Alternative Cell Culture-Based Methods for Animal use in Liver Oncology
Received Date: February 13, 2023 Accepted Date: March 13, 2023 Published Date: March 16, 2023
doi: 10.17303/jcrto.2023.11.102
Citation: Alex José de Melo Silva, Sheilla Andrade de Oliveira, Danielle Maria Nascimento Moura, Helotonio Carvalho,Cleonilde Maria do Nascimento et al. (2023) Alternative Cell Culture-Based Methods for Animal use in Liver Oncology. J Cancer Res Therap Oncol 11: 1-19
Abstract
The scientific progress required for biotechnological advances is associated with the need for more predictive alternative methods that can replace or reduce the number of animals used in biomedical research. In preclinical studies on hepatocarcinoma,cell cultures are used as a tool in research on cellular and molecular mechanisms, cytotoxicity assays, drug screening and new therapies. Cell cultures are mostly performed with commercially available immortalized cell lines. Cultures can be performed in two-dimensional monolayers (2D) or in spheroid cell cultures (3D), in which cells grow in a three-dimensional system with zones of cellular heterogeneity, microenvironment formation and differential exposure to gradients and nutrients.In scientific research, prior knowledge about the culture model and the cell lines to be used is relevant, since the findings must be analyzed in light of the biological profile of the method and cells being used.
Keywords: Cell Culture; Alternative Methods; Liver Oncology
Introduction
Hepatocellular carcinoma (HCC) is an epithelial neoplasm derived from hepatocytes. The development of HCC can be due to different risk factors, such as infection by viruses B, C or D, excessive alcohol consumption [1],metabolic syndrome [2], obesity [3], non-alcoholic steatohepatitis and autoimmune liver diseases [4,5]. Factors such asaflatoxin intake [4], smoking [6] and air pollution [7] also influence the biological behavior of liver tumors. In general,liver diseases result from persistent aggressive stimuli that incite an inflammatory response, generating liver damage that leads to the formation of fibrous scar tissue with possible evolution to cirrhosis, which may have an established association with HCC [8, 9]. There are different treatments for HCC, and they are associated with the stage of the patient’s disease; they can be curative (tumor resection) or palliative (chemotherapy, target therapy and radiation) [6].Chemotherapy is used when surgical resection of tumors is not possible. It can be administered by using a single drug or a combination of them [10]. Among the commercial chemotherapeutics, 5-fluorouracil (5-FU) [11], and kinase inhibitors such as sorafenib tosylate, lenvatinib, Ramucirumab are currently used [6,12]. This kind of therapy can inhibit the growth and proliferation of tumor cells [13]; however, the high cost, the side effects, the low response rate of patients with combined hepatocarcinoma, and a new perspective of individualized therapy led the community research to seek new therapeutic alternatives for liver tumor treatment [14].
A fundamental part in the search for new anticancer compounds (natural or synthetic) is to predict the toxic effects prior to the study of the therapeutic action. In this scenario, cell culture is an important technique in carrying out toxicological and drug mode of action assays, which can be conducted in a controlled manner and provide information on protein expression and transportation, enzyme regulation, hepatotoxicity, cytotoxicity, genotoxicity, cellular mechanisms, oxidative stress and drug action [15, 16].
For a long time, primary cultures of human or murine hepatocytes were used for in vitro studies; however, advances in this technique have brought alternatives, e.g., obtaining immortalized cell lines from liver carcinomas and/or hepatoblastomas [17]. In this context, the use of cell lines as biological models has become an alternative method to the use of animals in scientific research [18]. In the field of tissue engineering, one can carry out genetic manipulation of cells, use stem cells from different sources, as well as use induced pluripotent stem cells [16].
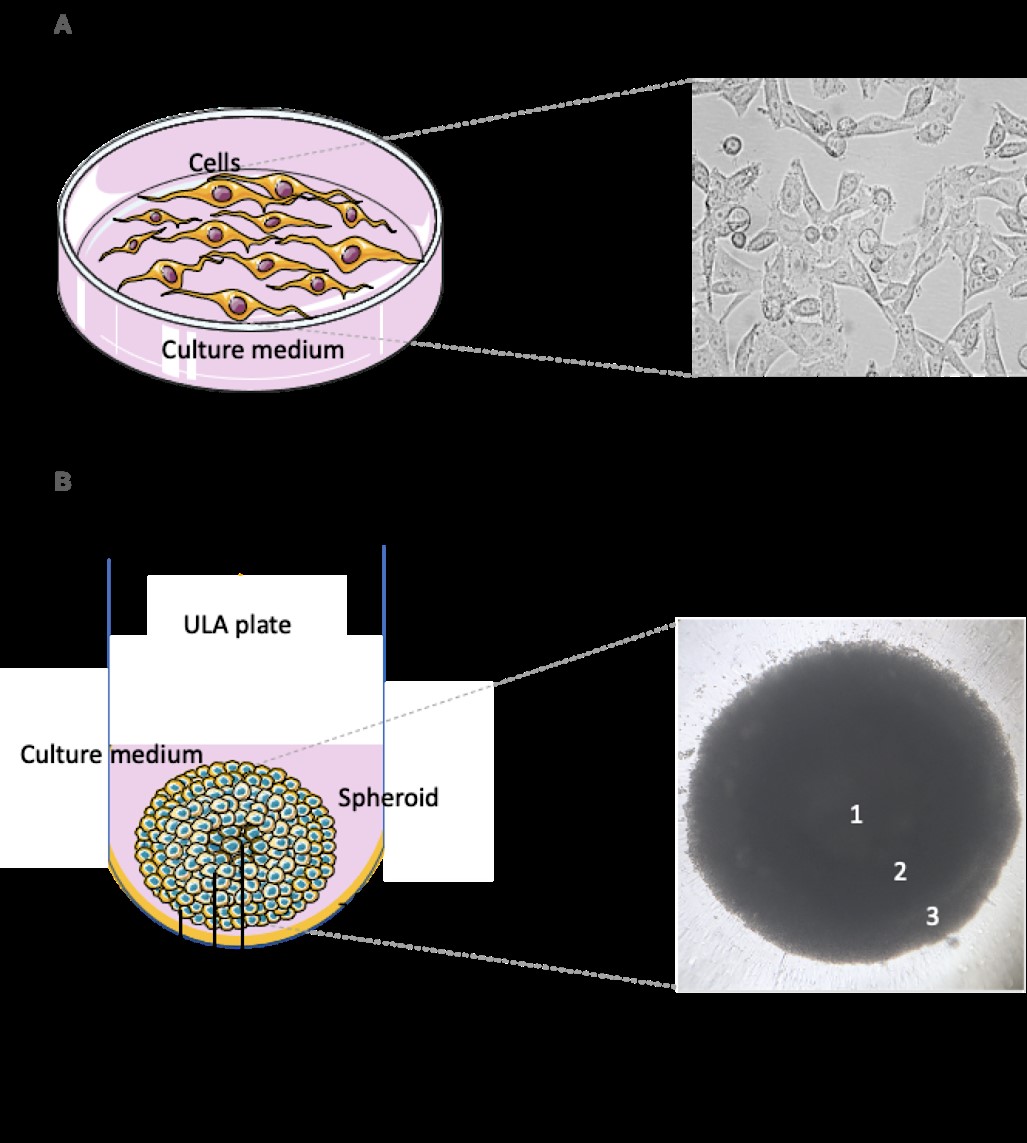
Different cell culture protocols are used to provide a better understanding of molecular and biochemical mechanisms and analysis of pharmacological targets and production of biological products [19]. Most of the research involved in the study of cancer is carried out using two-dimensional (2D) cultures. However, 2D cultures have important limitations, e.g., not mimicking the contact signal that occurs in vivo between cells and cells/extracellular matrix. This way, the three-dimensional (3D) cultures arose as an alternative to overcome those limitations [20]. New strategies such as cell co-cultures and three-dimensional (3D) cell culture systems are described below.
Cell types used in hepatocarcinoma studies
Hepatocellular carcinoma is a tumor with heterogeneous characteristics, and resistance to treatments makes curative therapy a great challenge [21]. Cell lines have been used as an important tool in studies seeking to broaden the knowledge of changes in cells and/or the identification of biomarkers for the diagnosis of hepatocarcinomas. It has been described that hepatocarcinoma cell lines retain the same genomic and transcriptomic background as primary human cancers [22]. However, it should be emphasized that cells from primary tumors have mutations, creating different scenarios for each cell of an established lineage [22, 23].
Currently, studies using cell lines have become an important tool in biotechnology, and different researchers have been describing a growing number of cell lines used in research involving hepatocarcinoma (Table 1. Suppl).
HepG2 is a line of epithelial cells isolated in 1975 from a liver biopsy of a 15-year-old Caucasian male with a well-differentiated hepatocellular carcinoma. As one of the most used cells in metabolism and hepatotoxicity studies, they are capable of synthesizing many plasma proteins, including albumin, alpha-fetoprotein and beta-lipoprotein [24]. Another cell line, Hep 3B, from a black African individual, with similar characteristics, was described in the same work. The Hep 3B strain contains an integrated genome of the hepatitis B virus; however, there is no evidence of production of infective viral particles. Regarding morphology, the Hep G2 and Hep 3B cell lines are similar to hepatocytes and distinguished only by their smaller size and architectural organization as irregular trabecular or pseudoglandular patterns.
HuH-6 is a line of Hepatoblastoma cells isolated from a one-year-old Japanese boy, and these cells can be used in genotoxicity studies; they have a karyotype that allows reproducibility of experiments [25, 26].
The HuH-1 cell line was isolated in 1981 from a 57-year-old Asian male with hepatocarcinoma carrying HBs antigen, and which maintains some liver-specific properties, such as inducible tyrosine aminotransferase activity. HuH-1 cells produce tumors in nude mice, with similar morphology to that of the original tumor. HuH-1 maintains the ability to metabolize benzo(a)pyrene (B(a)P), a potent carcinogenic polycyclic aromatic hydrocarbon [27]. Similarly, the HuH-7 cell line was established in 1982, also derived from a hepatocellular carcinoma of a 75-year-old Japanese male patient with no viral status. HuH-7 cells are highly susceptible to the hepatitis virus C and have a great potential to produce recombinant proteins such as erythropoietin (EPO); thus, the use of this cell line is suggested for the production of glycoproteins of therapeutic importance. Huh-7 and HepG2 cells support hepatitis B virus (HBV) replication when transfected with DNA from HBV. These two strains have been useful for studies of therapeutics and regulatory mechanisms of gene expression. Another strain that has been described to support HBV infection is HepaRG; this cell line is a human bipotent progenitor cell line capable of differentiating two different cell phenotypes (i.e., biliary-like and hepatocyte-like cells) [28]. They were isolated from a liver tumor of a female patient suffering from hepatocarcinoma and hepatitis C in France. It is noteworthy that differentiation and infectability are maintained only when these cells are cultured in the presence of corticoids and dimethyl sulfoxide [29].
PLC/PRF5, also known as Alexander Cell, was established in 1976 from a primary liver tumor of an individual of African origin [30]. Although it presents integrated HBV DNA, the ultrastructural examinations of the cells did not show any viral particles. It has been used in several studies to investigate mechanisms of drug resistance in hepatocellular carcinoma [31, 32].
In order to perform detailed analyses of cell interactions in tumor development, new epithelial and mesenchymal cell lines were established from human hepatocellular carcinoma by spontaneous growth in culture. Epithelial cell lines (HCC-1.1; HCC-1.2 and HCC-3) from European adult male patients were characterized by cell kinetics, genotype, tumorigenicity, expression of cell type-specific markers, and proteome patterns. The authors found many functions of preserved source cells [33].
Cell lines (SNU-182, SNU-354, SNU-368, SNU-387, SNU-398, SNU-423, SNU-449, SNU-475; SNU-739, SNU-761, SNU-878, SNU-886) of hepatocellular carcinoma established from primary tumors of Korean patients have been described. Hepatitis B virus (HBV) DNA has been integrated into the genomes of all strains. Two of the cell lines (SNU-354, SNU-368) showed expression of HBV transcripts. Most cultured cells retained many morphological features of the original tumors. SNU-354 strongly expressed albumin and SNU-368 transferrin and insulin-like growth factor II. None of these strains produced alpha-fetoprotein at the RNA and protein level [34, 35]. TGF-β treatment significantly enhances the viability of SNU-354, SNU-475, and SNU449 cell lines [35].
HLE and HLF are two strains established from hepatocellular carcinoma of a 68-year-old Japanese patient. HLE cells are of the epithelial type, demonstrate glycogen granules in the cytoplasm, and are capable of producing α-fetoprotein. HLF resembles fibroblasts in terms of morphology, but it does not produce α-fetoprotein [36]. HLE, HLF and SNU-449 cells are late-stage cell models poorly differentiated in relation to HUH7, HEPG2 and HEP3B cells (well differentiated), and they have properties of mesenchymal cells [37].
The cell lines JHH-1, 2, 4, 5, 6 and 7 were established from hepatocellular carcinoma derived from Asian adult patients seronegative for the hepatitis B surface antigen, which was not detected by radioimmunoassay; however, DNA integration of the hepatitis B virus was confirmed at two locations on the chromosomes of this strain by Southern blot hybridization [38].
MHCC97-H cells were established from the orthotopic inoculation of an intact tumor tissue of an intrahepatic disseminated lesion from a 39-year-old Chinese male patient with hepatocellular carcinoma; spontaneous pulmonary metastasis occurred in 100% of recipient nude mice after inoculation [39]. HCCLM3 cell lines were established from nude mouse lung metastasis, consisting of polygonal epithelial cells with hypotriploid karyotype [40,41].
Cell lines BEL-7402, BEL-7404 and BEL-7405 derived from liver carcinoma specimens from two males and one female from China, respectively. They present morphological aspects similar to those of epithelial cells, presence of fast growing and poorly differentiated alpha-fetoprotein [42]. A caution note on the hepatocellular origin of BEL7402 has been published [43].
The HA22T/VGH cell line is derived from a primary hepatocellular carcinoma from a 56-year-old Chinese male. This line presents different responses to the presence of epidermal growth factor (EGF), insulin and human growth hormone (hGH) in a serum-free culture medium. These cells contain the following liver associated enzymes: alanine amino transferase, tyrosine aminotransferase and gamma-glutamyl transferase. Alpha-fetoprotein was not detectable [44].
In addition to the cell types described in this topic, there are a variety of cell lines derived from human primary liver tumors in the literature. When proposing a new study, the importance of these cells as tools in pre-clinical studies must be taken into account, while paying attention to the specificities of each cell and, whenever possible, the use of more than one cell lineage.
Cell culture models for liver oncology studies
Cell culture techniques are an important tool for studying and understanding the behavior of in vivo organisms [45]. For many years, 2D cell culture was the main technique used for cell studies dedicated to oncogenic research, testing of new drugs, vaccine studies, and cell and gene therapies; in addition, it provided important information about biological processes and diseases [46, 47]. In this technique, cells usually grow as monolayers on a plastic adherent surface, allowing homogeneous growth, and there are significant advantages that justify the use of 2D cell culture in biomedical research. Major advantages are low cost of cultivation and reliability, as well as simple maintenance/manipulation for performing the experiments [48-50].
Despite the advances in cell culture technology, there are some disadvantages of the 2D technique, especially in studies focused on tumor models, e.g., drug trials, when compared to those performed with the 3D technique [50]. The cells grown in monolayers do not reliably represent the natural structures related to tumors and tumor tissues. In general, this kind of culture does not provide interactions such as those that occur between cells and the cells with the extracellular matrix of the tumor environment, as observed in vivo. These cellular communications are important because they enable the process of cell proliferation, survival and differentiation, response to microenvironment stimuli, expression of specific genes and proteins, and allow the observation of cell response to experimental drugs and metabolism, among other important functions in the study of tumor biology [20, 51-53].
Tumor-derived cells, when subjected to 2D cultivation conditions, present changes in cellular morphology, thus resulting in an important loss of phenotypic aspects and impairment of cell division. Morphological modification of these cells can lead to a change in their function and compromise their process of internal structural organization, signaling and secretion of bioactive molecules [20, 54-57]. In the 2D model, cells whose interactions are modified, whether cell-cell or matrix-cell ones, have their polarity changed owing to adherent growth on culture surfaces, which leads to changes in processes such as apoptosis [58-60].
Another disadvantage of the 2D method refers to the access of cells to nutrients in the medium, metabolites, oxygen and molecules for cell signaling pathways. In living organisms, this access is favored by the architectural arrangement of the cells that naturally form a tumor mass. Moreover, 2D cultivation also promotes changes in the process of gene expression and processing of RNA structures, during splicing, in addition to aspects related to cell biochemistry [20, 51, 61-63]. The study of the tumor microenvironment is also limited in 2D culture, since usually only one cell strain is used for growth in monolayers, which is a different condition from what occurs in vivo, when different cell types such as cancer initiator cells and other cell types are present altogether [64,65]. Owing to these disadvantages, the development of other methods has become increasingly necessary, for example, models that would be able to mimic the tumor microenvironment as well as the interactions that occur among tumor cells, as is the case with three-dimensional or 3D systems [20].
Three-dimensional (3D) culture models provide important tools to fill gaps between studies conducted in 2D models and foster the understanding of what happens in living systems [66]. The 3D model can reveal characteristics of the microenvironment in vivo and enable the understanding of several aspects about cell behavior and the processes involved in tumor development, as well as tissues and organs, providing a more reliable behavioral similarity with cells found in in vivo models [66, 67]. Also this model provides some advantages even over in vivo models related to the cross talk of the human tumor recapitulation, excluding the presence of incompatibilities of cross species patient-derived tumor xenografts (PDTX). Furthermore the in vivo machinery, in fact present some mechanism that configure some biologic limitation, once may impair mechanism leading to reduce their efficacy, and therefore its predictive value [66-68].
Thus, 3D models provide advantageous benefits that make them a good choice over 2D models. One of the significant advantages is the production of an extracellular matrix that enables both cell-cell and cell-matrix interplay, promoting a niche for this interaction [67]. In addition, it provides similar cell growth to the one found in vivo, and it has spherical morphology composed of cell aggregates [69,49]. Another important feature of this model is the similarity of gene and protein expression compared to in vivo models. In studies involving response to drugs, this is efficient because it represents the patterns of similar responses in clinical practice, e.g., the occurrence of resistance to a given agent [50, 70,71]. The cellular conformation found in the 3D models provides a flow of essential components to cell viability in a more feasible way, e.g., oxygen, metabolites, and nutrients, as well as the signaling molecules of specific processes resembling the living system [51, 72]. Furthermore, inside the spheroid, there are three different zones: a necrotic zone resulting from lower levels of nutrients accessing this area; a quiescent zone; and a proliferating zone, which is in contact with higher levels of nutrients and oxygen [67], as shown in Figure 1.
Thus, this model may provide relevant characteristics, thus contributing to the acceleration of research and development in the fields of tissue engineering and biological materials, and to a better understanding of cancer biology [66]. Advances have also occurred in studies of mechanisms of drug resistance, progression, metastases and tumor cell differentiation. Some perspectives for this model are based on ensuring the control and adjustment of substrates through more advanced technologies and materials. However, type of tumor and studies to be performed have to be taken into account [69].
Despite the multiple advantages, the use of 3D technology has shown some limitations, e.g., higher costs and a need for longer culture time when compared to 2D cultures [73]. In addition, in some cases, the ability of 3D cultures to mimic in vivo conditions may vary: for example, the immunological response to a given host under certain conditions and the lack of transport of small molecules within the microenvironment can be a limiting factor. The physiological conditions of in vivo systems are usually progressive, while in 3D culture systems, they are commonly mimic static and short-term conditions, which is a disadvantage for some studies because it is not exactly like a living machinery [66]. The choice for this technology is usually based on final purpose and applications [69]. This model also aims to validate results found in preclinical studies; thus, it is an alternative to the use of animals in research laboratories [67]. Another system such as three-dimensional printing provide a significant method for producing biosensors using eletrodes as gold, silver, platinum and others. These semiconductor elements together with the 3D printing provide a maximization of tumor cell adhesion, providing the control of the biosensor surface and their sensitivity and selective properties [74].
Cell cultures, oxidative stress and hepatocellular carcinoma
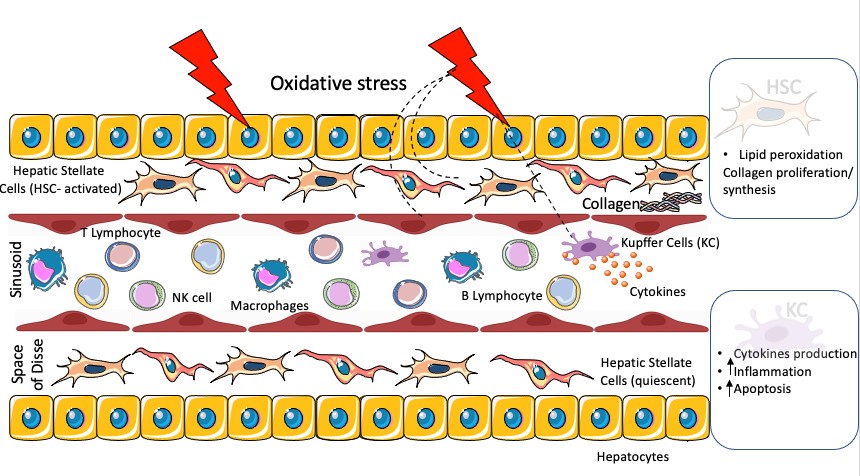
The liver is the most widely used organ in drug toxicity research [75], and the organ most affected by Reactive Oxygen Species (ROS) [76]. ROS production may be an early event of hepatotoxicity in liver damage and an indicative of hepatotoxic potential [73, 77]. Most of the activities related to the development of systems for hepatotoxicity evaluation in vitro are focused in the parenchymal cell, the hepatocyte [15], which is the major cell type sensitive to injury induced by oxidative stress in the liver. However, this liver microenvironment also presents circulating lymphocytes such as T (CD4+/CD8+), B and natural killer (NK) cells [78] (Figure 2).
The mitochondria, the microsomes and the peroxisomes in the parenchymal liver cells can produce ROS, regulating the peroxisome proliferator-activated receptor α (PPARα), a receptor that is related to gene expression which is associated with lipid metabolism in the liver [79]. In addition, Kupffer cells (KC), hepatic stellate cells (HSC) and endothelial cells are potentially more exposed or even more sensitive to oxidative stress, which induces the production of cytokines such as TNF-α by Kupffer cells, and may enhance inflammation and apoptosis (figure 2). In stellate cells, oxidative stress causes lipid peroxidation, promoting collagen proliferation and synthesis [75, 77, 79].
Oxidative stress is widely recognized as a response to initial stress related to liver injury progression and cancer [80]. Many factors such as alcohol, drugs, environmental pollutants and ionizing radiation can generate ROS and induce oxidative stress in the liver, resulting in severe liver diseases [75, 77]; thus, it plays an important role in several chronic, inflammatory and metabolic liver diseases, such as liver cirrhosis, hepatitis and nonalcoholic fatty liver disease (NAFLD); all of which are associated with oxidative imbalance, with higher production of ROS and reduction of antioxidant bioavailability [75, 76, 81-83]. In addition, in liver surgeries and transplantation, ROS are usually produced owing to the ischemia/reperfusion process [84].
ROS generation promotes the expression and secretion of pro-inflammatory cytokines, inducing inflammation and sustained oxidative stress, which, in turn, are crucial in the initiation and development of liver diseases, regardless of their etiology [75, 77, 80, 85]. Other studies have reported that ROS contribute to neoplastic transformation by various mechanisms, including interference in DNA repair systems responsible for removal of oxidized bases, i.e., triggering metabolic changes, glycolytic adaptation and increased lipidic biosynthesis, which promotes steatosis, which, in turn, leads to hepatocarcinogenesis [75, 82].
Hepatic carcinogenesis is characterized by deregulation of several enzymes involved in producing and eliminating ROS [82], and it is orchestrated by several ROS-mediated processes. The increase in the number of ROS, particularly in the nucleus, leads to DNA damage, mutations and genetic instability [86]. In many liver diseases that can progress to liver cancer, such as viral hepatitis, oxidative stress is one of the factors that drives the neoplastic transformation process, contributing to hepatocellular carcinoma (HCC) development [82].
Nanba et al. (2016)[87] demonstrated that levels of oxidative stress markers in patients with HCC are positively correlated with the likelihood of HCC development. On the other hand, recent findings indicate that, although oxidative stress is an initiation response to cancer, it can also be an antitumor cell response necessary to kill cancer cells [81]. Despite these advances, many aspects of the mechanisms involved in ROS participation in liver carcinogenesis, and the complex role of oxidative stress in the physiological and pathological processes of the disease, still need to be elucidated, requiring the establishment of an adequate study model - something that has been a barrier for a long time. In this respect, 3D cultures are an attempt to diminish these limitations, allowing significant advances in in vitro research, and providing a better understanding of the roles of ROS and oxidative stress in liver diseases [75, 77].
Kermanizadeh, et al., (2014)[88] demonstrated that 3D human liver microtissues are an efficient model for the mechanistic assessment of the toxicity of drugs or nanomaterials associated with inflammation. Corroborating this finding, Bhise et al. (2016) [89] used 3D bioprinting technology to produce liver spheroids that proved suitable for toxicity studies as they responded similarly to animal models (Bhise et al., [89,90]. The authors of these studies argue that the 3D model is an excellent candidate to replace some traditional in vitro liver models [88-90]. Hendriks et al. (2016)[91] evaluated two models of 3D hepatic spheroids: one composed of primary human hepatocytes (PHH) and another one of HepaRG cells, and they demonstrated that both of them can be used to detect and study compounds at risk of causing cholestatic hepatotoxicity, including those associated with increased oxidative stress and modulation of cell death receptor signaling. Thus, 3D cell cultures are promising systems, suitable for various purposes in scientific research, and an important tool for discovery of new medicines, identification of therapeutic targets, and investigation of compounds with antitumor activity [68, 89-91].
MSCs and liver cancer - in vitro models
Mesenchymal stem cells (MSCs), also called mesenchymal stromal cells, are known for their capacity of in vitro differentiation in osteoblasts, adipocytes, chondrocytes, and other cell types. Additionally, they can perform a number of biological activities, including immunomodulatory and anti-inflammatory ones [92]. MSCs have been studied as an alternative for the treatment of liver cancer. In vitro models with different liver tumor cell lines are important to better understand the mechanisms involved; however, the role of MSCs in the occurrence, development and treatment of hepatocellular carcinoma (HCC) is still controversial [9].
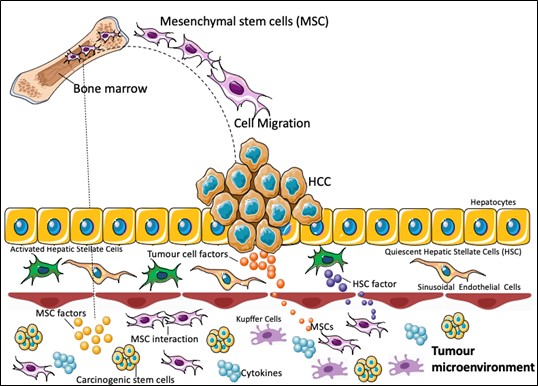
It is known that MSCs can migrate to the tumor, as demonstrated by Garcia et al., (2011)[93] in an in vitro study. They observed increased migration of human bone marrow derived MSC (hBMSC) towards the conditioned medium of different HCC cell lines and hepatic stellate cells, showing that factors produced by the tumor stroma may also be able to promote migration of these cells.
In addition to being able to act in the tumor microenvironment through cell-cell interactions and by the secretion of factors at the site, MSCs can act at a distance by secreting paracrine factors (figure 3). For this reason, many of the in vitro studies with hepatic tumor strains have focused on the use of conditioned medium, in which only secreted factors are present.
Zhao et al., (2012)[94] observed a reduction in cell viability of different lines of hepatocellular carcinoma (HepG2, HuH7, 1, Bel7402 and) when cultured with conditioned medium of human adipose tissue mesenchymal stem cells (CM-ADMSC) for 3 days. In addition, they also reported inhibition of SMM7721 proliferation and increased apoptosis 48 h after incubation with CM-ADMSC. Since culture conditions interfere in MSC properties, Xie, et al., (2018)[95] evaluated the effect of rat CM-ADMSC obtained through 3 different culture protocols (2D, spheres or 3D). They found that the conditioned medium obtained in the 3D cultivation conditions inhibited the proliferation of HCCLM3clm3 cells (human hepatocellular carcinoma), but only the conditioned medium obtained from 3D culture was able to inhibit the proliferation of HepG2 cells (human hepatoblastoma). The treatment with 3D CM-ADMSC also outperformed other conditions regarding apoptosis induction in HepG2 cells, although all CM-ADMSC conditions significantly increased apoptosis in comparison to the untreated control for both tumor cell lines. Additionally, inhibition of liver cancer cell migration, adhesion, and invasion occurred when tumor cells were treated with all types of CM-ADMSC; the 3D culture method was the one producing the most pronounced effect. The processes of migration and invasion of tumor cells have important involvement of epithelial mesenchymal transition (EMT), and there was also downregulation of EMT signaling in this study. Since 3D culture methods more closely reproduce the environment in vivo compared to the 2D culture method, this may have been the cause for the better result of 3D CM-ADMSC for the different parameters analyzed.
In another study, Serhal et al. (2019)[96] evaluated the effects of human ADMSC on liver tumor cells, testing not only the conditioned medium, but also the direct and indirect coculture with HCC cells (HepG2 and PLC-PRF-5). They found that both cocultures and the conditioned medium were able to reduce the number of HCC cells and increase the apoptosis rate, as well as induce the expression of p53 and RB tumor suppressor genes. They also evaluated the biochemical markers alpha fetoprotein (AFP) and des-gamma-carboxy-prothrombin (DCP) in the supernatant of the HCC cells co-cultured directly with ADMSC or treated with CM-ADMSC, and they found a reduction in these two serum markers, which are used to detect tumor progression and malignant proliferation in patients with HCC.
The processes of migration and invasion are related to the ability of tumor cells to promote metastases, and they are controlled by the imbalance between metalloproteinases (MMPs) and their inhibitors (TIMPs). An increase in MMPs has been correlated with the promotion of HCC metastases, while the increase in TIMPs was shown to inhibit this process [96]. Li et al. (2010)[ 48] reported a reduction in gene expression and protein levels of MMP-2 in MHCC97-H cells treated with conditioned medium of hBMSC, although an increase in tumor cell proliferation was also found. In a study with ADMSC, Serhal et al. (2019)[96] found an increase in gene expression of TIMP1 and TIMP3 in HepG2 and PLC-PRF-5 cell lines, both after co-cultivation and treatment with a conditioned medium of ADMSC. Regarding the migration and invasion, the results varied according to the type of tumor cell and the form of interaction with the ADMSC; there was a reduction in both parameters in HepG2 and PLC-PRF-5 cells with coculture, as well as incubation in the presence of a conditioned medium in the experiments with HepG2.
Thus, the results may vary not only according to the conditions of stem cells, but also in the way they interact with tumor cells (direct, indirect or medium-conditioned contact). Additionally, different tumor cell lines respond in different ways, as demonstrated by Garcia et al. (2011)[93] while using a conditioning medium of hBMSC; they reported a heterogeneous effect on tumor cell proliferation: no alterations in HuH7 cells, inhibition in Hep3B cells, and increase in PLC-PRF-5 cells.
Although the above-mentioned studies suggest beneficial effects of MSCs on liver tumor cells, this is still a controversial field. Some studies have shown that MSCs can increase the proliferation and invasive capacity of HCC cells. Liu et al. (2016)[97], using 3D culture of HCCLM3 cells, found that co-cultivation with umbilical cord (UC)MSC promoted an increase in gene expression of MMPs 2, 7 and 14 and higher expression of MMP2 secreted in the active form. This finding suggests an increase in the metastatic properties of HCCLM3 cells. In addition, the authors reported an increased migration capacity and expression of genes related to EMT (N-cadherin and vimentin). EMT plays an important role in the progression of HCC owing to its role in the increase of cancer stem-like cells (CSCs), which promote metastasis and drug resistance [98].
Although the co-cultivation with UCMSC did not interfere in the proliferation of HCCLM3 cells, treatment with a conditioned medium under normal conditions or hypoxia of different tumor lines (Bel-7402 and Hep3B) resulted in a significant increase in proliferation in both strains [99]. In addition to the participation of MMPs and TIMPs in migration and invasion processes, Mi and Gong (2017)[100] demonstrated that secretion of IL-6 by hBMSC also plays an important role in this process. Treatment of Bel-7402 and Bel-7404 cells with hBMSC conditioning increased the invasion rate of this cells, which was reduced when a neutralizing antibody against IL-6 was added. The same effect was not observed in HepG2 cells, probably owing to the higher level of endogenous IL-6 produced by this cell line. Additionally, hB-MSCs can also increase the migration and invasion of SNU-398 cells through different mechanisms, including CXCL12/CXCR4 (Fontanella et al., 2016)[101] and aquaporin (AQP1), a protein related to tumor progression that regulates water and solute transport through the membrane (Pelagalli et al., 2016)[102]. The effect through both pathways was reversed in the presence of their respective inhibitors (AMD3100 and Peptide R for CXCR4, and tetraethylammonium chloride for AQP1).
These controversial results may also be due to the different tumor cell lines used, as well as to the different origins of MSCs (bone marrow, adipose tissue and umbilical cord) and culture conditions (2D and 3D). According to Serhal et al. (2019)[96], tumor microenvironment directs the type of response that will be promoted by MSCs, which can be pro or antitumor, and it is also influenced by the source of each MSC. Therefore, in vitro studies are important tools to better understand the best source of MSCs and their therapeutic potential, for each type and stage of HCC.
As previously mentioned, tissue engineering has been seeking to develop and provide viable biological tools for the study of liver diseases for a few years. The difficulties in these systems, when using cells obtained from liver tissue, are associated with the induced microenvironment, which is often not able to mimic the biological conditions of the liver, thus compromising the maintenance of these cultures [103]. In this context, several studies have invested in the use of cell populations with greater plasticity, which can provide favorable conditions for in vitro studies [104].
A promising alternative for the study of hepatic oncogenesis is the use of induced pluripotent stem cell technology (iPSCs) to establish in vitro models [105]. This cellular population has gained prominence in the academic scene in recent years. This is due to the fact that they are cells that, when compared to other cell types, such as liver cells and embryonic stem cells, are easy to obtain: they are acquired from adult biological tissues through a phenomenon known as somatic reprogramming. In hepatology studies, iPSCs have been used, in the vast majority, to obtain hepatocytes and cholangiocytes [106].
The use of iPSCs to obtain cells to study hepatic oncogenesis can be highly advantageous, since this modality of cell culture allows an almost unlimited expansion of cells before the process of cell differentiation. Additionally, the cell line will maintain the genetic profile of the donor, ensuring a personalized study. In studies of liver diseases, either for understanding the cellular and molecular mechanisms associated with the pathogenesis of liver diseases, or for investigation of therapeutic alternatives, cell culture assays using iPSCs are very promising to obtain liver tumor cells when using specific inducing factors, although there is a need for techniques to validate the efficiency of the cell phenotype obtained [107].
The establishment of 3D co-cultures for production of hepatic organoids using iPSCs was initially described by Takebe et al., (2014)[108], and it has been used in protocols as an alternative for studies of liver diseases [109]. Cultures of 3D iPSCs, maintained in extracellular matrix support, are induced to hepatic differentiation through specific modulating factors, which results in hepatic organoids formed by a single or several cell lines [110].
In recent years, iPSCs have been used in preclinical trials for different pathological liver disorders, such as cystic fibrosis [110]; Alagille syndrome, [111]; HBV infection [113]; citrullinemia type I [112]; steatosis, steatohepatitis and Wolman's disease [114]. These studies used iPSCs derived from human skin fibroblasts and human peripheral blood cells [110]. Some findings in the literature investigate the use of iPSCs in studies in hepatic oncogenesis.
A recent study investigated iPSCs as a promising tool in the study of liver cancer. Experiments conducted by Afify et al., (2020)[115] developed a new model of liver cancer stem cells (CSCs) from iPSCs obtained from mouse fibroblasts (miPSCs), induced by a conditioned medium obtained from a hepatocarcinoma cell lineage culture (Huh7 cells). MiPSCs exhibited significant expression of molecular markers of liver cancer, such as glypican 3, alpha-fetoprotein (AFP) and arginase-1. In the study, it was found that the conditioned medium, enriched with pro-inflammatory cytokines and chemokines, was able to activate tumorigenic receptors coupled to g protein and phosphoinositide 3-kinase, resulting in the conversion of miPSCs into CSCs [115].
It is noteworthy that the use of organoid platforms produced from iPSCs technology allows investigations regarding the heterogeneity of liver tumors, in addition to providing safe results regarding the mechanisms involved in mutations in genes associated with hepatic oncogenesis [116]. A study conducted by Artegiani et al. (2019)[117] used normal iPSC-derived tissue organoids, with mutations induced by the CRISPR/Cas9 system, in specific genes associated with the development of cholangiocarcinoma (TP53, PTEN, SMAD4 and NF1) and in the BAP1 gene, which resulted in changes in cell junctions and consequent impairment of epithelial tissue arrangement. Such phenomena are directly associated with the emergence of malignant characteristics.
Biomaterial scaffolds for cell cultures and tissue engineering
Scaffold-based systems are biocompatible artificial structures that can be used to maintain 3D cell cultures since they mimic some of the most important characteristics of the extracellular matrix, e.g., permeability, porosity, mechanical stability and surface properties [118,119]. Thereby, it promotes a favorable biochemical and biophysical microenvironment for cell growth, proliferation and differentiation [120, 121].
Several natural polymers such as cellulose, chitin/chitosan, collagen, alginate, silk and hyaluronic acid, have been tested as scaffolds for 3D culture because of their high biocompatibility and low cost [122,123]. Similarly, synthetic/manufactured polymers (PCL, PVA, PEG, PLGA, Matrigel® etc.) are other interesting alternatives because their designed properties may enhance cell culture attributes [124]. Lee et al., (2021)[125] successfully stablished 3D models of hepatocellular carcinoma (HCC) using Matrigel® (solubilized basement membrane matrix secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells) and reported high cell proliferation, angiogenesis and increase of cell-to-cell interactions compared to 2D culture.
Hydrogels are polymeric networks with high permeability and swelling capacity that allow the flow of oxygen, water, nutrients and cell metabolites [126]. Moreover, hydrogels exhibit similar mechanical characteristics to that of different soft and wet tissues, allowing modeling of physiological and pathological states [127]. Ozkan et al., (2021)[128] used hydrogel-based scaffolds to investigate the response of HCC cell lines to chemotherapy, demonstrating that the microenvironment produced by 3D cell culture had a role in the differential therapeutic response of HCC cells.
Final considerations
The use of cell cultures has been considered as an important tool in replacing or reducing the use of animals in biomedical research on HCC. Biotechnological advances in this area have broadened the knowledge of cellular pathophysiology and molecular and cellular changes in liver cancer. Scientific advances in cell culture studies have resulted in new therapeutic, diagnostic and treatment approaches, for example, testing of new drugs and the use of stem cells and iPSC. Although highly relevant, 2D culture models have limitations in the interpretation of results, mostly because not all the signals that occur between cells in the in vivo model are found in 2D model. In an attempt to mimic the complex structural and functional interactions of cells, the 3D model has been currently used and has contributed significantly to cancer studies. This model allows cell-to-cell interactions as well as interactions between them and the extracellular matrix (of natural or synthetic sources); however, its main disadvantages are the high cost and the need for development of specific protocols for each study. We conclude, therefore, that 2D and 3D cell culture models can be used as alternative methods on animals use in scientific research of HCC; however, the choice of study model must be based on the proposed scientific question with attention to the cell types and appropriate methodologies that enable the effective development of the approaches considered in the particular study.
Funding
The present study was funded by the Oswaldo Cruz Foundation (FIOCRUZ), the Foundation for Science and Technology Support of Pernambuco (FACEPE), and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Funding Code 001
The figures were designed using the graphical elements provided by smart Servier Medical Art (https://smart.servier.com).
Conflicts of interest
The authors declare no conflict of interest.
Authors Contributions
The authors contributed equally to this work.
- El Serag HB (2011) Hepatocellular Carcinoma. The New England Journal of Medicine 365: 1118-27.
- Marquardt JU, Nguyen Tat M, Peter R, Galle PR, Wörns MA (2016) Surveillance of Hepatocellular Carcinoma and Diagnostic Algorithms in Patients with Liver Cirrhosis. Visceral Medicine 32: 110-5.
- Forner A, Llovet JM, Bruix J (2012) Hepatocellular carcinoma.Lancet 379: 1245-55.
- Yapali S, Tozun N (2018) Epidemiology and viral riskfactors for hepatocellular carcinoma in the Eastern Mediterranean countries. Hepatoma Research 4.
- Natarajan Y, El Serag HB (2021) Risk Factors for Hepatocellular Carcinoma: a Historical Perspective. Clinical Liver Diseases 18: 1-13.
- Llovet JM, Kelley RK, Villanueva A, Singal AG,Pikarsky E et al. (2021) Hepatocellular carcinoma. Nature Reviews Disease Primers 7.
- Deng H, Eckel SP, Liu L, Lurmann FW, Cockburn MGet al. (2017) Particulate matter air pollution and liver cancer survival. International Journal of Cancer 141: 744-9.
- Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M (2019) From NASH to HCC: Current Concepts and Future Challenges. Nature Reviews in Gastroenterology and Hepatology 16: 411-28.
- Zhang Y, Wang H, Xiao H (2021) Metformin Actions on the Liver: Protection Mechanisms Emerging in Hepatocytes and Immune Cells against NASH-Related HCC. International Journal of Molecular Sciences 22: 5016.
- Rinaldi L, Vetrano E, Rinaldi B, Galiero R, CaturanoA et al. (2021) HCC and Molecular Targeting Therapies: Back to the Future. Biomedicines 9: 1345.
- Sethy C, Kundu CN (2021) 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition.Biomedicine & Pharmacotherapy 137.
- Wang JMD, Pillai AMD (2021) Systemic Therapy for Hepatocellular Carcinoma. Clinical Liver Disease 17: 337-40.
- Wang CI, Chu PM, Chen YI, Lin YH, Chen CY (2021) Chemotherapeutic Drug-Regulated Cytokines Might Influence Therapeutic Efficacy in HCC. International Journal of Molecular Sciences 22: 13627.
- Gua YS, He Q (2013) A Current Update on the Rule of Alternative and Complementary Medicine in the Treatment of Liver Diseases. Evidence Based Complementary and Alternative Medicine.
- Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N et al. (2013) Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Archives in Toxicology 87: 1315-530.
- Kammerer S (2021) Three-Dimensional Liver Culture Systems to Maintain Primary Hepatic Properties for Toxicological Sciences 22: 10214.
- Zeilinger K, Freyer N, Damm G, Seehofer D, Knöspel F (2016) Cell sources for in vitro human liver cell culture models. Experimental Biology and Medicine (Maywood) 241: 1684-98.
- Doke SK, Dhawale SC (2015) Alternatives to animal testing: A review. Saudi Pharmacology Journal 23: 223-9.
- Jensen C, Teng Y (2020) Is It Time to Start Transitioning From 2D to 3D Cell Culture? Frontiers in Molecular Bioscience 7.
- Kapałczyńska M, Kolenda T, Przybyła W, Zajączkowska M, Teresiak A et al. (2018) 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Archives of Medical Science 14: 910-9.
- Llovet JM, Hernandez-Gea V (2014) Hepatocellular carcinoma: reasons for phase III failure and novel perspectives on trial design. Clinical Cancer Research 20: 2072-9.
- Qiu Z, Zou K, Zhuang L, Qin J, Li H et al. (2016) Hepatocellular carcinoma cell lines retain the genomic and transcriptomic landscapes of primary human cancers. Scientific Reports 6.
- Caruso S, Calatayud AL, Pilet J, La Bella T, Rekik S etal. (2019) Analysis of Liver Cancer Cell Lines Identifies Agents With Likely Efficacy Against Hepatocellular Carcinoma and Markers of Response. Gastroenterology 157: 760-76.
- Aden DP, Fogel A, Plotkin S, Damjanov I, Knowles BB (1979) Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature 282: 615-6.
- Doi I (1976) Establishment of a cell line and its clonal sublines from a patient with hepatoblastoma. Gan 67: 1-10.
- Waldherr M, Mišík M, Ferk F, Tomc J, Žegura B etal. (2018) Use of HuH6 and other human-derived hepatoma lines for the detection of genotoxins: a new hope for laboratory animals? Archives of Toxicology 92: 921-34.
- Huh N, Utakoji T (1981) Production of HBs-antigen by two new human hepatoma cell lines and its enhancement by dexamethasone. Gan 72: 178-9.
- Marion MJ, Hantz O, Durantel D (2010) The HepaRG cell line: biological properties and relevance as a tool for cell biology, drug metabolism, and virology studies. Methods in Molecular Biology 640: 261-72.
- Gripon P, Rumin S, Urban S, Le Seyec J, Glaise D etal. (2002) Infection of a human hepatoma cell line by hepatitis B virus. Proceedings of the National Academy of Sciences of the U S A 99: 15655-60.
- Alexander JJ, Bey EM, Geddes EW, Lecatsas G (1976) Establishment of a continuously growing cell line from primary carcinoma of the liver. South African Medical Journal 50: 2124-8.
- Pan S, Cheng X, Chen H, Castro PD, Ittmann MM etal. (2013) ERManI is a target of miR-125b and promotes transformation phenotypes in hepatocellular carcinoma (HCC).PLoS One 8: e72829.
- Tomonari T, Takeishi S, Taniguchi T, Tanaka T, Tanaka H (2016) MRP as a novel resistance factor for sorafenib in hepatocellular carcinoma. Oncotarget 7: 7207-15.
- Sagmeister S, Eisenbauer M, Pirker C, Mohr T, HolzmannK et al. (2008) New cellular tools reveal complex epithelial-mesenchymal interactions in hepatocarcinogenesis. British Journal of Cancer 99: 151-9.
- Park JG, Lee JH, Kang MS, Park KJ, Jeon YM et al.(1995) Characterization of cell lines established from human hepatocellular carcinoma. International Journal of Cancer 62:276-82.
- Ku JL, Park JG (2005) Biology of SNU cell lines. Cancer Research and Treatment 37: 1-19.
- Dor I, Namba M, Sato J (1975) Establishment and some biological characteristics of human hepatoma cell lines. Gan 66: 385-92.
- Nwosu ZC, Battello N, Rothley M, Pioronska W,Sitek B et al. (2018) Liver cancer cell lines distinctly mimic the metabolic gene expression pattern of the corresponding human tumours. Journal of Experimental & Clinical Cancer Research, 37: 211.
- Fujise K, Nagamori S, Hasumura S, Homma S, Sujino H et al. (1990) Integration of hepatitis B virus DNA into cells of six established human hepatocellular carcinoma cell lines.Hepatogastroenterology 37: 457-60.
- Tian J, Tang ZY, Ye SL, Liu YK, Lin ZY, Chen J, Xue Q (1999). New human hepatocellular carcinoma (HCC) cell line with highly metastatic potential (MHCC97) and its expressions of the factors associated with metastasis. British Journal of Cancer 81: 814-21.
- Yang J, Qin LX, Li Y, Ye SL, Liu YK (2005) Molecular cytogenetic characteristics of the human hepatocellular carcinoma cell line HCCLM3 with high metastatic potential: comparative genomic hybridization and multiplex fluorescence in situ hybridization. Cancer Genetics and Cytogenetics 158: 180-3.
- Li Y, Tang Z, Ye S, Liu Y, Chen J et al. (2002) [Establishmentof human hepatocellular carcinoma cell line with spontaneous pulmonary metastasis through in vivo selection].Zhonghua Yi Xue Za Zhi 82: 601-5.
- Chen R, Zhu D, Ye X, Shen D, Lu R (1980) Establishment of three human liver carcinoma cell lines and some oftheir biological characteristics in vitro. Scientia Sinica 23:236-47.
- Rebouissou S, Zucman-Rossi J, Moreau R, Qiu Z, Hui L (2017) Note of caution: Contaminations of hepatocellular cell lines. Journal of Hepatology 67: 896-7.
- Lin YM, Hu CP, Chou CK, O-Lee TW, Wuu KT (1982) [A new human hepatoma cell line: establishment and characterization]. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi 15: 193-201.
- Duval K, Grover H, Han LH, Mou Y, Pegoraro AF etal. (2017) Modeling Physiological Events in 2D vs. 3D Cell Culture. Physyology (Bethesda) 32: 266-77.
- Choi SW, Yeh YC, Zhang Y, Sung HW, Xia Y (2010) Uniform beads with controllable pore sizes for biomedical applications. Small 6: 1492-8.
- Langhans SA (2018) Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Frontiers in Pharmacology 9.
- Li GC, Ye QH, Xue YH, Sun HJ, Zhou HJ (2010) Human mesenchymal stem cells inhibit metastasis of a hepatocellular carcinoma model using the MHCC97-H cell line. Cancer Sciences 101: 2546-53.
- 49.Breslin S, O’Driscoll L (2013) Three-dimensional cell culture: the missing link in drug discovery. Drug Discovery Today 18: 240-9.
- Hoarau Véchot J, Rafii A, Touboul C, Pasquier J (2018) Halfway between 2D and Animal Models: Are 3D Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? International Journal of Molecular Sciences 19:181.
- Pampaloni F, Reynaud EG, Stelzer EH (2007) The third dimension bridges the gap between cell culture and live tissue. Nature Reviews of Molecular Cell Biology 8: 839-45.
- Baker BM, Chen CS (2012) Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. Journal of Cell Science 125: 3015-24.
- Hickman JA, Graeser R, de Hoogt R, Vidic S, Brito Cet al. (2014) Three-dimensional models of cancer for pharmacology and cancer cell biology: capturing tumor complexity in vitro/ex vivo. Biotechnology Journal 9: 1115-28.
- Von der Mark K, Gauss V, von der Mark H, Müller P (1977) Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 267: 531-2.
- Petersen OW, Rønnov-Jessen L, Howlett AR, Bissel MJ (1992). Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proceedings of the National Academy of Sciences of the U S A 89: 9064-8.
- Kilian KA, Bugarija B, Lahn BT, Mrksich M (2010) Geometric cues for directing the differentiation of mesenchymal stem cells. Proceedings of the National Academy of Sciences of the U S A 107: 4872-7.
- Nelson CM, Bissell MJ (2006) Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development,homeostasis, and cancer. Annual Reviews Cell Development Biology 22: 287-309.
- Weaver VM, Lelièvre S, Lakins JN, Chrenek MA,Jones JC et al. (2002) Beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium.Cancer Cell 2: 205-16.
- Meyers J, Craig J, Odde DJ (2006) Potential for control of signaling pathways via cell size and shape. Current Biology 16: 1685-93.
- Mseka T, Bamburg JR, Cramer LP (2007) ADF/cofilin family proteins control formation of oriented actin-filament bundles in the cell body to trigger fibroblast polarization. Journal of Cell Sciences 120: 4332-44.
- Fuchs E, Tumbar T, Guasch G (2004) Socializing with the neighbors: stem cells and their niche. Cell 116: 769-78.
- Birgersdotter A, Sandberg R, Ernberg I (2005) Gene expression perturbation in vitro – a growing case for three-dimensional (3D) culture systems. Seminars in Cancer Biology 15: 405-12.
- Li C, Kato M, Shiue L, Shively JE, Ares MJr (2006) Cell type and culture condition-dependent alternative splicing in human breast cancer cells revealed by splicing-sensitive microarrays. Cancer Research 66: 1990-9.
- Fischbach C, Chen R, Matsumoto T, Schmelzle T,Brugge JS, Polverini PJ, Mooney DJ (2007). Engineering tumors with 3D scaffolds. Nature Methods 4: 855-60.
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi Na et al. (2010) Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329: 1078-81.
- Yamada KM, Cukierman E (2007) Modeling tissue morphogenesis and cancer in 3D. Cell 130: 601-10.
- Chaicharoenaudomrung N, Kunhorm P, Noisa P (2019) Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World Journal of Stem Cells 11: 1065-83.
- Unger C, Kramer N, Walzl A, Scherzer M, Hengstschläger M et al. (2014) Modeling human carcinomas: Physiologically relevant 3D models to improve anti-cancer drug development. Advanced. Drug Delivery. Review 79: 50-67.
- Lv D, Hu Z, Lu L, Lu H, Xu X (2017) Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncology Letters 14: 6999-7010.
- Bingel C, Koeneke E, Ridinger J, Bittmann A, Sill Met al. (2017) Three-dimensional tumor cell growth stimulates autophagic flux and recapitulates chemotherapy resistance. Cell Death & Disease 8: e3013.
- Ravi M, Paramesh V, Kaviya SR, Anuradha E, Solomon FD (2015) 3D cell culture systems: advantages and applications.Journal of Cellular Physiology 230: 16-26.
- Senkowski W, Jarvius M, Rubin J, Lengqvist J, Gustafsson MG et al. (2016) Large-Scale Gene Expression Profiling Platform for Identification of Context-Dependent Drug Responses in Multicellular Tumor Spheroids. Cell Chemical Biology 23: 1428-38.
- Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA (2009) Spheroid-based drug screen: considerations and practical approach. Nature Protocols 4: 309-24.
- Neto JBMR, Carvalho HF, Soares JC et al. (2022) Three-Dimensional Printing and Its Potential to Develop Sensors for Cancer with Improved Performance. Biosensors 12:685.
- Li S, Tan HY, Wang N, Zhang ZJ, Lao L (2015) The Role of Oxidative Stress and Antioxidants in Liver Diseases. International Journal of Molecular Sciences 16: 26087-124.
- Sanchez-Valle, V., Chavez-Tapia, N. C., Uribe, M.,Mendez-Sanchez, N. (2012). Role of oxidative stress and molecular changes in liver fibrosis: A review. Current Medicinal Chemistry 19: 4850-60.
- Li S, Hong M, Tan HY, Wang N, Feng Y (2016) Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxidative Medicine and Cellular Longevity 21.
- Hofmann J, Hackl V, Esser H, Meszaros AT, Fodor M et al. (2021) Cell-Based Regeneration and Treatment of Liver Diseases. International Journal of Molecular Sciences 22: 10276.
- Kersten S, Stienstra R (2017) The role and regulation of the peroxisome proliferator activated receptor alpha in human liver. Biochimie 136: 75-84.
- Cichoz-Lach H, Michalak A (2014) Oxidative stress as a crucial factor in liver diseases. World Journal of Gastroenterology 20: 8082-91.
- Uchida D, Takaki A, Oyama A, Adachi T, Wada N etal. (2020) Oxidative Stress Management in Chronic Liver Diseases and Hepatocellular Carcinoma. Nutrients 12: 1576.
- Ivanov AV, Valuev-Elliston VT, Tyurina DA, Ivanova ON, Kochetkov SN et al. (2017) Oxidative stress, a trigger of hepatitis C and B virus-induced liver carcinogenesis. Oncotarget8: 3895-932.
- Monserrat-Mesquida M, Quetglas-Llabrés M, Abbate M, Montemayor S, Mascaró CM et al. (2020) Oxidative Stress and Pro-Inflammatory Status in Patients with Non-Alcoholic Fatty Liver Disease. Antioxidants (Basel) 9: 759.
- Senoner T, Schindler S, Stättner S, Öfner D, Troppmair J et al. (2019) Associations of Oxidative Stress and Postoperative Outcome in Liver Surgery with an Outlook to Future Potential Therapeutic Options. Oxidative Medicine and Cellular Longevity 3950818.
- De Andrade KQ, Moura FA, dos Santos JM, de Araújo OR, de Farias Santos JC et al. (2015) Oxidative Stress and Inflammation in Hepatic Diseases: Therapeutic Possibilities of N-Acetylcysteine. International Journal of Molecular Sciences 16: 30269-308.
- Kryston TB, Georgiev AB, Pissis P, Georgakilas AG (2011) Role of oxidative stress and DNA damage in human carcinogenesis. Mutation Research 711: 193-201.
- Nanba S, Ikeda F, Baba N, Takaguchi K, Senoh T (2016) Association of hepatic oxidative stress and iron dysregulation with HCC development after interferon therapy in chronic hepatitis. Journal of Clinical Pathology 69: 226-33.
- Kermanizadeh A, Hr ML, Roursgaard M, Messner S,Gunness P et al. (2014) Hepatic toxicology following single and multiple exposure of engineered nanomaterials utilising a novel primary human 3D liver microtissue model. Particle and Fibre Toxicology 11.
- Bhise NS, Manoharan V, Massa S, Tamayol A, Ghaderi M et al. (2016) A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 8: 014101.
- Jiang J, Messner S, Kelm JM, van Herwijnen M, Jennen D et al. (2019) Human 3D multicellular microtissues: An upgraded model for the in vitro mechanistic investigation of inflammation-associated drug toxicity. Toxicology Letters 312: 34-44. 91.Hendriks DFG, Puigvert LF, Messner S, Mortiz W, Ingelman-Sundberg M (2016) Hepatic 3D spheroid models for the detection and study of compounds with cholestatic liability. Scientific Reports 6: 35434.
- Zhao Q, Ren H, Han Z (2016) Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. Journal of Cellular Immunotherapy 2: 3-20.
- Garcia MG, Bayo J, Bolontrade MF, Sganga L, Malvicini M et al. (2011) Hepatocellular Carcinoma Cells and Their Fibrotic Microenvironment Modulate Bone Marrow- Derived Mesenchymal Stromal Cell Migration in Vitro and in Vivo. Molecular Pharmaceutics 8: 1538-48.
- Zhao W, Ren G, Zhang L, Zhang Z, Liu J et al. (2012) Efficacy of Mesenchymal Stem Cells Derived from Human Adipose Tissue in Inhibition of Hepatocellular Carcinoma Cells In Vitro. Cancer Biotherapy Radiopharmaceuticals 27: 606-13.
- Xie H, Liao N, Lan F, Cai Z, Liu X et al. (2018) 3D cultured adipose tissue derived stem cells inhibit liver cancer cell migration and invasion through suppressing epithelial mesenchymal transition. International Journal of Molecular Medicine 41: 1385-96.
- Serhal R, Saliba N, Hilal G, Moussa M, Hassan GS etal. (2019) Effect of adipose-derived mesenchymal stem cells on hepatocellular carcinoma: In vitro inhibition of carcinogenesis. World Journal of Gastroenterology 25: 567-83.
- Liu C, Liu Y, Xu XX, Guo X, Sun GW et al. (2016) Mesenchymal stem cells enhance the metastasis of 3D-cultured hepatocellular carcinoma cells. BMC Cancer 16: 3-9.
- Yin Z, Jiang K, Li R, Dong C, Wang L (2018) Multipotent mesenchymal stromal cells play critical roles in hepatocellular carcinoma initiation, progression and therapy. Molecular Cancer 17: 178.
- Liu Y, Ren H, Zhou Y, Shang L, Zhang Y et al. (2019) The hypoxia conditioned mesenchymal stem cells promote hepatocellular carcinoma progression through YAP mediated lipogenesis reprogramming. Journal of Experimental & Clinical Cancer Research 38.
- Mi F, Gong L (2017) Secretion of interleukin-6 by bone marrow mesenchymal stem cells promotes metastasis in hepatocellular carcinoma. Bioscience Report 37: BSR20170181.
- Fontanella R, Pelagalli A, Nardelli A, D'Alterio C, Ieranò C et al. (2016) A novel antagonist of CXCR4 prevents bone marrow-derived mesenchymal stem cell-mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion. Cancer Letters 370: 100-7.
- Pelagalli A, Nardelli A, Fontanella R, Zannetti A (2016) Inhibition of AQP1 Hampers Osteosarcoma and Hepatocellular Carcinoma Progression Mediated by Bone Marrow- Derived Mesenchymal Stem Cells. International Journal of Molecular Sciences 17: 1102.
- Katari R, Peloso A, Orlando G (2015) Tissue engineering and regenerative medicine: semantic considerations for an evolving paradigm. Frontiers in Bioengineering and Biotechnology 2.
- Camp JG, Sekine K, Gerber T, Loeffler-Wirth H, Binder H et al. (2017) Multilineage communication regulates human liver bud development from pluripotency. Nature 546: 533-8.
- Nuciforo S, Heim MH (2020) Organoids to model liver disease. JHEP Reports 3: 100198.
- Oliveira AG, Fiorotto R (2021) Novel approaches to liver disease diagnosis and modeling. Translational Gastroenterology and Hepatology 6.
- Caiazza C, Parisi S, Caiazzo M (2021) Liver Organoids: Updates on Disease Modeling and Biomedical Applications. Biology (Basel) 10: 835.
- Takebe T, Zhang RR, Koike H, Kimura M, YoshizawaE et al. (2014) Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature Protocols 9: 396-409.
- Worsdorfer P, Dalda N, Kern A, Krüger S, Wagner N et al. (2019) Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Scientific Reports 9: 15663.
- Sampaziotis F, de Brito MC, Madrigal P, Bertero A, Saeb-Parsy K et al. (2015) Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nature Biotechnology 33: 845-52.
- Guan Y, Xu D, Garfin PM, Ehmer U, Hurwitz M etal. (2017) Human hepatic organoids for the analysis of human genetic diseases. JCI Insight 2: e94954.
- Nie YZ, Zheng YW, Miyakawa K, Murata S, Zhang RR et al. (2018) Recapitulation of hepatitis B virus-host interactions in liver organoids from human induced pluripotent stem cells. EBioMedicine 35: 114-23.
- Akbari S, Sevinc GG, Ersoy N, Basak O, Kaplan K etal. (2019) Robust, long-term culture of endoderm-derived hepatic organoids for disease modeling. Stem Cell Reports 13:627-41.
- Ouchi R, Togo S, Kimura M, Shinozawa T, Koido Met al. (2019) Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metabolism 30: 374-84.
- Afify SM, Calle AS, Hassan G, Kumon K, NawaraHM et al. (2020) A novel model of liver cancer stem cells developed from induced pluripotent stem cells. British Journal of Cancer 122: 1378-90.
- Nguyen R, Da Won Bae S, Qiao L, George J (2021) Developing liver organoids from induced pluripotent stem cells (iPSCs): An alternative source of organoid generation for liver cancer research. Cancer Letters 508: 13-7.
- Artegiani B, Van Voorthuijsen L, Lindeboom RGH, Seinstra D, Heo I et al. (2019) Probing the Tumor Suppressor Function of BAP1 in CRISPR-Engineered Human Liver Organoids. Cell Stem Cell 24: 927-43.
- Saydé T, El Hamoui O, Alies B, Gaudin K, Lespes Get al. (2021) Biomaterials for three-dimensional cell culture: From applications in oncology to nanotechnology. Nanomaterials 11: 481.
- Zhao Y, Li M, Liu B, Xiang J, Cui Z et al. (2018) Ultra-tough injectable cytocompatible hydrogel for 3D cell culture and cartilage repair. Journal of Materials Chemistry B 6: 1351-8.
- Fontoura JC, Viezzer C, dos Santos FG, Ligabue RA, Weinlich R et al. (2020) Comparison of 2D and 3D cell culture models for cell growth, gene expression and drug resistance.Materials Science and Engineering 107
- Miao S, Cui H, Esworthy T, Mahadik B, Lee SJ et al. (2020) 4D self‐morphing culture substrate for modulating cell differentiation. Advanced Science 7: 1902403.
- Campuzano S, Pelling AE (2019) Scaffolds for 3D cell culture and cellular agriculture applications derived from non-animal sources. Frontiers in Sustainable Food Systems 3.
- Passos MF, Carvalho NMS, Rodrigues AA, Bavaresco VP, Jardini AL et al. (2019) PHEMA hydrogels obtained by infrared radiation for cartilage tissue engineering. International Journal of Chemical Engineering.
- Badekila AK, Kini S, Jaiswal AK (2021) Fabrication techniques of biomimetic scaffolds in three‐dimensional cell culture: A review. Journal of Cell Physiology 236: 741-62.
- Lee S, Teng Y, Son M, Ku B, Whang HJ et al. (2021) 3D-Hepatocellular Carcinoma (3D-HCC) Models of Diffused and Aggregated Spheroids using a 96-Pillar/Well Plate. Research Square.
- Park Y, Huh KM, Kang MH (2021) Applications of biomaterials in 3D cell culture and contributions of 3D cell culture to drug development and basic biomedical research. International Journal of Molecular Sciences 22: 2491.
- Muduli S, Chen LH, Li MP, Heish ZW, Liu CH et al. (2017) Stem cell culture on polyvinyl alcohol hydrogels having different elasticity and immobilized with ECM-derived oligopeptides. Journal of Polymer Engineering 37: 647-60.
- Ozkan A, Stolley DL, Cressman ENK, McMillin M,DeMorrow S et al. (2021) Tumor Microenvironment Alters Chemoresistance of Hepatocellular Carcinoma Through CYP3A4 Metabolic Activity. Frontiers in Oncology 11.

Figures at a glance