Radiotherapy for Esophageal Squamous Cell Carcinoma Concomitant with Hypofibrinogenemia: A Case Report
Received Date: June 01, 2024 Accepted Date: July 01, 2024 Published Date: July 04, 2024
doi: 10.17303/jcrto.2024.12.303
Citation: Zhongfei Jia, Wenxi Wang, Jie Yang, Meng Song, Yuxiang Wang (2024) Radiotherapy for Esophageal Squamous Cell Carcinoma Concomitant with Hypofibrinogenemia: A Case Report. J Cancer Res Therap Oncol 12: 1-8
Abstract
Background: Malignant tumors are frequently concomitant with hyperfibrinogenemia, but only rarely with hypofibrinogenemia.
Case Summary:This study reports an unusual case of a 60-year-old male who had mid-thoracic esophageal squamous cell carcinoma (ESCC) with hypofibrinogenemia and presented at our hospital because of a swallowing disorder and dull pain in the upper abdomen. An initial test indicated his plasma fibrinogen (FIB) level was 0.88 g/L (reference range: 2.38–4.98 g/L). After multiple infusions of fresh plasma and supplements of FIB and cryoprecipitate, he maintained a FIB level above 1.0 g/L. We administered radical radiotherapy (RT) for the ESCC, and his FIB level gradually normalized during the RT period. The symptoms from ESCC gradually resolved, and we classified the patient as having stable disease at the end of the RT period. After 10 months follow-up, the patient achieved partial response (PR). At that time, he had no increased tendency for bleeding and his FIB level was 0.97 g/L. At the last follow-up (18 months), the patient remained alive.
Conclusions: We considered the hypofibrinogenemia in this ESCC patient to be a consequence of paraneoplastic syndrome.
Keywords: Esophageal Squamous Cell Carcinoma; Hypofibrinogenemia; Radiotherapy
Core Tip: We reported a male patient who had mid-thoracic esophageal squamous cell carcinoma (ESCC) combined with hypoproteinemia. His initial plasma fibrinogen (FIB) level was 0.88 g/L. After multiple infusions of fresh plasma and supplements of FIB and cryoprecipitate, he maintained a FIB level above 1.0 g/L and received radiotherapy (RT). His FIB level gradually normalized and the symptoms from ESCC gradually resolved. The patient achieved stable disease at the end of RT and obtained partial response after 10 months follow-up, when his FIB level was 0.97 g/L. At the last follow-up (18 months), the patient remained alive.
Introduction
Esophageal cancer is one of the most common malignant neoplasms and these patients typically have poor outcomes. Esophageal squamous cell carcinoma (ESCC) accounts for about 80% of all esophageal cancers in Eastern countries [1]. Fibrinogen (FIB) is a glycoprotein synthesized in hepatocytes that functions in the final steps of the blood coagulation cascade and as a precursor monomer of the fibrin hemostatic plug. FIB also functions in tumorigenesis, formation of stroma, angiogenesis, and tumor metastasis [2]. In general, tumor patients have increased risk for hypercoagulability and thrombosis. Studies of ESCC patients indicated that hyperfibrinogenemia was associated with lymph node metastasis, distant organ metastasis, worse survival, and a higher rate of relapse [3,4]. However, ESCC with concomitant hypofibrinogenemia has only been rarely reported. This paper reports a rare case of ESCC with concomitant hypofibrinogenemia.
Case Presentation
In April, 2019, we admitted a 60-year-old male patient to the Department of Thoracic Surgery in our hospital. The patient reported having a swallowing disorder and dull pain in the upper abdomen for more than 1 month. The results of gastroscopy indicated esophageal mucosal erythema that was 30–35 cm from the incisors and light-stained spots after iodine staining of those sites. Examination of the biopsy specimens indicated a diagnosis of well-differentiated ESCC.
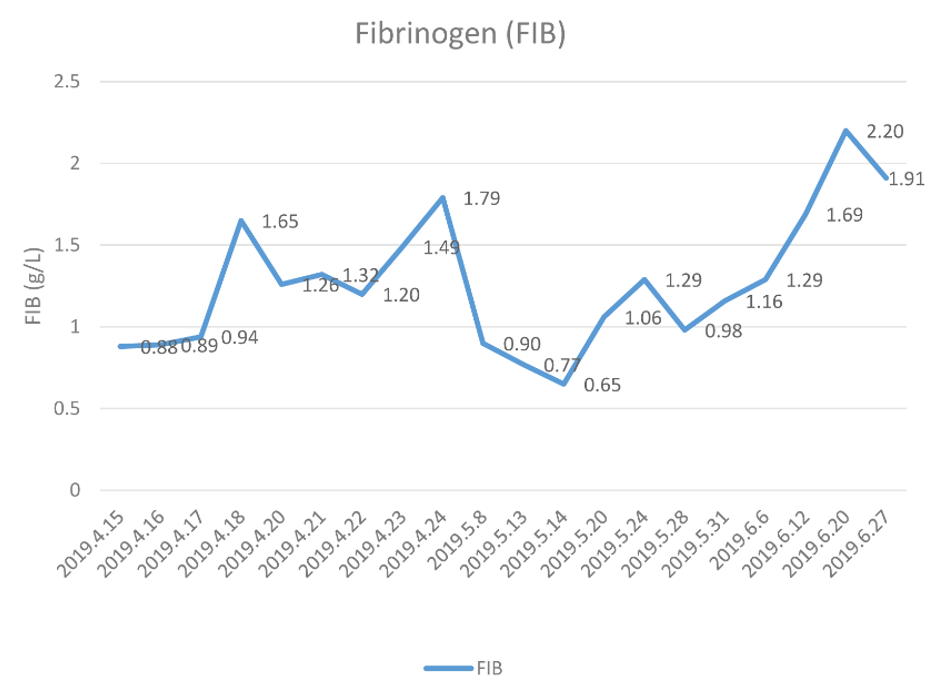
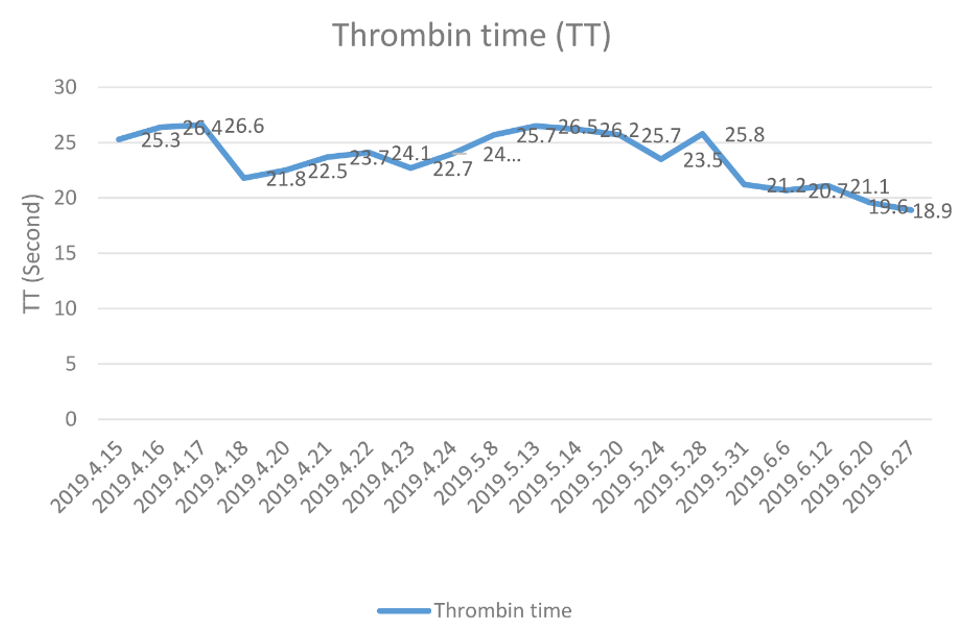
Barium esophageal radiography showed there was a 5 cm mucosal lesion in the mid-thoracic esophagus. Computed tomography (CT) was then performed of the chest and abdomen, and confirmed the clinical stage as T2N1M0 according to the AJCC staging criteria for ESCC (9th edition). The results of routine tests for blood, liver function, and kidney function were all normal. However, measurement of blood coagulation on April 16 indicated an abnormally long thrombin time (TT: 26.4 s, reference range: 10.3–16.6 s) and an abnormally low level of FIB (0.88 g/L, reference range: 2.38–4.98 g/L). A bone marrow biopsy on the same day indicated no reason for the hypofibrinogenemia. The patient also had no tendency for increased bleeding.
To correct the hypofibrinogenemia, we infused 600 mL of fresh plasma, but the FIB level remained low (0.94 g/L) on April 17. Considering the high-risk of surgery, the patient was transferred to the Department of Hematology in our hospital. After FIB infusion, his plasma FIB level was 1.79 g/L on April 24. However, thoracic surgery was still considered a high-risk and the patient refused the operation. Thus, the patient was transferred to Tianjin Institute of Hematology for further diagnosis, and the cause of the hypofibrinogenemia was still unknown. The patient subsequently reported a gradually increasing difficulty in swallowing and dull pain in the upper abdomen. He was admitted to the Department of Radiotherapy in our hospital on May 8. At this time the patient received a semi-liquid diet. Reexamination indicated the plasma FIB level was 0.90 g/L, the TT was 15.7 s, and the international normalized ratio (INR) was 1.77 (reference range: 0.80–1.40). The preliminary diagnosis was ESCC combined with hypofibrinogenemia.
We infused the patient with 10 units of cryoprecipitate on May 10, and with 200 mL of leukocyte-free frozen plasma infusion on May 13. However, on May 14 his FIB level was 0.65 g/L, so we transferred him to the Department of Hematology once more. Following continuous infusion with FIB and treatment for anti-fibrinolysis, his FIB level reached about 1 g/L. After the patient's family signed an informed consent agreement, the patient was positioned under a CT simulator. The gross tumor volume (GTV) was defined as the primary tumor in the mid-thoracic segment of the esophagus and the mediastinal metastatic lymph nodes. The clinical target volume (CTV) included the GTV and the area of corresponding lymphatic drainage in the middle and superior mediastinum. The planning target volume (PTV) was then established by appropriate enlargement of the CTV according with therapeutic principles. The dose at 95% coverage of the PTV was defined as 60 Gy in 30 fractions, 2 Gy per fraction, administered 5 days per week for 6 weeks. After the planning of intensity-modulated radiotherapy (IMRT) by the medical physicist and approval by the physician, it was initiated on May 20
During the RT period, the patient experienced no skin congestion, ecchymosis, or subcutaneous hemorrhage, and his swallowing symptoms and dull pain in the upper abdomen gradually resolved. At the same time, the level of FIB (Figure 1) and the TT (Figure 2) both gradually normalized. Upon completion of the RT (June 28), the patient was easily able eat a soft diet without swallowing disorders or abdominal pain. At the end of the RT, the curative effect was evaluated as stable disease (SD) based on CT imaging of the chest and abdomen and barium esophagography.
One month after the RT, the patient reported continued intake of soft food and the curative effect was assessed as partial response (PR) based on reexamination of CT images of the chest and abdomen and a barium esophagogram. The next examination of the patient in April 2020 indicated he continued easy intake of soft food, and the therapeutic effect was confirmed as PR. Importantly, his plasma FIB level at that time was 0.97 g/L. Because of the COVID-2019 pandemic, medical resources are strained, with the primary effort directed at combating the epidemic. People's movements are restricted, making it difficult to access regular medical care. the patient has not recently visited the hospital during 3years, but we connected by telephone follow-up every half a year. He remained a stable condition. With the epidemic fade away, On November 23, 2023 (40 months after RT), the patient contact us at hospital, and reported continued easy intake of soft food, on May 14 his FIB level was 2.58 g/L, the curative effect was PR.
Discussion
Most patients with malignant tumors have abnormalities in one or more coagulation indicators, such as a shortened prothrombin time (PT), an increased plasma FIB level, or an increased D-dimer level [5]. FIB and D-dimer are specific indicators of hypercoagulability. Hypercoagulability in patients with malignant tumors can promote the formation of tumor thrombi and cause secondary hyperfibrinolysis [6]. FIB is the main coagulation factor in plasma, and its normal concentration is about 2 to 4 g/L [7]. Fibrinogen is a hexameric glycoprotein composed of Aα,Bβ, and γ chains. A high level of plasma FIB in patients with lung cancer, ESCC, gastric cancer, colorectal cancer, ovarian cancer, and other cancers is independently associated with poor prognosis [8-11]. A reduced FIB level occurs in bleeding, disseminated intravascular coagulation (DIC), severe hepatitis, cirrhosis, thrombolytic therapy, primary fibrinolysis, and several other diseases. Patients with esophageal squamous cell carcinoma have a high incidence of bleeding, Coagulopathy is a major clinical issue. It is characterized by tissue injury and low tissue perfusion. This results in coagulation factor (CF) depletion [12], Primary hypofibrinogenemia is an autosomal genetic disease and identification of the mutant gene is the gold standard for diagnosis, but this is difficult in clinical practice [13]. Secondary hyperfibrinolysis is a thrombo-hemorrhage syndrome that is the consequence of a primary disease and manifests as local or diffuse intravascular coagulation [14].
Plasma fibrin precipitates in blood vessels, thereby promoting the release of plasminogen activator in the circulating blood, leading to hyperfibrinolysis and an increased level of D-dimer. Several factors, such as severe trauma, postpartum hemorrhage, and liver transplantation, can lead to hyperfibrinolysis, and administration of tranexamic acid can reduce the risk of bleeding and death [15]. A multi-center study by Hagemo et al. [16] showed that various factors, such as hyperfibrinolysis, severe blood loss, blood dilution after rehydration, acidosis, and hypothermia, could lead to a decreased FIB level, and that the most direct and effective treatment was intravenous infusion of plasma, cryoprecipitate, and FIB. In addition, Hess et al. [17] reported that hypofibrinogenemia caused by trauma was related to a more favorable prognosis.
fibrinogen has been shown to participate in tumorigenesis, formation of stroma, angiogenesis, and tumor metastasis [18]. The decrease in fibrinogen may occur due to synthesis disorder, increased consumption, or hyperfibrinolysis. Hypofibrinogenemia can lead to spontaneous bleeding. Hypofibrinogenemia secondary to a malignant tumor is rare, and there are only a few case reports with this finding. Paraneoplastic syndrome (PNS) refers to a series of diseases caused by malignant tumors that are not associated with direct tumor invasion; it is caused by immune cross-reactivity between tumor-produced bioactive substances (e.g., hormones, peptides and cytokines) and normal tissues . PNS may affect multiple organ systems throughout the body, especially the endocrine, nervous, rheumatic and hematological systems. Some patients with malignant tumors such as small cell lung cancer often present with PNS as the first manifestation before the diagnosis of the tumor is confirmed. Correct identification of PNS can allow the timely diagnosis of the primary disease and avoid a missed diagnosis [19] Rapaport et al. [20] described a patient who had prostate cancer with concurrent hypercoagulability and hypofibrinogenemia. Libek et al. [21] described a patient who had prostate cancer and a subcutaneous hematoma due to hyperfibrinolysis, and they considered this to be paraneoplastic syndrome (PNS). Aulmann et al. [22] described a patient who had metastatic breast cancer combined with thrombocytopenia and hyperfibrinolysis, and they also considered this to be PNS. Recently, Ma et al. [23] described hypofibrinogenemia in patient who had relapsed gastric cancer after surgery. Hunault-Berger et al. [24] examined 214 patients with acute T lymphoblastic leukemia and T lymphoblastic lymphoma, and reported that administration of L-asparaginase chemotherapy inhibited the biosynthesis of liver L-asparagine-dependent protein, leading to acquired hypofibrinogenemia. Acute promyelocytic leukemia (APL) can also cause secondary hypofibrinogenemia [25]. These patients have increased levels of urokinase-type plasminogen activator, tissue-type PA, and annexin-α2 in APL cells, leading to synthesis and activation of plasminogen, metabolism of FIB, and hypofibrinogenemia. Liu et al. [26] studied patients with APL and reported that administration of all-trans retinoic acid (ATRA) induced APL cell differentiation, down-regulated annexin-α2, and corrected the hyperfibrinolysis [26]. The main supportive treatments for these patients are infusion of fresh frozen plasma (FFP), cryoprecipitate, and/or concentrated FIB to maintain an FIB level above 1.0 to 1.5 g/L [27].
The patient described here had ESCC combined with hypofibrinogenemia. After fresh plasma infusion, FIB supplementation, and cryoprecipitate, his FIB level increased slightly to about 1 g/L. The patient accepted radical RT. His FIB level gradually rose during the RT period, and reached a maximum of 2.20 g/L. His symptoms gradually resolved, and the response was evaluated as SD at the end of the RT. One month after RT, the patient’s response was evaluated as PR and this status was maintained for more than 10 months. The FIB level was 0.97g/L at this time of this follow-up. The patient had no tendency for bleeding during the entire course of disease, treatment, and recovery. We therefore considered the hypofibrinogenemia in this patient to be a consequence of PNS, although the mechanistic relationship of PNS with ESCC remains unknown and needs further study.
This is a case of esophageal squamous cell carcinoma with hypofibrinogenemia. At the initial stage of treatment, the fibrinogen level of the patient was extremely low. With the effective treatment to the primary esophageal tumor, the fibrinogen level also gradually increased. However, just one case, and the Individualized factors which could not be excluded, the causal relationship between the hypofibrinogenemia and the tumor could not be concluded. It is also necessary to widely collect medical records, verify the conclusion after excluding the interference factors, and further explore the treatment to hypofibrinogenemia related to esophageal carcinoma.
Acknowledgements
Not applicable.
Authors' Contributions
Data collection: ZJ, JY, MS
Paper writing: ZJ, WW, YW
Paper design and direction: YW, JY
All authors have read and approved the final manuscript.
Informed Consent Statement
Written informed consent was obtained from the patient and the designated caregiver for publication of this case report and accompanying images.
Conflict-of-Interest Statement
The authors declare that they have no conflict of interest.
CARE Checklist (2016) Statement
The authors have read the CARE Checklist (2016),and the manuscript was prepared and revised accordingly.
- Napier KJ, Scheerer M, Misra S (2014) Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol; 6: 112-20.
- Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, et al. (2005) Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood; 105: 178-85.
- Zhang D, Zhou X, Bao W, Chen Y, Cheng L, et al. (2015) Plasma fibrinogen levels are correlated with postoperative distant metastasis and prognosis in esophageal squamous cell carcinoma. Oncotarget; 6: 38410-20.
- Takeuchi H, Ikeuchi S, Kitagawa Y, Shimada A, Oishi T, et al. (2007) Pretreatment plasma fibrinogen level correlates with tumor progression and metastasis in patients with squamous cell carcinoma of the esophagus. J Gastroenterol Hepatol; 22: 2222-7.
- Gouin-Thibault I, Samama MM (1999) Laboratory diagnosis of the thrombophilic state in cancer patients. Semin Thromb Hemost; 25: 167-72.
- Kwietniak M, Al-Amawi T, Błaszkowski T, Sulżyc-- Bielicka V, Kładny J (2017) The usefulness of D-dimer in diagnosis and prediction of venous thromboembolism in patients with abdominal malignancy. Pol Przegl Chir; 89: 27-30.
- Ilhan-Mutlu A, Starlinger P, Perkmann T, Schoppmann SF, Preusser M, Birner P (2015) Plasma fibrinogen and blood platelet counts are associated with response to neoadjuvant therapy in esophageal cancer. Biomark Med; 9: 327-35.
- Zhang Y, Cao J (2020) Pretreatment plasma fibrinogen level as a prognostic biomarker for patients with lung cancer. Clinics (Sao Paulo); 75: e993.
- Zhao LY, Zhao YL, Wang JJ, Zhao QD, Yi WQ, et al. (2020) Is Preoperative Fibrinogen Associated with the Survival Prognosis of Gastric Cancer Patients? A Multi-centered, Propensity Score-Matched Retrospective Study. World J Surg; 44: 213-22.
- Li M, Wu Y, Zhang J, Huang L, Wu X, Yuan Y (2019) Prognostic value of pretreatment plasma fibrinogen in patients with colorectal cancer: A systematic review and meta-analysis. Medicine (Baltimore); 98: e16974.
- Hefler-Frischmuth K, Lafleur J, Hefler L, Polterauer S, Seebacher V, et al. (2015) Plasma fibrinogen levels in patients with benign and malignant ovarian tumors. Gynecol Oncol; 136: 567-70.
- Bialkower M, Garnier G (2022) Fibrinogen Diagnostics in Major Hemorrhage. Crit Rev Anal Chem. 52: 194-209.
- Asselta R, Duga S, Tenchini ML (2006) The molecular basis of quantitative fibrinogen disorders. J Thromb Haemost; 4: 2115-29.
- Li GH, Lu MP, Ye LZ, et al. (2015) Analysis of Clinical Characteristics of low Fibrinogen of Patients. Chinese Journal of Thrombosis and Hemostasis, 21: 5.
- Pabinger I, Fries D, Schöchl H, Streif W, Toller W (2017) Tranexamic acid for treatment and prophylaxis of bleeding and hyperfibrinolysis. Wien Klin Wochenschr; 129: 303-16.
- Hagemo JS, Stanworth S, Juffermans NP, Brohi K, Cohen M, et al. (2014) Prevalence, predictors and outcome of hypofibrinogenaemia in trauma: a multicentre observational study. Crit Care; 18: R52.
- Hess JR, Brohi K, Dutton RP, Hauser CJ, Holcomb JB, et al. (2008) The coagulopathy of trauma: a review of mechanisms. J Trauma; 65: 748-54.
- Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. (2005) Platelets and fibrin(ogen) increase metastatic potential by impedingnatural killer cell-mediated elimination of tumor cells. Blood. 105: 178-85
- Soomro Z, Youssef M, Yust-Katz S, Jalali A, Patel AJ, Mandel J (2020) Paraneoplastic syndromes in small cell lung cancer. J Thorac Dis.12: 6253-63.
- Rapaport SI, Chapman CG (1959) Coexistent hypercoagulability and acute hypofibrinogenemia in a patient with prostatic carcinoma. Am J Med; 27: 144-53.
- Kulić A, Cvetković Z, Libek V (2016) Primary hyperfibrinolysis as the presenting sign of prostate cancer: A case report. Vojnosanit Pregl; 73: 877-80.
- Aulmann C, Seufert P, Sandherr M, Schlimok G, Schulze R, Oruzio D (2007) A 65-year-old female patient with breast cancer accompanied by thrombocytopenia and hyperfibrinolysis. Internist (Berl); 48: 1015-9.
- Ma S, Dang Q, Yang Y, Liu Y, Sun Y, Sun M (2020) Sintilimab, a PD-1 Inhibitor, Completely Reversed Rarely Refractory Hypofibrinogenemia in a Gastric Cancer Patient: A Case Report and Review of the Literature. Front Oncol; 10: 526096.
- Hunault-Berger M, Chevallier P, Delain M, Bulabois CE, Bologna S, (2008) Changes in antithrombin and fibrinogen levels during induction chemotherapy with L-asparaginase in adult patients with acute lymphoblastic leukemia or lymphoblastic lymphoma. Use of supportive coagulation therapy and clinical outcome: the CAPELAL study. Haematologica; 93: 1488-94
- Franchini M, Mannucci PM (2018) Primary hyperfibrinolysis: Facts and fancies. Thromb Res; 166: 71-5.
- Liu Y, Wang Z, Jiang M, Dai L, Zhang W, (2011) The expression of annexin II and its role in the fibrinolytic activity in acute promyelocytic leukemia. Leuk Res; 35: 879-84.
- Sanz MA, Grimwade D, Tallman MS, Lowenberg B, Fenaux P, et al. (2009) Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood; 113: 1875-91.

Figures at a glance