that of a massive biloma surrounded by a fibrinous pseudo-capsule (Figure 2). The fibrinous wall was adherent to the bowel and liver and encased the ovaries (Figure 1). An area of bile extrusion was found at the porta-hepatis. Due to surrounding inflammation and adherent cyst wall no further anatomy was delineated. A feeding tube cholecystostomy was flushed with saline to confirm bile duct perforation and was left in situ. After washout, a drain was placed at the porta.
Histology revealed an inflamed fibrous pseudocyst wall with no epithelium, consisting of fibroblastic and granulation tissue, acute haemorrhage and inflammatory infiltrate. Histology of liver showed subcapsular haemorrhage, cholestasis and granulation. There was sinusoidal congestion, periportal inflammation and necrosis
Discussion
Spontaneous bile duct perforation (SBDP) in infants is rare, with limited numbers reported in the literature [1]. With a highly variable presentation, diagnosis and management is challenging, and outcomes can bare significant morbidity.
The presentation varies with age. Younger patients present with abdominal distension due to free biliary ascites, failure to thrive and jaundice [2]. Older patients commonly have an acute presentation with biliary peritonitis or sepsis. Paracentesis and/or HIDA scan can aid in the diagnosis [3], however, in localized biliary ascites, preoperative diagnosis is more difficult.
The most commonly reported site of SBDP is at the junction of the common hepatic and cystic ducts [4]. Possible aetiologies include congenital pancreaticobiliary anomalies, cholelithiasis, infection, pancreatitis, trauma, and ampullary stenosis, with none proven as a single cause and a multifactorial causation is most likely [5]. There are similar suggested aetiologies for spontaneous rupture of choledochal cyst indicating that these two entities might have a common source [6].
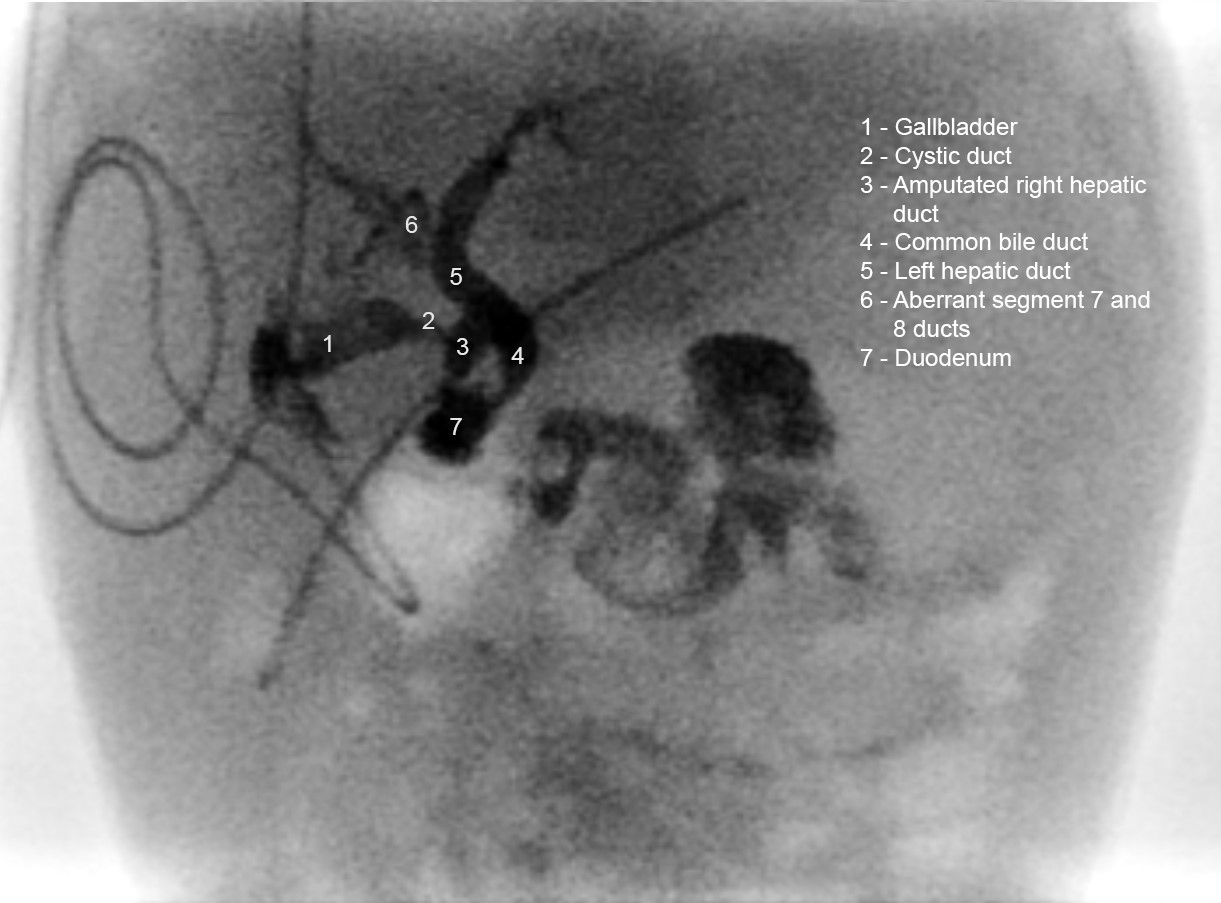
The post-operative course was uneventful. Cultures were negative, and antibiotics were stopped. Feeds were commenced. Initially bile drainage averaged 30ml/kg/day, which subsided over the following 4 weeks. A cholangiogram (Figure 3) performed via the cholecystostomy revealed no leak, but signs of aberrant biliary anatomy with a shortened right hepatic duct and segment 7 and 8 biliary drainage via the left hepatic duct. The patient was discharged after drain removal. The child was well at 1-month follow-up.
Early surgical management with the priority of drainage of biliary peritonitis and sepsis prevention is indicated [7]. Management varies from open or percutaneous drainage with or without suture closure, T-tube drainage, and/or cholecystostomy. An intra-operative cholangiogram may confirm the site of leakage and rule out distal obstruction or a pancreatico-biliary malformation [1].
Portal vein thrombosis is a known complication and can be due to chemical irritation or pressure from extravasated bile near the porta-hepatis, and is more common when the perforation is posterior [7]. The role of anticoagulation in addition to the long-term prognosis of this complication needs further study. Long-term follow-up with ultrasound monitoring for portal hypertension and its sequelae is essential [7].
This case differs from most reported cases due to the encysted bile on presentation. Pre-operative imaging was non-conclusive. Determination of whether the ascites is biliary can possibly be made by hepatobiliary scintigraphy [5]. From a radiological perspective, it is difficult to evaluate the origin of such a large intra-abdominal cyst thus more common causes of cysts were considered. The post-operative cholangiogram shows abnormal biliary anatomy which may have been the underlying cause of the perforation.
The reported case is an example of conservative management with spontaneous healing as opposed to initial bile duct repair. We suggest that, dissection and surgery to the distorted biliary tree are not always required provided that the diagnosis is confirmed, and that simple drainage can be successful. Stricture formation is however a risk and meticulous follow up is suggested.
Conclusion
SBDP represents a diagnostic challenge. Surgical drainage is the mainstay of management. Extensive biliary dissection should be avoided in acute phase. The condition carries favourable prognosis.
- Davenport M, Heaton ND, Howard ER (1991) Spontaneous perforation of the bile duct in infants. The British journal of surgery 78: 1068-70.
- Jeanty C, Derderian SC, Hirose S, Lee H, Padilla BE (2015) Spontaneous biliary perforation in infancy: Management strategies and outcomes. Journal of pediatric surgery 50: 1137-41.
- Malik HS, Cheema HA, Fayyaz Z, Hashmi MA, Parkash A et al. (2016) Spontaneous Perforation Of Bile Duct, Clinical Presentation, Laboratory Work Up, Treatment And Outcome. J Ayub Med Coll Abbottabad 28: 518-22.
- Murphy JT, Koral K, Soeken T, Megison S (2013) Complex spontaneous bile duct perforation: an alternative approach to standard porta hepatis drainage therapy. Journal of pediatric surgery 48: 893-8.
- Goldberg D, Rosenfeld D, Underberg-Davis S (2000) Spontaneous biliary perforation: biloma resembling a small bowel duplication cyst. Journal of pediatric gastroenterology and nutrition 31: 201-3.
- Steiner Z, Dimitrov D (2006) Spontaneous perforation of the common bile duct mimicking choledochal cyst. The Israel Medical Association journal: IMAJ 8: 655-6.
- Pereira ECMV, Yan J, Asaid M, Ferguson P, Clarnette T (2012) Conservative management of spontaneous bile duct perforation in infancy: case report and literature review. Journal of pediatric surgery 47: 1757-9.

Figures at a glance