Metformin-Associated Lactic Acidosis Following Dangerous Interaction with Xigduo: A Case Report
Received Date: January 27, 2024 Accepted Date: February 27, 2024 Published Date: March 01, 2024
doi: 10.17303/croa.2024.9.101
Citation: Sherley M. Rosa MD, Maria C. Perez MD, Jennifer Ramirez MD, Noel Torres MD, Monica Santiago MD, et al. (2024) Metformin-Associated Lactic Acidosis Following Dangerous Interaction with Xigduo: A Case Report. Case Reports: Open Access 9: 1-6
Abstract
Metformin is an oral antidiabetic drug frequently used among specialists and subspecialists. Its toxicity is rare, but it may occur in the setting of supratherapeutic levels, especially in patients with renal failure. Metformin-associated lactic acidosis (MALA) is a complication characterized by elevated lactate and hydrogen metabolism, increasing the mortality rate by up to 50% if not identified and treated. We reported a 64-year-old female with a history of diabetes mellitus type 2 who presented to the Emergency Room with anuria of two days of evolution. Medication reconciliation provided vital information about the concomitant use of metformin 1000 mg orally twice a day with dapagliflozin/metformin (Xigduo) 5/1000 mg orally daily. The patient was hemodynamically stable and he had level 2 hypoglycemia. Chemistry was remarkable for an acute kidney injury stage III with creatinine levels more than 3-fold of her baseline with hyperlactatemia (10 mM) along with severe hyperkalemia with ECG changes. The patient was admitted to the intensive care unit for emergent hemodialysis. Finally, she was discharged home with improving renal function, adequate urine output, normalization of electrolytes, and acidemia. Clinicians need to be aware of possible side effects occurring when combining antidiabetic medications.
Keywords: Metformin; Lactic Acidosis; Xigduo; Antidiabetic Drug; Metabolism
Introduction
Metformin is an oral antidiabetic drug, approved in 1994 for treating Type 2 Diabetes Mellitus by the U.S. Food and Drug Administration [1]. A common side effect is lactic acidosis which is a condition that is commonly induced by a medical overdose or pharmacological combination. Patients at risk include those with a serious infection, kidney or liver failure, recent surgical procedures, hypoxemia, arterial or venous insufficiency, heavy alcohol use, dehydration, imaging procedures that require an injectable iodinated contrast drug, and the elderly. Metformin-associated lactic acidosis (MALA) occurs when there is an imbalance between increased lactate production and impaired metabolism/reduced clearance [1].
Metformin toxicity is rare, but it may occur in the setting of supratherapeutic levels, especially in patients with hepatic or renal dysfunction due to decreased excretion. The expected incidence of hyperlactatemia in patients with metformin prescriptions annually is 6 cases per 100,000 patients. The fatality rate of this uncommon but dangerous adverse effect is more than 30% [2]. Metformin is mostly eliminated by the kidneys and follows a multiphasic elimination; in patients with normal renal function, the half-life is initially 4 to 8 hours, and the terminal half-life is approximately 20 hours [3].
Case Report
We discussed a 64-year-old female with a history of diabetes mellitus type 2 who presented to the emergency room with hypoglycemia and anuria for the past 2 days. A review of the systems was negative for abdominal pain, nausea, vomiting, diarrhea, and urinary symptoms. The patient's physical examination was unremarkable and hemodynamically stable. Her vital signs were 135/95 mm Hg, 85 beats per minute, 16 breaths per minute, and 36.7°F.
Patient’s persistent hypoglycemia was refractory to frequent replacements provided in the emergency department. This unusual presentation of refractory hypoglycemia made the medical team rule out the multiple causes of acute hypoglycemia in this patient. Such as Imaging, physical exam, and inflammatory markers were negative for possible infectious processes. The hepatic panel, amylase, and lipase were negative which ruled out autoimmune conditions. The patient consumed a high-carbohydrate meal in the presence of the medical team which ruled out starvation as the etiology of her hypoglycemia. Physical examination ruled out gastroparesis diagnosis. Medication reconciliation provided vital information about the concomitant use of metformin 1000 mg orally twice daily with dapagliflozin/metformin (Xigduo) 5/1000 mg daily for the past 5 days
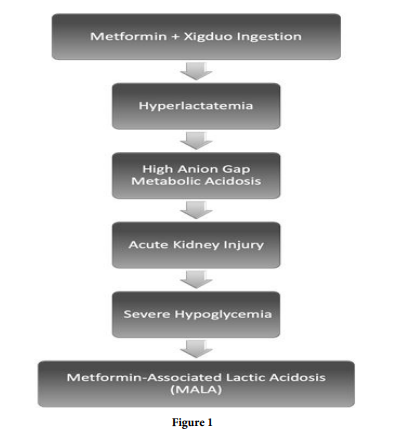
Patient was admitted to the Medical Intensive Care Unit where a Foley catheter was placed and urine output remained less than 0.5cc/kg/hr. The arterial blood gasses reported a compensated high anion gap metabolic acidosis with a pH of 7.17, a bicarbonate of 7.5 and partial pressure of carbon dioxide (pCO2) of 20.9. Chemistry was remarkable for an acute kidney injury stage III with creatinine levels more than 3-fold of her baseline from 3 weeks ago (Table 1). The patient also presented with hyperlactatemia of 10 mmol/L and severe hyperkalemia with peaked T waves. The toxicology and ketones were negative which excluded intentional ingestion or isolated diabetic ketoacidosis (DKA). The abdominal computerized tomography scan was unremarkable as well. Aggressive intravenous hydration was provided to evaluate the possibility of occult shock but lactic levels increased and renal function worsened despite resuscitative measures. Incidental overdose of metformin, refractory hypoglycemia, lactic acidosis, acute kidney injury stage 3, and gradual onset of symptoms provide strong evidence for Metformin-associated lactic acidosis or MALA (Figure 1).
Nephrology service was consulted and after failure to improve despite standard treatment, she was admitted to the intensive care unit for emergent hemodialysis. Finally, she was discharged home with improving renal function, adequate urine output, and normalization of electrolytes and acidemia (Table 1). The patient’s renal function did not recover completely, her creatinine is still not at her baseline, and is being closely followed up outpatient due to diagnosis of Chronic kidney disease stage.
Discussion
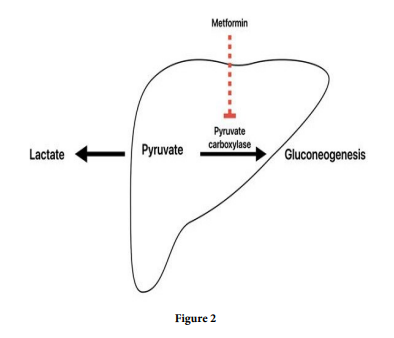
MALA is diagnosed when blood lactate concentration is >5 mmol/L and pH blood level is < 7.35 [3], which our patient meets criteria. MALA occurs when there is an imbalance between increased lactate production and impaired metabolism/reduced clearance which can occur acutely in an overdose but usually has a more gradual onset, typically causing nausea, abdominal pain, tachycardia, hypotension, and tachypnea. This complication is characterized by elevated lactate and hydrogen metabolism, increasing the mortality rate. The lactic acidosis from metformin is a mixed type A and B is seen in most cases. MALA pathophysiology results from the blockage of pyruvate carboxylase, the initial step in gluconeogenesis, which converts pyruvate to oxaloacetate, leading to the accumulation of lactic acid (Figure 2). The resultant metabolic acidosis can be severe and lead to a shock state with depressed hemodynamics [4]. The acidotic state can lead to impaired myocardial contractility, decreased function of catecholamines, and subsequent multiorgan dysfunction [5]. As the acidosis progresses and shock develops, concurrent renal and liver failure may contribute to the onset of MALA by decreased metformin and lactate clearance, respectively [4]. In individuals with severe lactic acidosis caused by metformin, hemodialysis improves acid-base balance and clinical outcomes.
Xigduo and Metformin combination therapy is generally discouraged due to one of the principal components of Xigduo being Metformin itself. The complication of this dual therapy has yet to be studied in the general literature since they are not advised to be taken together due to the increased risk of MALA and nephrotoxicity. A lawsuit was launched in June 2020 against AstraZeneca, the developer of Xigduo, for receiving information about infections and other health issues linked to the medication for years but failing to alert the medical community [6]. Necrotizing fasciitis of the genital/perianal/gluteal areas, ketoacidosis, severe renal failure, and lower limb amputations are among the adverse consequences found [7]. Multiple preventive measures can be implemented in the population to prevent these cases from occurring. A single electronic health records for patient's prescription medications can be implemented in hospitals nationwide to strengthen medication reconciliation between providers.
Conclusion
Excessive plasma levels of metformin are a required predisposing factor for MALA, alternate strategies of administering metformin to high-risk diabetic patients that reduce systemic exposure while preserving glycemic effectiveness are recommended [1]. Metformin can still be used in patients with chronic kidney disease without dose adjustment if the glomerular filtration rate (GFR) is above 30 ml/min/1.73 m⋀ 2, but kidney function must be monitored every 3 to 6 months. Patients should avoid ethanol consumption while taking metformin to avoid the development of MALA and hepatotoxicity [4]. Future therapeutic strategies that target pH might be a new approach to lactic acidosis management [6].
We aim to highlight the importance of medication reconciliation, interdisciplinary communication, and collaboration between physicians, and an adverse effect that could have been avoided. Physicians should be diligent when prescribing to account for other medications, medical comorbidities, age, and nutrition status [4]. We hope to prevent a similar outcome in future patients by raising awareness.
Acknowledgments
None
Declaration of Conflicting Interests
There is no conflict of interest in this article
Funding
This case report did not receive any specific grant from any funding agency in the public, commercial or nonprofit sectors.
- DeFronzo R, Fleming GA, Chen K, Bicsak TA (2016) Metformin-associated lactic acidosis: Current perspectives on causes and risk. Metabolism: clinical and experimental, 65: 20-9.
- Blough B, Moreland A, Adan Mora J (2015) Metformin-induced lactic acidosis with emphasis on the anion gap. Proceedings (Baylor University. Medical Center), 28: 31-3.
- Ayoub P, Hétu PO, Cormier M, Benoit A, Palumbo A, et al. (2017) Toxicokinetics of Metformin During Hemodialysis. Kidney international reports, 2: 759-62.
- Dyatlova N, Tobarran NV, Kannan L, et al. (2023) Metformin Associated Lactic Acidosis (MALA) [Updated 2022 Sep 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan. MALA
- Kimmoun A, Novy E, Auchet T, Ducrocq N, Levy B (2015) Hemodynamic consequences of severe lactic acidosis in shock states: from bench to bedside. Critical care (London, England), 19: 175.
- Newton Media. (2023, August 12). AstraZeneca defends diabetes medications in triple lawsuits. Life Sciences IP Review. https://www.lifesciencesipreview.com/news/astrazen eca-defends-diabetes-medications-in-triple-lawsuits-5316
- U.S. Food And Drug Administration. (2018, September 29). FDA warns about rare occurrences of a serious infection of the genital area with SGLT2 inhibitors for diabetes. Drug Safety Communications. https://www.fda.gov /drugs/- drug-safety-and-availability/fda-warns-about-rare-occurren

Tables at a glance
Figures at a glance