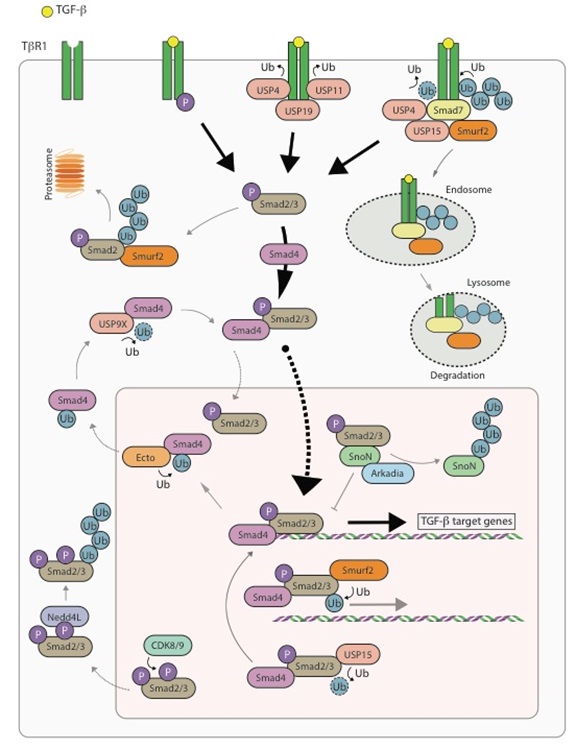
Figure 1: Ubiquitin regulation in the TGF-β pathway. Upon TGF-β stimulation, transforming growth factor β-receptor II (TβRII) binds and phosphorylates type I receptor (TβRI). Phosphorylation of TβRI leads to the recruitment and phosphorylation of regulatory SMADs, SMAD2/3, which then bind to SMAD4. The SMAD2/3-SMAD4 complex is translocated to the nucleus where it binds to SMAD binding elements on the chromatin and initiates transcription of TGF-β target genes. One of the target genes is inhibitory SMAD, SMAD7. Through a negative feedback loop, SMAD7 binds to the E3 ubiquitin ligase, SMURF2, which ubiquitinates the TβR complex leading to ubiquitin- mediated degradation of complex. SMAD7 also recruits the deubiquitinating enzyme USP15, which removes ubiquitin moieties off of TβRI. The balance between USP15 and SMURF2 activities determines the stability of the TGF-β receptor complex. High USP15 concentrations promote TβRI stability, high TGF-β activity and tumour progression in cancer. Like USP15, other deubiquitinating enzymes have been shown to deubiquitinate the TβR complex such as USP4, USP11 and USP19. Activated SMAD2/3 are polyubiquitinated by SMURF2 in the cytoplasm and mono-ubiquitinated in the nucleus, both events lead to attenuation of downstream signalling. Inside the nucleus, SMAD4 can be mono-ubiquitinated by yet another E3 ubiquitin ligase named ECTODERMIN that sends SMAD4 back to the cytoplasm. Herein, the ubiquitin moiety can be removed by USP9X, which recycles Smad4 for further downstream signalling. The transcriptional corepressor SnoN can bind to SMAD2/3 thereby preventing it from transcribing TGF-β target genes. ARKADIA has been shown to positively regulate the TGF-β signalling by binding to and polyubiquitinating SnoN. Activated SMAD2/3 can also be phosphorylated by CDK8/9, an event that temporarily leads to enhanced signalling, however, CDK8/9 phosphorylated SMAD2 is specifically recognized by NEDD4L in the cytoplasm, which mediates its ubiquitin-dependent degradation.