Adjunctive Therapy with DLBS 1033 Following Catheter-Directed Thrombectomy and Thrombolysis in Acute Pulmonary Thromboembolism
Received Date: May 12, 2023 Accepted Date: June 12, 2023 Published Date: June 15, 2023
doi: 10.17303/croa.2023.8.201
Citation: Muhammad Munawar, Beny Hartono, Intan Toekan, Dian Larasati Munawar (2023) Adjunctive Therapy with DLBS 1033 Following Catheter-Directed Thrombectomy and Thrombolysis in Acute Pulmonary Thromboembolism. Case Reports: Open Access 8: 1-8
Abstract
Pulmonary thromboembolism (PTE) is a life-threatening condition with a high early mortality rate caused by acute right ventricular failure and cardiogenic shock. We reported two patients who presented with acute submassive PTE. They were successfully treated by simple catheter-directed thrombectomy and intra-pulmonary arterial thrombolysis followed by oral DLBS 1030 for 12 weeks. We conclude that oral thrombolytic DLBS 1033 seems promising as an adjunctive therapy for the management of acute PTE after catheter-directed thrombectomy and thrombolysis. The dosing and safety of treatment are yet to be evaluated further in a large randomised clinical trial.
Learning Objective
Acute treatment of PTE remains challenging, because the remaining thrombus after thrombolytic and mechanical thrombectomy can built up over time. The oral thrombolytic DLBS 1033 seems promising as an adjunctive therapy for the management of subacute PTE after catheter-directed thrombectomy and thrombolysis.
Keywords: Pulmonary Thromboembolism; Thrombectomy; Intra-Pulmonary Arterial Thrombolysis; Oral Thrombolysis
Introduction
Pulmonary thromboembolism (PTE) is one of the most common causes of cardiovascular morbidity and mortality behind myocardial infarction and stroke [1]. Trend towards case fatality will decline following the effective therapies, interventions, and adherence to guidelines protocol [2]. In a massive PE, the cornerstones of treatment are reperfusion strategy with systemic and percutaneous catheter-directed thrombolysis, percutaneous embolectomy and surgical embolectomy. There are some reports of an oral thrombolytic showed beneficial as an adjunctive therapy to completely resolve the remaining thrombus after catheter directed thrombectomy [3]. One of the local preparation in Indonesia is DLBS1033 (Disolf ®, Dexa Medica, Jakarta, Indonesia). DLBS1033 is a bioactive protein fraction derived from Lumbricus earthworm through a patented technology of extraction. The earthworm extract possesses eight major proteins with molecular weight below 100 kDa. DLBS1033 has been shown to have antithrombosis activities due to its fibrinogen degradation assay,antiplatelet aggregation, and ex vivo antithrombosis assay; and thrombolytic activities demonstrated by fibrin plate assay and clot lysis assay [4,5].Each tablet contains DLBS1033 Lumbricus rubellus 490 mg. The specific activity was found 1180 U/mg protein.
Here we reported two case series of patient with acute submassive PTE who underwent catheter-based thrombectomy and thereafter continued with oral DLBS1033 for 12 weeks, resulting in a total recovery. The combination of catheter-based thrombectomy and oral DLBS1033 represents a novel and comprehensive treatment strategy for PTE, potentially improving outcomes
Case Background
Case 1
A 46-year-old male with progressing shortness of breath for 12 days before admission. He had no history of hypertension and diabetes, and was heavy smoker since his age of 12 years. He was hemodynamically stable, but had lower peripheral oxygen saturation 88% in the room air.
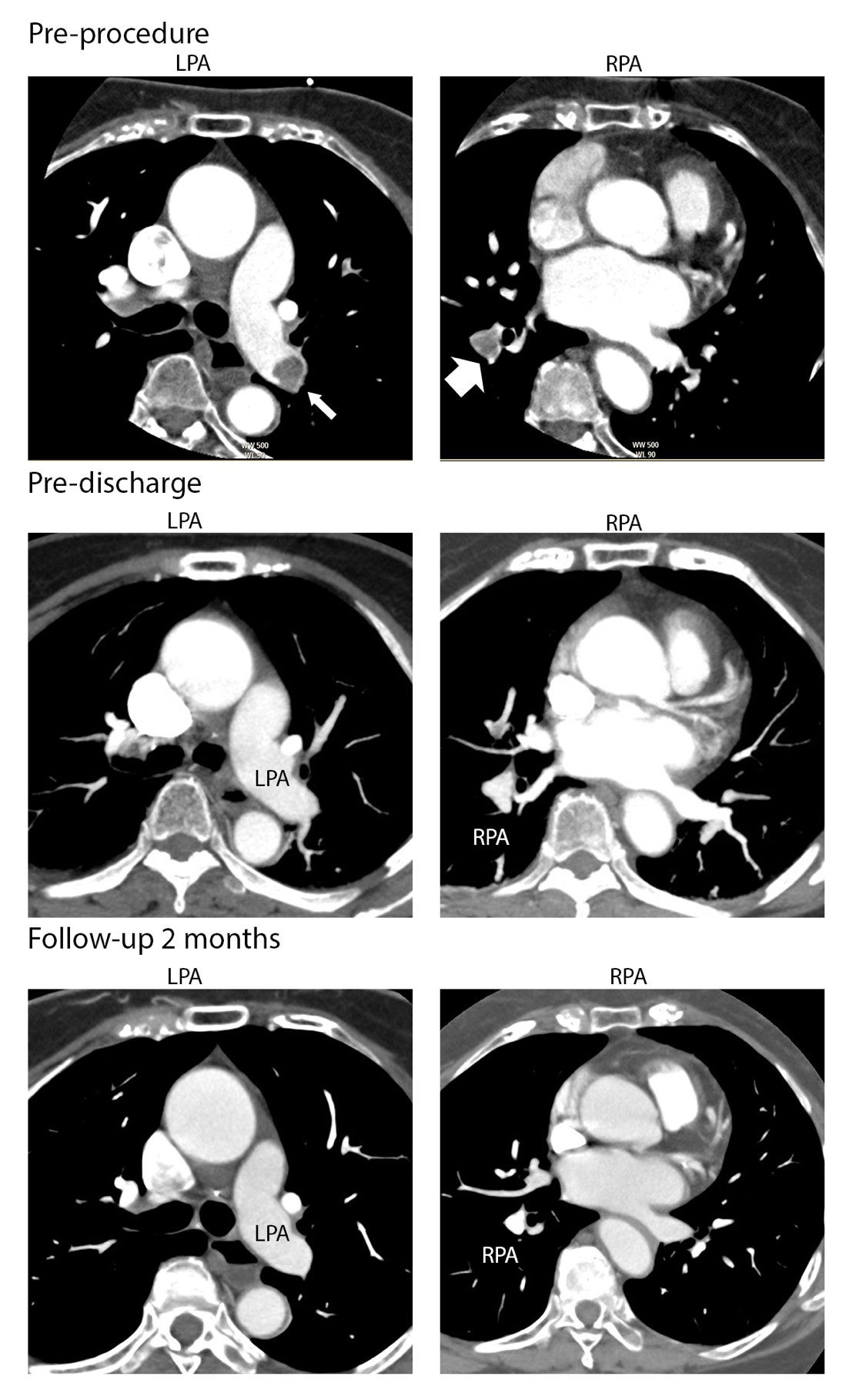
There was normal jugular venous pulsation (JVP) and had no clinical evidence of deep vein thrombosis (DVT). The electrocardiogram (ECG) showed T wave inversion in inferior lead and anterior lead (V2-5). Laboratory results showed a high level of D-dimer (4800 ng/ml), normal value of platelet counts, bleeding time, clothing time, prothrombin time (PT), activated partial thromboplastin time (aPTT) and increased of NT-proBNP (547 pg/ml). The computed-- tomography of pulmonary angiogram (CTPA) demonstrated dilatation of the main pulmonary artery (PA) and extensive thrombi in both right and left PA (Figure 1, pre-procedure).Thrombectomy and intra-pulmonary arterial thrombolysis followed by oral administration of DLBS 1033 was planned and the written informed consent was obtained from patient. Simple catheter-directed thrombectomy followed intra-pulmonary arterial thrombolysis was performed and was reported elsewhere [6]. The basic principle of procedure described as follow. Cardiac catheterization was performed via right femoral vein access and an 8Fr vascular sheath was inserted. A guiding catheter (GC) 8Fr Multipurpose A-1 (MPA-1) (Cordis, Johnson & Johnson Company, USA) was advanced to main PA and PA pressure of 61/25 mmHg was recorded. Catheter-directed intervention was proceeded and guided by CTPA finding. A selective right pulmonary arteriography was performed using the GC MPA-1/8Fr that also revealed the presence of extensive thrombus. Aspiration and fragmentation of the thrombus was performed first with GC MPA-1/8Fr and then replaced with JR3.5/8 Fr (Terumo Co., Tokyo, Japan). The reason why we used both GCs MPA-1 and JR3.5 was because each of these two GCs has preference for reaching to certain branches of PA. With this technique, most of the major branches of PA could be reached and therefore more thrombus could be fragmented and aspirated. This manoeuvre was repeated in the left PA. Following aspiration, a significant amount of thrombus material was collected. Then followed by catheter-directed thrombolytic intra-main pulmonary arterial with Streptokinase 500.000 unit within 15 minutes and 100.000 unit per hour for the next 10 hours. Mean PA pressure was decreased significantly from 37 mmHg to 24 mmHg after the IV thrombolytic.
Repeated CTPA 5 days after the thrombectomy showed minimal remaining thrombus at the inferior segment of the right and left PA (Figure 1, pre-discharge). He was discharged with DLBS1033 at a dose as recommended by Dexa Medica Company i.e. 1 tablet three times daily,along with dual antiplatelets (clopidogrel and aspirin). After a month follow-up, the repeated CTPA still showed increasing thrombus size at the inferior segment of the left PA that caused 80% stenosis and minimal thrombus at the inferior segment of the right PA (Figure 1, follow-up 1 month). We increased the dose of DBLS1033 3 tablets three times daily, considering the remain high level of d-Dimer (3810 ng/mL) and normal value of conventional coagulation test i.e. platelet counts, PT and aPTT. Follow-up CTPA 2 months later showed a minimal thrombus at the inferior segment of left PA and complete resolution at the right PA (Figure 1, follow-up 3 month). The patient tolerated well with the dosing and did not report any clinical adverse and bleeding event. The patient was then advised for doing regular exercise.
Case 2
An 80-year-old female with progressing shortness of breath since a week before being admitted to a hospital was then referred to us for further evaluation and treatment. At presentation she was hemodynamically stable, but had shortness of breath with lowered oxygen saturation of 90% in room air. There was normal JVP and had no clinical evidence of DVT. The ECG showed normal sinus rhythm, with S1Q3T3 sign. Laboratory results demonstrated a high level of D-Dimer (>5000 ng/ml), normal value of platelet counts, PT, aPTT, and increased of NT-proBNP (559 pg/ml).The echocardiography noted a sign of pulmonary hypertension with the right ventricular dilatation. The CTPA showed a large thrombus covering the right PA and left inferior segment of the PA (Figure 2, Pre-procedure). Catheter based pulmonary thrombectomy was performed as described above and followed by intra-pulmonary arterial thrombolysis with Streptokinase for the next 10 hours. Following aspiration, a significant amount of thrombus material was collected. After thrombus aspiration, there was a significant decrease of the mean PA pressure from 26 mmHg to 19 mmHg. However, there were remnant thrombus in the both bilateral inferior segment of PA. Ten hours after catheter- directed thrombolysis, the mean PA pressure slightly sloped down to 18 mmHg.
A follow-up CTPA 3 days after thrombectomy andintra-pulmonary arterial thrombolysis, still demonstrated residual thrombus in the inferior segment of left PA (Figure 2, pre-discharge). She was discharged with continuing DLBS 1033 dose 3 tablets three times daily for 12 weeks, along with the dual antiplatelet: aspirin 80 mg and clopidogrel 75 mg daily. The conventional coagulation tests were within normal. The written informed consent was obtained from the patient.
The CTPA 2 months later showed a complete thrombus resolution at the right PA and left inferior segment of PA (Figure 2, follow-up 2 months). During the treatment period, she tolerated well with DLBS1033 and reported no clinical adverse event. She was advised for doing regular exercise.
Discussion
Acute PTE can be devastating and fatal if it is not treated aggressively. Initial risk assessment to assess clinical symptoms and hemodynamic instability determined further reperfusion treatment. According to the simplified Pulmonary Emboli Severity Index, both of our patients had the score>1, which was correlated to a mortality risk of 10.9 % [7], and would be categorized as high risk or intermediate high risk. In such cases, a reperfusion strategy with catheter- directed thrombectomy and thrombolysis should be the strategy of choice in the availability of expertise and facility [2]. The percutaneous catheter-directed reperfusion are used for mechanical fragmentation and thrombus aspiration to reduce quickly the PA pressure and RV dilatation.6 This strategy would also reduce the dose of catheter-directed thrombolysis following the fragmentation of thrombus [2]. An adjuvant therapy with an anticoagulant is recommended for further dissolve the remaining thrombi.
In our cases, both patients underwent percutaneous catheter-directed treatment following intra-pulmonary artery thrombolysis. However, that procedure alone did not completely resolve all thrombi, and the remaining thrombi could be reaccumulated to cause subacute and chronic PTE. In previous report, the use of oral Lumbrokinase (Thromboles®, The Institute of Biophysics, Chinese Academy of Sciences, Beijing, China) for 12 weeks after discharged completely resolved of thrombi in the PA [3]. In our current cases, we demonstrated the use of DLBS 1033, which is a bioactive protein fraction containing lumbrokinase derived from Lumbricus rubellus, a local earthworm. Despite the pharmacological similarity of DLBS 1033 with previous Lumbrokinase derived from earthworm of China,a study reported that DLBS 1033 had an unique characteristic, owing its specific protein profile, that differentiate this bioactive protein fraction from previously available Lumbrokinase [4]. DLBS 1033 possessed quadruple activities that inhibit platelet aggregation, induces fibrinogenolysis, fibrinolysis, and thrombolysis [4], with a longer biological half-life than the conventional thrombolytic agents [5]. A previous study reported that DLBS 1033 at the dose 490 mg three times daily was evidently safe and tolerable in healthy adult patients [5]. In our cases, we tripled the dose to increase the efficacy but apparently such a higher dose did not increase the bleeding event. In Case 1, the recommended dose of three times 1 tablet daily of 490 mg showed less efficacious with the remaining thrombi built up. But after increasing the daily dose to three times of 3 tablets (1470 mg), the complete resolution of all thrombi was observed. In the ex-vivo coagulation model using human blood clot induced by thrombin demonstrated that the consistency of blood clot is significantly reduced with dose dependent of DLBS 1033.5 Studies have shown that DLBS 1033 inhibits platelet activation and aggregation and blocks the intrinsic coagulation pathway.4 Unlike t-PA, DLBS 1033 exhibits thrombolytic activity only in the presence of fibrin [4,5]. The mechanism underlying this is complex, however from the models develop using human blood clot where the main component is fibrin showed that the DLBS 1033 could penetrate complex environment of thrombus and resulted in fibrindegradation [5]. Therefore, DLBS 1033 has the advantage of minimizing the excessive bleeding.
In the ESC guideline 2019 for the management of PTE, anticoagulant treatment after an acute phase of PE should be continued until 3 months [2]. The use of vitamin K antagonist (VKA) or a novel oral anticoagulant (NOAC) are recommended. In our cases, we substituted the oral anticoagulant with the oral DLBS 1033, which showed a satisfaction result without excess of bleeding. The rational of using oral DLBS 1033 are the unique properties of plasminogen activator and fibrin-specific thrombolytic agent, that will dissolve remaining thrombus in the subacute phase, and inhibition of platelet activation and intrinsic pathway to prevent the clot built up in the certain using time. However further clinical trials with sufficient number of patients to conclude this finding is necessary. There is still no consensus of the ideal therapeutic dose of DLBS1033 as an adjunct treatment of PTE and the long-term safety for the high dose of DLBS 1033 is still to be evaluated. But from our experience the dose of 3 times 3 tablets of DLBS 1033 daily (each tablet contain 490 mg DLBS 1033) is effective to completely resolve the remaining thrombus in the PA without an excessive bleeding risk.
There are some challenges and limitation in these case series report. First, the assessment of patients bleeding risk profile only from the conventional coagulation test i.e. platetlet counts, BT, CT, PT and aPTT test. We did not assess the thromboelastography before increasing the dose to triple due to unavailability in our laboratory. Although it might potentially underestimate the patient’s bleeding risk profile, the increment of the dose did not cause any bleeding events. Second, our finding is based on only two patients, so our procedure and observations need further evaluation and verification in a greater number of patients.
In conclusion, adjunctive therapy with oral thrombolytic DLBS 1033 seems promising as an adjunctive therapy for the management of acute PE after catheter directed thrombectomy and thrombolysis. The dosing and safety of DLBS 1033 yet to be tested further in a large randomised clinical trial.
Conflict of Interest
All authors have no conflict of interest.
- Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A et al. (2014) Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol 34: 2363-71.
- Konstantinides SV, Meyer G, Becattini C, Bueno H,Geersing GJ (2020) 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J 41: 543-603.
- Ahmed K, Munawar M, Munawar DA, Hartono B (2015) Impact of oral thrombolysis after catheter-based thrombectomy in acute and subacute submassive pulmonary thromboembolism. Chin Med J (Engl) 128: 401-3.
- Tjandrawinata RR, Yunaidi DA, Susanto LW (2016) The Safety and Tolerability of Lumbrokinase DLBS1033 in Healthy Adult Subjects. Drug Res (Stuttg) 66: 293-9.
- Trisina J, Sunardi F, Suhartono MT, Tjandrawinata RR (2011) DLBS1033, a protein extract from Lumbricus rubellus, possesses antithrombotic and thrombolytic activities. J Biomed Biotechnol 2011: 519652.
- Ahmed K, Munawar M, Andina Munawar D, Hartono B (2014) Simple mechanical thrombectomy with intrapulmonary arterial thrombolysis in pulmonary thromboembolism: a small case series. J Geriatr Cardiol 11: 349-53.
- Jimenez D, Aujesky D, Moores L, Gomez V, Lobo JL et al. (2010) Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 170: 1383-9.


Figures at a glance