Mitigation of CRPC Cells Invasiveness via the Downregulation of Hsp27 Transcription and Stress-induced Signaling Pathways with JOMD-1307 Theranostic Nanoparticle
Received Date: August 06, 2024 Accepted Date: September 06, 2024 Published Date: September 09, 2024
doi: 10.17303/jcrto.2024.12.401
Citation: Jerry A. Ombor, Eni-yimini S. Agoro, George S. Gborienemi, Jonathan Nyebuchi, James E. Omietimi (2024) Mitigation of CRPC Cells Invasiveness via the Downregulation of Hsp27 Transcription and Stress-induced Signaling Pathways with JOMD-1307 Theranostic Nanoparticle.jCancer Res Threap Oncol 12: 1-23
Abstract
Prostate cancer (PC) represents one of the most commonly diagnosed cancers, and the over-expression of cell survival genes has been described to underlie castration-resistant prostate cancer (CRPC) emergence and resistance to chemotherapies post-androgen withdrawal. Although several patients initially respond well to standard treatments but later become unresponsive and recur within two years, leading to therapy-resistant prostate cancer (TRPC), which has become a major cause of PC deaths worldwide. There is an urgent need to find a novel class of anticancer agents that will reverse the therapy-resistant phenotype of PC. However, drug-like small molecules that exert their cytotoxic activity through mechanisms of inclusive gene inhibition potentiate an excellent modality for CRPC treatment with overt therapeutic advantage. In this study, an improved conjugated small molecule, a theranostic nanoparticle agent (JOMD-1307) was identified as a promising treatment strategy against the progression of TRPC cells. JOMD-1307 downregulates increased transcription and expression of Hsp27 stress-induced signaling pathways involved in PC cell (PC-3 and LNCaP) survival and blockage of apoptotic PC cell death. Therefore, the mitigation of Hsp27 oncogenic functions restores the sensitivity of CRPC cells to treatment.
Keywords: CRPC Cells; TRPC; Theranostic Nanoparticle; JOMD-1307; Hsp27 Transcription and Stress-induced Signaling Pathways
Introduction
Prostate cancer (PC) is one of the most frequently occurring cancers and the fifth cause of male death worldwide, with approximately 7% cancer mortality rate [1,2]. Patients within the advanced stage are treated with androgen deprivation therapy (ADT) known as castration. They initially respond to ADT but relapse to experience progression of PC disease that will subsequently lead to castration resistance to treatment. The use of therapies like radiotherapies and chemotherapies has shown adverse side effects on patients especially those with comorbid conditions, high chronological age, and/or a poor performance status due to the therapy's limitations [2-4]. Progression to castration- resistant prostate cancer (CRPC) mainly occurs after 2-3 years of castration therapy. Unfortunately, CRPC has been a menace to treat, and ultimately, patients in this category usually have an overall survival (OS) of 12-18 months [5,6]. Clinical phase III trials on the docetaxel-based treatment of advanced prostate cancer cells showed proof of improved patient survival. However, the treatment benefit was short-lived for 2.7 months due to the progression to therapy-resistant phenotypes [7]. This has made CRPC a serious challenge for clinicians and drug developers to elicit cancer therapy. Despite the recent arrays of therapies, CRPC and metastasized CRPC (mCRPC) remain a significant medical challenge, and understanding the underlying mechanisms of CRPC progression and developing anticancer agents, will improve patient outcomes [8]. However, recent findings show that the bypass pathways (Non-AR-related pathways) mechanism, increases the cascade of P13K/AKT/TOR and MAPK signaling activity to stimulate alternative growth pathways or enhances bypass survival signaling pathways involved in CRPC progression [8-10]. These signaling pathways bind to the increased levels of insulin-like growth factor 1 and its receptor (IGF-1 and IGF-1R) in PC to stimulate its transduction signal cascade and activate the expression of survival proteins responsible for the cellular growth and invasiveness in CRPC cells [11-13]. Importantly, the alternative pathways also involve the increased expression of heat shock proteins 27 (Hsp27) stress-induced signaling pathways [8]. This chaperone protein interacts and prevents the partner proteins (Menin, TCTP, DDX5, elF4E) from undergoing proteasome degradation [14-16], and the mitigation of Hsp27 expression inhibits the expression of the partner proteins [17]. Therefore, a promising therapeutic approach to improve the treatment of therapy-resistant prostate cancer (TRPC) cells invasiveness via targeting and mitigating Hsp27 stress-induced signaling pathways, inhibits the partner proteins expression, to prevent, delay, and treat the emergence of the castration-refractory phenotype [14-16,18,19]. It has been shown that the Hsp27 upregulation via the inducement of c-Myc and heat shock factor-1(HSF-1), which are transcription factors responsible for elevated regulation of survival genes are aggressively involved in TRPC progression [20-23]. In addition, Hsp27 is an ATP-independent molecular chaperon and its stress-induced signaling to cytoprotects and interactions with the partner proteins contributes to resistance to anticancer therapies leading to TRPC [8,24]. While cMyc is a Myc-proto-oncogene and a multifunctional nuclear phosphoprotein, thus its increased inducement and expression regulate metabolic changes (survival proteins) involved in TRPC progression [25-28]. Also, HSF-1 is a transcription factor that upregulates the genes of heat shock protein (such as HSPB1) expression in response to cellular stress [22,23]. Therefore, inhibiting molecular chaperon functions of Hsp27 and its transcriptional amplification by c-Myc and HSF-1 transcription has been seen as a pivotal pointer in arresting CRPC progression. Moreover, studies have previously shown antisense oligonucleotide (OGX-427), small molecules such as NA49, and phenazine #14 delays PC progression, and restore castration after chemotherapy in mice xenografts by targeting and inhibiting Hsp27 stress-induced signaling and its cytoprotecting interaction with the partner proteins expression from ubiquitin proteasome-degradation [29,30,16]. While c-Myc increased transcription regulation of genes is induced by survival proteins to enhance their oncogenicity, and the inhibition of c-Myc elevated transcriptional functions with c-Myc inhibitors, such as S2L-PI-41, WEN-3694, FTY720, and Omomyc, obstructs transcription of survival proteins expression [26,28,31]. Also, the inhibition of HSF-1 increased transcriptional regulation impairs the elevated transcription and expression of stress- induced survival genes in CRPC cells [23,32,33]. In addition, survival proteins are known to trigger the survival pathways and have been identified to have the potential mechanisms of action to hinder apoptotic cell death and induce mutant p53 expression after anticancer therapy, thereby suppressing the expression of wild-type p53 [34-36]. They are often implicated in several physiological and metabolic alterations, such as the survival and the downstream signaling pathways, and long-term/continuous inhibition of these survival proteins' expression might be accompanied by adverse cytotoxic side effects [16,37]. Thus, novel anticancer agents with an improved mechanism of action, high target specificity, and selectivity on PC cell progression, with insignificant adverse effects are urgently needed. Howbeit, previous studies have shown an effective therapeutic strategy that will rely on the use of small molecules and their analogs that can identify, target, disrupt, and inhibit survival protein-protein interactions and expression, and the downstream signaling pathways involved in TRPC [38-41]. Some of these small molecules are designed as theranostic agents with alpha-emitting properties that have a site-specific affinity to target and inhibit the survival proteins genome thereby causing DNA damage [42-47].
Targeting and inhibiting increased transcription of Hsp27-stress-induced signaling pathways and its cellular interactions with the partner proteins brings huge clinical significance to resolving TRPC progression [28,35,48].
However, most small molecules have poor solubility and penetration into the cell, limiting their therapeutic capability. Previous studies have shown naturopathic compounds, and their derivatives have therapeutic efficacy and high affinity for cancer cells [49,50]. Therefore, we used JOMD-1307 which is synthesized as a theranostic agent or chemical compound (nanoparticle) that is water-soluble and can easily attach and penetrate the cell membrane of cancer cells than non-cancerous cells to target and inhibit the increased expression of Hsp27, and its interactions with partner proteins, including inhibiting the targeted transcriptional factors (c-Myc and HSF-1) of Hsp27 to enhance treatment of TRPC [26,28,48,51].
JOMD-1307 can form micelle when exposed to cancer cells, interact with cell DNA molecules, and target the mitochondria causing cellular stress (ROS) [50,52-57]. Due to its chemical composition, it can also intercalative bind to single-stranded polyadenylic acid (poly A) to inhibit the mRNA- poly-A synthesis and auto-regulate poly- A binding to impair the target protein synthesis in some human cancers, and an option for multidrug resistance reversal and increase cancer cells' sensitivity to treatment [50,54,57-59].
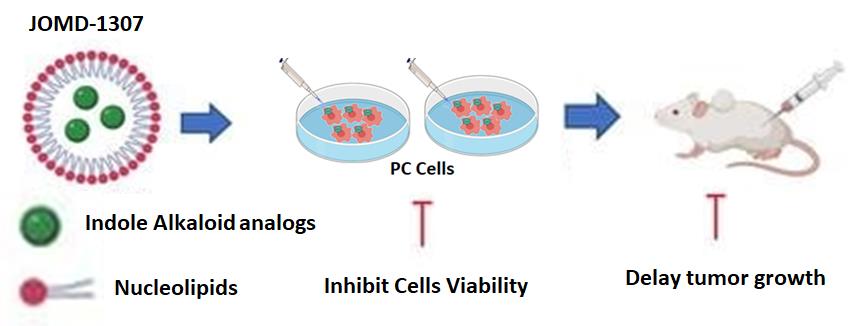
To elucidate the cellular anticancer ability of the JOMD-1307, we performed in vitro, in vivo, and ex vivo experimental analyses on PC cell lines, such as androgen-independent PC-3, androgen-dependent LNCaP, and normal human epithelial prostate cell line PNT1A, as a corresponding non-cancerous cell to determine its efficiency on the cells. The results showed JOMD-1307 anticancer therapeutic abilities to inhibit the target proteins' interaction and expression, decrease mRNA expression and PC cell viability, distort cancer PC cell division, induce apoptotic PC cell death, and delay tumor growth. Therefore, inhibiting TRPC cells via mitochondrial targeting and inhibition of cell survival proteins, and downregulating the cellular survival pathways to potentiate an efficient anti-cancer therapy on CRPC cells.
In this study, the JOMD-1307 was able to target and mitigate the expression and interaction of Hsp27 stress-induced signaling pathways and downregulate its transcription factors and gene upregulation to enhance the treatment strategy for CRPC cells.
Materials and Methods
Synthesis of JOMD-1307JOMD-1307 is derived from the indole alkaloid group synthesized with DOTAU nucleolipids (DOU-PEG-2000) formulated in a pharmacology adjustment dose to form nucleoside-lipid-base nanoparticles (Figure 7). JOMD-1307 stock concentration of 10mM was dissolved and diluted in phosphate-buffered saline (PBS) to the working concentrations used for the experiments.
Cell Lines and Cell CultureThe androgen-independent prostate cancer (AIPC) cell line PC-3, androgen-sensitive prostate cancer (ASPC) cell line LNCaP, and normal human epithelial prostate cell line PNT1A were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in Nitrogen oxide (NO2). Dulbecco’s Modified Eagle’s Medium (DMEM) and Roswell Park Memorial Institute 1640 (RPMI 1640) from (Life Technology, Inc., Saint Aubin, France) supplemented with 10% fetal bovine serum (FBS) from (Invitrogen, Paisley, UK) were used to cultivate PC-3, LNCaP, and PNT1A cell lines at 37ₒC in 5% CO2. Trypsin-EDTA for trypsinization was purchased from Gibco (Life Technologies, Villebon-sur-Yvette, France), and harvested cells were washed with 1% phosphate-buffered saline (PBS) purchased from Gibco (Life Technologies, Villebon-sur- Yvette, France).
Treatment of PC-3, LNCaP, and PNT1A Cells with JOMD-1307PC-3, LNCaP, and PNT1A cells were seeded into 10 cm dishes (1,250,000 cells/well) or 12-well plates (50,000 to 100,000 cells/well) according to the different experiments to be carried out. After 24 h, the cells were treated with the JOMD-1307, according to the different experiments to be carried out, while the untreated cells were used as controls. The effects of the treatment with JOMD-1307 were analyzed after 72 h compared with the control(s).
Cell Viability and Proliferation AssayFor cell viability and proliferation studies, cells (PC-3, LNCaP, and PNT1A) were seeded in 12-well plates at a density of 50,000 to 100,000 cells per well. After 24 h, cells were incubated with different concentrations of JOMD-1307, and DMSO and untreated cells were used as controls in a total volume of 500 µL/ well each in triplicate. After 72 h of incubating the treatments, the medium was removed, cells were stained with 100 µL of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) solution (from 5mg/ml stock solution in 1% PBS, pH 7.4) in each well of the plate for 2 h at 37 ◦C in a humidified of 5% CO2 incubation. After removing the supernatant, the cells are re- suspended in DMSO at room temperature to dissolve the formazan crystals. The plate(s) were agitated to mix the reaction content and transferred to 96 well plates for the optical density to be read at 595 nm by spectrometry using an ELISA reader from Multiskan SkyHigh (Life Technologies, Villebon-sur-Yvette, France). The assay was performed in triplicate. The absorbance was converted into a percentage of cell proliferation compared to the controls, and this protocol was also used to estimate IC50 (50% inhibitory concentration) values for JOMD-1307. The cell proliferation and anticancer activities of DMSO and untreated cells are used as positive and negative controls respectively. The IC50 value(s) of JOMD-1307 obtained was used for subsequent experiments.
Cell Cycle Analysis using Flow CytometryThe cytostatic effects of the JOMD-1307 compound on PC-3 and LNCaP cells were tested using Phospho H-3 and PI Staining Method for cell cycle assay. The cells are cultured for 24 h and later treated with JOMD-1307 compound, and their corresponding controls untreated. After incubation, the cells were harvested and washed with PBS 1X and re- suspended by fixing the cells with 75% ice-cold ethanol at 4 oC for a minimum of 1 h or overnight. The cells spun at 2000 rpm for 10’, the supernatant was removed, and the cells washed twice with PBS 1X (2000 rpm 5’). The cells were counted and adjusted to 1.106 cells/tube, and cells were re-suspended in 200µl PBS 0,2% Triton (Sigma-Aldrich, St. Louis, MO, USA) for permeabilization on ice for 10’. The cells were spun at 2000 rpm for 10’, the supernatant removed, and 100ul of anti-Phospho H-3 (Sigma-Aldrich, St. Louis, MO, USA) were added to dilute to 1/500 in PBS 1X + 0.2% triton + 0.5% BSA buffer. It was incubated for 30’ at RT, washed with PBS 1X + 0.2% triton + 0.5% BSA buffer, and spun at 2000 rpm for 5’. We added 100µl of anti-Rabbit Alexa 647 (Invitrogen, Paisley, UK) in 1/250 dilution in PBS 1X + 0.2% triton + 0.5% BSA buffer and incubated for 20’ in RT, protected from light. Then washed with PBS 1X + 0.2% triton + 0.5% BSA buffer and spun at 2000 rpm for 5’. Add 0.5ml of PI/RNase (Sigma-Aldrich, St. Louis, MO, USA) staining solution containing 50µg/mL of PI and 100 µg/mL of RNase for 1.106 cells, and incubate for 30’ at 37o C, protected from light. The cells were transferred into a 5ml FACS tube on ice for acquisition in the FACS machine. The cell cycle distribution according to the cell cycle phases was examined with a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA), and Cell Quest (BD Biosciences, San Jose, CA, USA). The cell cycle was analyzed with Multi-Cycle AnalysisTM (Phoenix Flow Systems, San Diego, CA, USA). The analysis is used to determine the content of DNA, and based on the results produced, the phase(s) of the cell cycle arrest was determined.
Apoptosis Analysis using Flow CytometryDetermination of apoptosis was analyzed using the APC Annexin V/PI staining method, Apoptosis Kit (Life Technologies Corporation, USA). The PC-3 and LNCaP cells were cultured for 24 h and later treated with JOMD-1307, and their corresponding controls untreated. After incubation, the cells were harvested and washed with PBS 1X twice and then once in 1X binding buffer. The cells were re-suspended in 1X binding buffer at 1-5 x106 cells/ml and add 5µl of fluorochrome-conjugated Annexin V was to 100µl of cell suspension. Incubate for 10-15’ at RT, and protect the cells from light. We added 2 ml of 1X binding buffer and centrifuged at 400-600 rpm for 5’ at RT. The supernatant was discarded and cells re-suspended in 200 µl of 1X binding buffer. 5 µl of prepared FITC dye was added from the manufacturer’s instructions and incubated for 5-15’ on ice or at RT. The cells were analyzed within 4 h of initial incubation stored at 2-8oC, and protected from light before acquisition with the FACS machine. Approximately 10,000 events were collected from each sample using flow cytometry (BD LSR II Flow Cytometry Analyzer) and the rate of apoptosis was analyzed by FlowJo software (Tree Star, Inc., Ashland, USA).
Confocal Microscopy ImagingTo observe, the cellular distribution of the JOMD-1307 on the PC cell line, PC-3, and LNCaP cells were cultured into a 12-well plate containing cover glasses covered at a density of 100,000 cells/well. After 24 h, the cells were treated with 2.0 µM JOMD-1307, and their controls untreated. After incubation, cells were harvested and washed with PBS1X and fixed with formaldehyde 4% (ThermoFisher Scientific, Strasbourg, France) for 15-30’ RT. The Cells are washed with PBS 1X twice and incubated with a prepared 1: 10,000 dilutions of 4’,6-diamidino-2-phenylindole (DAPI) solution in a checker at RT for at least 10’. The cover slides containing the cells are mounted on glass slides using Prolong Gold anti-fade reagent (Life Technologies, Villebon-sur-Yvette, France). The Samples were kept to dry in the dark for at least 24 h at RT, and sealed with nail polish. Images were captured with Zeiss 510 META fluorescence confocal microscope plan 40×/1.4 (Carl Zeiss, Paris, France) for JOMD-1307 and controls (absorption; 570 nm, emission; 790 nm) and DAPI (absorption; 350 nm, emission; 450 nm - 490 nm. Nuclei were visualized by DAPI fluorescence incorporated in the mounting media (Vectashield, Vector Laboratories, Burlingame, CA). Results were representative of random pictures taken from three independent experiments.
Western Blot AnalysisThe protein expression levels on PC cell lines were tested using western blot analysis. PC- 3 and LNCaP cells are cultured in 6 x 105 cells/dish in culture medium supplemented with 10% FBS and incubated overnight in a 5% CO2 incubator at 37°C and 95% humidity. After 24 h, the cells were treated with JOMD-1307, and the untreated controls were all incubated. After incubation, harvested cells were transferred into falcon tubes washed with PBS 1X, and lysed on ice for 30’ to form lysates by using a constituted lysis buffer added with protease and phosphates enzyme inhibitors according to the quantity of harvested cells. The cell's protein lysates are mixed and spun at 13,000 rpm at 4°C for 1 h and the supernatant is separated into Eppendorf tubes. The Protein concentrations were quantified using Pierce BCA Protein Assay (ThermoFisher Scientific, Strasbourg, France). Equal amounts of protein concentration undergo electrophoreses on a 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (Amersham, GE Healthcare Life Sciences, Freiburg, Germany) using vertical electrophoresis apparatus Mini-PROTEAN® 3 Cell (Bio-Rad, Hercules, CA, USA). Then membranes were placed on a flat flask and blocked with a blocking solution of 5% milk for 1 hour. The membranes are washed with TBS X1 buffer trice in checkers for 10’, and they were incubated with the following primary antibodies that were used: rabbit Heat Shock Protein 27 (Hsp27) antibody (Assay Designs, Villeurbanne, France, 1/5000), rabbit c-Myc (anti-c-Myc) antibody (Cell Signaling Technology, 1/1000, Massachusetts, USA), rabbit antibody Caspase-3 (Cell Signaling Technology, 1/1000, Massachusetts, USA), rabbit antibody Caspase-8 (Cell Signaling Technology, 1/1000, Massachusetts, USA), rabbit Poly(ADP-ribose) polymerase (anti- PARP) antibody (Cell Signaling Technology, 1/1000, Massachusetts, USA), rabbit Apoptosis-Inducing Factor antibody (anti-AIF) (Cell Signaling Technology, 1/1000, Massachusetts, USA), rabbit Heat Shock Factor 1(HSF-1) antibody (Cell Signaling Technology, 1/1000, Massachusetts, USA), mouse anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) antibody (Santa Cruz Biotechnology, Heidelberg, Germany 1/5000.) as an internal control, anti-rabbit IgG HRP conjugate antibody (Santa Cruz Biotechnology, Heidelberg, Germany, 1/5000), and anti-rabbit True blot IgG HRP conjugate antibody (eBiosciences, 1/1000, Villebon-sur-Yvette, France) according to the manufacturer’s protocols at 4°C overnight. Re-blot Plus Mild Solution (Millipore, Molsheim, France) was used for membrane stripping for 10’ at RT. Then the membranes were washed trice with TBS 1X buffer in checkers for 10’ and incubated with the corresponding secondary antibodies for 2 h at RT. Primary and secondary antibodies are constituted in TBS 1X of 0.5% Milk and BSA. After washing the membranes with TBS 1X buffer for 10’, the membrane bands were revealed by enhanced chemiluminescence (ECL; ThermoFisher Scientific, USA). Protein bands were characterized using the ImageJ (Sun Microsystems, Santa Clara, CA, USA) analysis software.
Co- Immunoprecipitation (co-IP) AssayThe protein interactions of Hsp27/c-Myc and Hsp27/HSF-1 expression levels on PC progression were performed using co-immunoprecipitation (Co-IP). The processes of protein lysate quantification for PC-3 and LNCaP cells treated with JOMD-1307 were conducted as previously described in western blot analysis. The lysate (1 mg) was diluted in the lysis buffer to obtain a working volume of 500 µL and incubated with 5-8 µL (1/50) of primary antibodies of rabbit anti-Hsp27 antibody (Stressgen, Victoria, British Columbia, Canada), rabbit c-Myc (anti-c-Myc) antibody (Cell Signaling Technology, 1/1000, Massachusetts, USA), rabbit Heat Shock Factor 1(HSF-1) antibody (Cell Signaling Technology, 1/1000, Massachusetts, USA) and rabbit anti-IgG (ThermoFisher Scientific, USA) as a control to rotate overnight in 4o C. The immune complexes were precipitated by incubating with 30 µL of True blot anti-rabbit Ig IP beads (eBiosciences, Paris, France) for 1 h at 4o C, and centrifuged with 10,000 rpm for 1’. The supernatant was removed and cells were washed three times with the cold lysis buffer. The beads were re-suspended in 8 µL of protein sample buffer (Bio-Rad, Marnes-la-Coquette, France) and heated for 5’ at 95o C before carrying out Western Blot analysis from the SDS-PAGE electrophoreses stage.
Messenger RNA (mRNA) Quantitative Real-time RT-PCR AnalysisTotal RNA was extracted from harvested PC-3 and LNCaP cells treated with JOMD-1307 for 72 h using the protocols of Maxwell RSC simply RNA cells kit (Cat. # AS1390) and instrument (Promega, USA). The extracted RNA concentrations were determined by NanoDrop Spectrophotometer ND-1000 (ThermoFisher, USA). Their viability/quality was observed using the Agilent RNA ScreenTape system (Agilent Technologies, Waldbronn, Germany). The RNAs were synthesized with Thermal cycler Eppendorf PCR Gradient (ThermoFisher, Strasbourg, France) to first-strand cDNA by using SuperScript III Reverse Transcriptase (RT) - SuperScript Vilo Master Mix (Invitrogen, ThermoFisher Scientific, USA) reagent and protocol. Then, the Real-time RT-PCR amplification of the cDNA was performed in triplicate on a StepOnePlus system (Life Technologies, Germany) according to manufactural protocols using primers pairs and probes of the Hsp27 Probe (Oligo #: 8819069316-000010); 5’- [ 6FAM] AGCCATGCTCGTCCTGCCGC [TAM], Hsp27_FW (Oligo #: 8819069316-000020); 5’-GAGATCACCGGCAAGCAC and Hsp27_RV (Oligo #: 8819069316-000030); 5’-ACAGGGAGGAGGAAACTTGG, and GAPDH – an oligo Mix pre-developed TaqMan Assay Reagents ( applied biosystems, Life Technologies LTD, Warrington, UK) respectively, which was mixed with a TaqMan Universal Gene Expression Master Mix (applied biosystems, Warrington, UK). The targeted genes Hsp27 and TCTP expressions were normalized with the GAPDH levels as an internal control on all the analyzed samples, while the non-treated samples of each cell line acted as the comparing factor.
An In Vivo Experimental Analysis on Tumor Growth EvaluationFor the in vivo study, 10,000,000 PC-3 cells were inoculated subcutaneously with 0.1 ml of DPBSX1 in the flank region of 6-week-old male Bal-C Nude mice (NOD SCID) through a 27-gauge needle under halothane anesthesia. The ethical approval for animal handling and experimentation was from the Directorate of Research and Quality Assurance, Federal University Otuoke, Nigeria. Tumor volumes were measured weekly with a caliper which is as follows: length × width × depth × 0.5236. PC-3 cells were inoculated intradermally into the mice to form a tumor volume of 90–100 mm3, mice were randomly selected from their cages for treatment by injecting intraperitoneal with PBS (control) and JOMD-1307(treatment). We had two experimental groups of 9 mice each for controls and treatments, which were mixed and randomly distributed into cages. JOMD-1307 treatment concentration was at 2mg/kg, corresponding with its maximum solubility. The administration was done over 4 weeks with two injections per week. and the tumor volume/size measurements are analyzed. Data points from the experiments were expressed as mean ± SEM. The harvested xenografts were incised into parts for ex vivo analysis. For target protein expressions, using the western blotting analysis method, xenografts were homogenized with a vibra-cellTM Ultrasonic processor (Bio block Scientific, ThermoFisher, Strasbourg, France), and the supernatant was used for investigation. For cell proliferation analysis with the Ki 67 method [69].
Statistical Analysis
Statistical analysis was conducted using Graph Pad Prism v 6.0 software. The data in the figures are either representative experiments or the mean +/− SEM (standard error of the mean) represents results. *: p ≤ 0.05, **: p ≤ 0.01, ***: p ≤ 0.001, were considered significant.
RESULTS
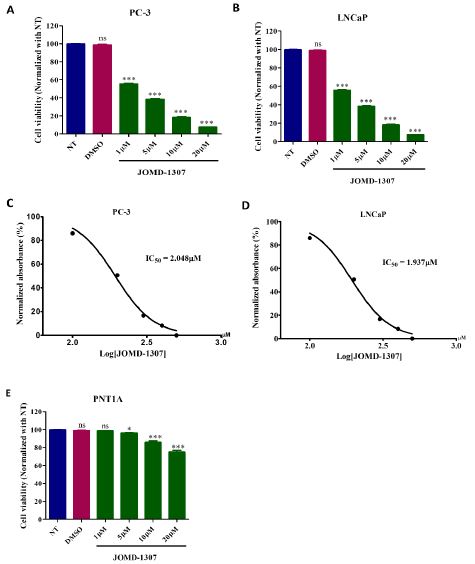
JOMD-1307 inhibits PC cell growth and progression.The therapeutic effect of JOMD-1307 on cell growth was evaluated on two PC cell lines PC-3 and LNCaP cells, as well as on the normal prostatic cell line PNT1A cells, which were measured using the MTT assay at increasing concentrations (from 1 to 20 µM). JOMD- 1307 exhibited a dose-dependent inhibition response on PC-3 and LNCaP cellular proliferation. The viability of the PC cell lines was reduced by 40% when treated at the lowest doses (1µM), and the IC50 values were approximately 2.0µM (Figureure. 1A-D). In PNT1A cells, JOMD-1307 had a low inhibition response on cellular proliferation in increasing concentrations (from 1 to 20 µM), and we observed a reduced inhibitory effect when compared to that seen in PC-3 and LNCaP cells (Figure. 1E). The viability of PNT1A cells has no significant inhibition when treated at lower doses (1µM). Therefore, these findings show that JOMD-1307 has more affinity to target and inhibit PC-3 and LNCaP cell proliferation and viability when compared to PNT1A cells. It revealed JOMD-1307 high affinity for PC cells and its ability to easily penetrate into the PC cells spearing or less affinity for the normal prostatic cell. However, it maybe the normal prostatic cell could resistant cellular stress induced by JOMD-1307 due to the reduce amount of the drug that penetrates into cell and heterogeneously distributed to distort the normal cell viability.
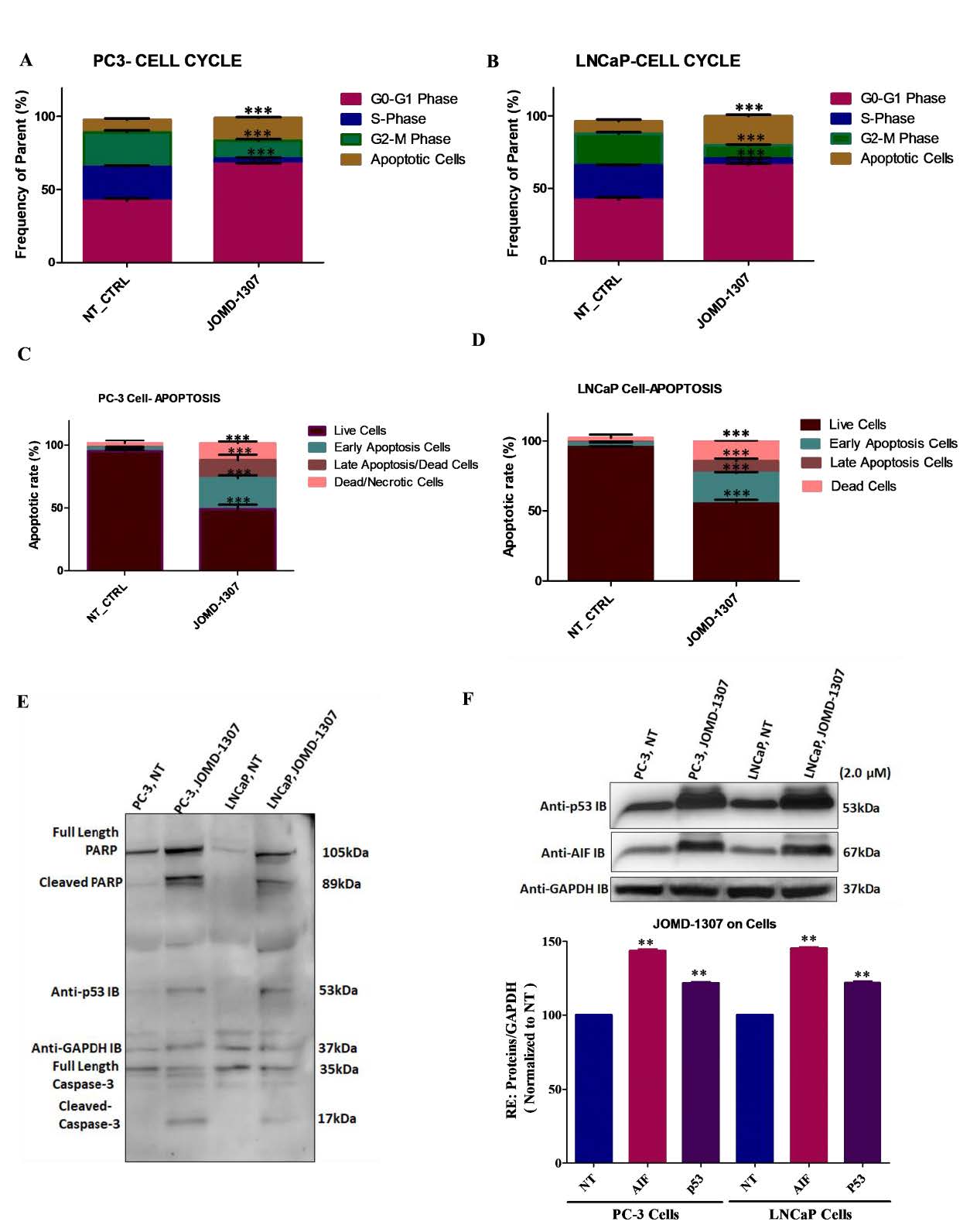
JOMD-1307 disrupts PC cell division and causes death through apoptotic inductionTo elucidate JOMD-1307stipulates anticancer activity, its effect on PC cell division and cell death was examined on PC-3 and LNCaP cells using Phospho H-3 and PI Staining method. The cells were treated with the IC50 concentration (2.0µM). Results revealed that PC-3 and LNCaP cell lines have an increased significant level of cellular population in the G0- G1 phase of 68.0% (Figure. 2A, ***P< 0.001) and 63.0% (Figure. 2B, **P< 0.001) respectively. This indicates that JOMD-1307 induces cell cycle arrest more in the G0/G1 phase than in other phases compared to non-treated cells as controls, thereby enhancing anti- proliferative activity in the PC cells (Figure. 2A-B).
To verify JOMD-1307 apoptotic induction, the fraction of apoptotic cells was significantly increased in both PC-3 and LNCaP cells by 54.6% and 51.8% respectively compared to the non-treated controls (Figure. 2 C-D, **P< 0.01). Also, western blotting analysis revealed JOMD-1307 significant induction of apoptotic proteins such as cleaved PARP, cleaved caspase-8 and -3, apoptotic-inducing factor (AIF), a mitochondrial apoptotic protein that induces programmed apoptotic cell death, and p53 expressions (Figure. 2C-F *P< 0.05; **P< 0.01). Previous studies have shown that induction of apoptotic cancer cell death is of integral importance to the objective of anticancer therapy [37-39].
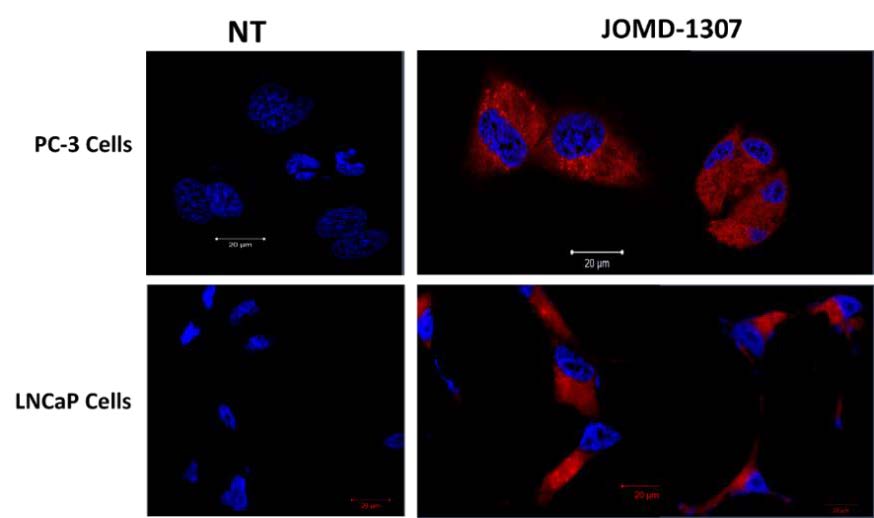
JOMD-1307 increased cellular uptake and heterogeneous distribution in PC cellsJOMD-1307 is water-soluble, absorbent, and has high affinity to penetrates PC cells. The theranostic nanoparticle has auto-fluorescence properties that emit far-red fluorescence which eases its detection when transfected on PC cells. Intracellular internalization and distribution were performed using the immunofluorescence (IF) method, confocal- microscopy to view JOMD-1307 localization after treatment concentration (2.0 µM) for 72 hrs in PC cells. It revealed significantly increased internalization, localization, and heterogeneously distributions of JOMD-1307 in the PC cell lines (PC-3 and LNCaP cells), however, there were more in the cell’s cytoplasm (Figure. 3). The increased concentration of the compound in the cytoplasm and the activation of the mitochondria protein AIF gives credence to JOMD-1307’s high mitochondrial targeting capacity in PC cells [52-56,60].
The internalization and heterogenous distribution of JOMD-1307 treatment concentration (2.0 µM) for 72 hrs in PC cell lines PC-3 and LNCaP cells. The PC cells were stained with DAPI after being treated with the auto-fluorescence JOMD-1307 compound with Bar = 20μm, which was observed using the immunofluorescence (IF) method, confocal-microscopy to view. The result revealed that treated cells show cellular distribution predominantly in the cytoplasm revealing the presence of far-red colorations and the nucleus picking bluish DAPI colorations compared to the untreated cells as controls.
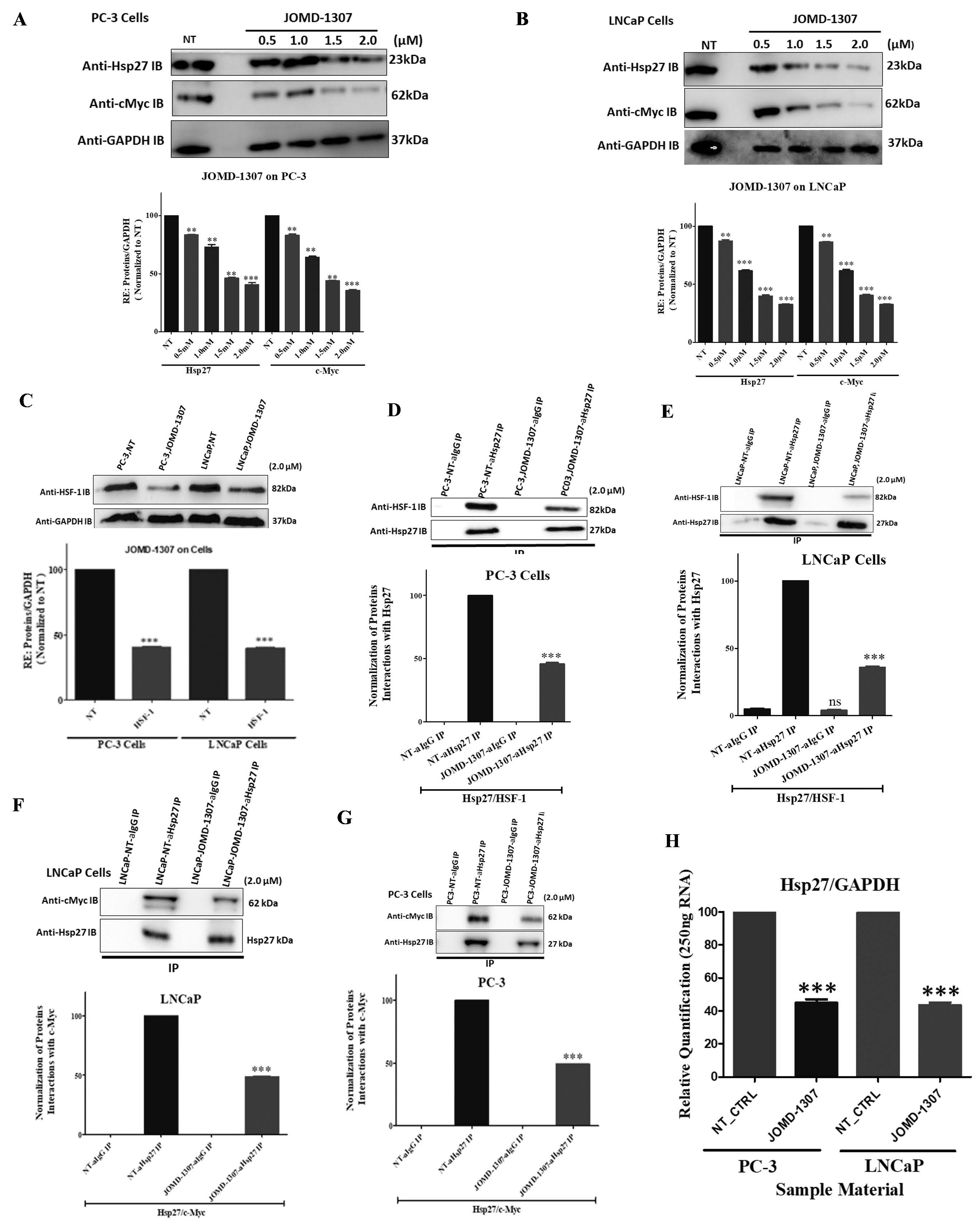
JOMD-1307 decreases Hsp27 expression and interactions and down-regulates its increased transcription in PC cellsTo elucidate and decipher the molecular mechanism of JOMD-1307 therapeutic effect on the interaction and expression of Hsp27 stress-induced signaling, and the transcription factors c-Myc and HSF-1 involvement in TRPC progression. This study interrogated the expression levels of Hsp27, c-Myc, and HSF-1, and the interaction of Hsp27 with c-Myc and HSF-1 in PC cells after JOMD-1307 treatment concentrations. It revealed suppression of Hsp27 and c-Myc expressions in PC cells (LNCaP and PC-3) in a dose-dependent manner, with increased significant inhibition in concentration (2.0µM) (Figure. 4A-B). Also, there was a significantly reduced expression level in the transcription protein HSF-1 in PC cells (Figure. 4C). While JOMD-1307 significantly disrupts protein-protein interactions (PPIs) of Hsp27 and c-Myc, and Hsp27 and HSF-1 in PC cells with equal protein extract concentrations for analysis after JOMD-1307 treatment concentration (2.0 µM), using co- immunoprecipitation (co-IP) assay (Figure. 4D-G). There was also disruption of Hsp27 mRNA expression in PC cells after treatment of JOMD-1307 concentration (2.0 µM) using the RT-qPCR method with significant effect *** p < 0.0001 (Figure. 4H).
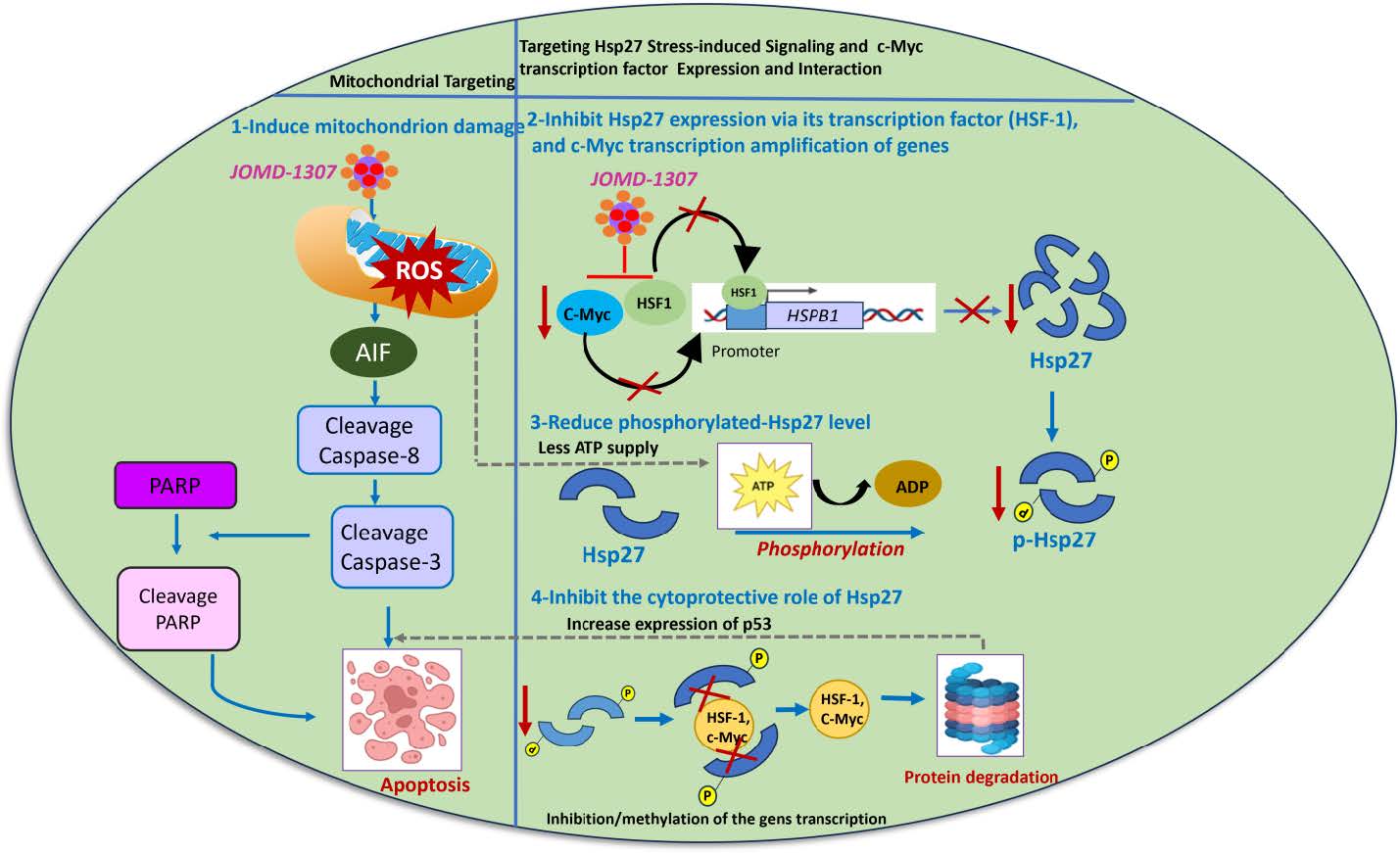
Therefore, JOMD-1307’s molecular mechanism of action from these results suggests its ability to disrupt PC cell division and induce apoptotic cell death via inhibiting Hsp27 stress-induced signaling pathways, responsible for its expression and interactions with the partner and transcriptional proteins that are involved in the downstream signaling pathways responsible for TRPC cell progression which could be efficacious due to the effective downregulation of the transcription factors c-Myc and HSF-1, which impairs the elevated transcription and phosphorylation of Hsp27 [17,23,24,27,32,33,61]. Interestingly, JOMD-1307 can intercalative bind to the nonpolar interior of the DNA helix and the single-stranded polyadenylic acid (poly-A), to inhibit and impair the mRNA-poly- A over-synthesis of target survival genes such as Hsp27(HSPB1) [30,40,50,55,62-65]. This indicates that JOMD-1307 could downregulate the Hsp27 transcription level and affect the mRNA stability translation processes. Also, previous studies have shown the inhibition of Hsp27 stress-induced signaling oncogenic functions will affect the partner proteins survival and expressions, and their increased transcription respectively in CRPC cells [27,30,33,62,66]. Therefore, the results from Figure. 4 confirm the inhibition of Hsp27 and its transcription factors could stimulate the apoptotic proteins' upregulation to induce apoptotic PC cell death [29,64,61,67,35,68,32,23,33].
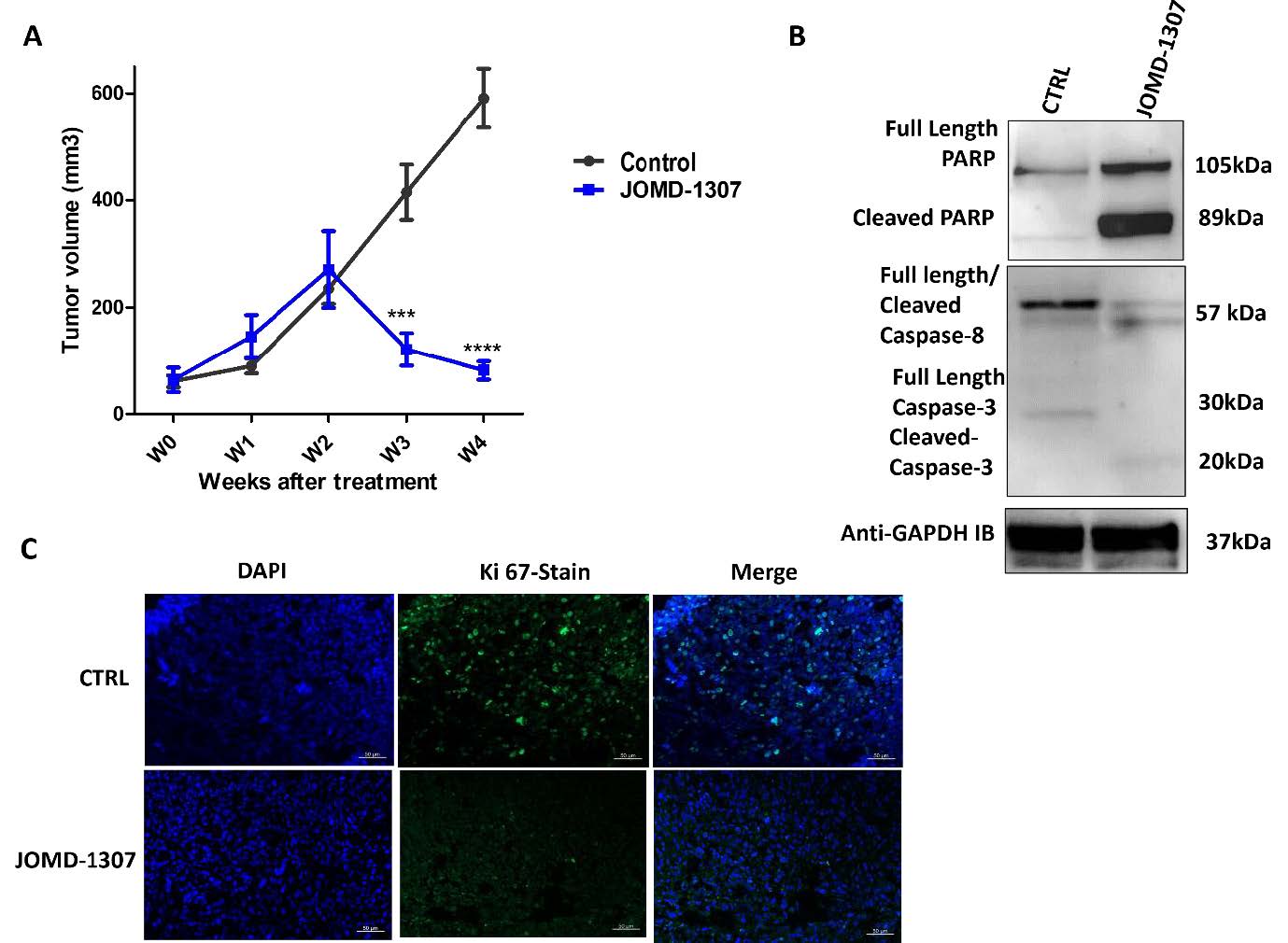
JOMD-1307 delays CR prostate tumor growth in preclinical mouse models triggers apoptotic induction, and significantly reduces proliferating tumor cellsTo further assess the efficacy of JOMD-1307's effect on delaying tumor (PC) growth and preclinical analysis of disrupting the survival proteins interaction, and expression involved in angiogenesis in tumor growth. An in vivo experiment was conducted with six- weeks-old male Bal-C Nude mice inoculated with an androgen-independent PC model (PC-3) to form 90 to 100mm3 tumor growth, and treated with JOMD-1307 concentrations (2mg/kg) for four weeks. The treatment of the mice showed statistical results from the periodic measurements demonstrating a significant delay of more than 50% tumor growth after three weeks of JOMD-1307-treated mice compared to the control mice (Figure. 5A ***P< 0.001). The measurement (in centimeters) shows a relatively significant reduction of tumor growth in JOMD-1307 treatments compared with the controls, which revealed the therapeutic efficiency of JOMD-1307 in delaying PC growth. Also, the ex vivo analysis with western blotting using extracts from JOMD-1307-treated homogenized xenograft revealed a relatively significant inducement of the apoptotic cascade (cleaved PARP, and cleaved caspase-8 and -3 expressions) (Figure. 5B). In addition, Ki 67 cells proliferation analysis using the immunofluorescence (IF) method, also shows a relatively significant reduction in proliferating cells in JOMD-1307 and Docetaxel treatments compared to the control, which reveals that there was significant induction of apoptosis after treatment with JOMD-1307 compared to the controls as shown in Figure. 5b (Figure. 5C). Therefore, these findings potentiate JOMD-1307 as an improved and promising chemotherapy agent with high therapeutic efficacy to restore the sensitivity of CRPC cells to treatment [56,69-71].
Discussion
Progression of PC, especially in the case of CRPC that occurs approximately 18-24 months after ADT has been a huge menace that needs urgent intervention [72-74]. The cellular and molecular mechanisms of action of CRPC progression are multifactorial and complex, and there are metabolic changes in the expression of survival genes to regulate apoptosis [24,75-77]. Moreover, targeting these survival genes and the signaling pathways responsible for CRPC progression is pivotal for clinicians and drug developers to improve anticancer therapy for PC progression. However, several therapeutic approaches and trials have been approved to target and inhibit these genes to treat PC progression, which are marred by noticeable bottlenecks in finding effective and safe therapeutic strategies without having adverse side effects on the patients has been the main clinical challenge. Presently, clinical trials are investigating efficient small molecule- based drugs in combination with other anti-PC therapies. Nano-vehicles for anti-PC therapy delivery are developed to control these potential side effects [78-82]. Nanoparticles are promising tools for improving cellular and molecular anticancer drug delivery to inhibit advanced PC progression [83-87]. These nano-vehicle compounds' composition is associated with their effective therapeutic efficiency, and are used as improved anticancer agents to inhibit PC cell growth [88-90]. In addition, theranostic nanoparticles or chemical compounds with high fluorescence of far-red and photodynamic therapeutic (PDT) properties, have remarkable therapeutic effects, and high affinity for cancer cells [50,52-54,56]. They are mostly mitochondria targeting causing cellular stress and can interact with cancer cell genomic molecules to disrupt survival gene synthesis in cancer cells leading to apoptotic cell death [50,52-57,60].
Therefore, a synthesized theranostic nanoparticle as an effective anticancer agent for CRPC therapy known as JOMD-1307 was elucidated in the project. JOMD-1307 has improved therapeutic functions and robust anticancer properties, that have been well characterized and can form micelle in increased volume when transfected to cancer cells [44,79,80,91]. The formation of micelle increases its cellular uptake and heterogenous distribution in cancer cells, due to its high solubility, and, the ability to easily attach and penetrate cancer cell membranes than non-cancerous cells, with reduced cytotoxic therapeutic side-effects of multidrug administration, making the JOMD-1307 a promising theranostic nanoparticle agent to be considered for cancer therapy [92-95]. Studies have shown most analogs that possess the planar phenazine ring are mitochondrial targeting such as JOMD-1307 and its high fluorescence emission can cause cellular stress when exposed to PC cells [54,55,60,96,97]. These anticancer properties may have JOMD-1307 to be used as a multifaceted anticancer agent, functioning as a diagnostic agent, photodynamic (PDT) agent, and chemotherapy agent, therefore having a combined therapy effect on cancer cells.
Experimentally, JOMD-1307's therapeutic efficacy as an anticancer agent to inhibit CRPC cell invasiveness was investigated on in vitro analysis to determine its anticancer abilities, which revealed a significant decrease in PC cell growth, and insignificant effect in normal prostatic cells (Figure. 1), and disrupts PC cell division, through apoptotic inducement (Figure. 2). This implies JOMD-1307 could disrupt cellular functions in PC cells by its ability to regulate PC cell growth, through disrupting cell cycle and induce of apoptosis, with insignificant effect on the normal epithelial prostate cells in a relative amount of the half- maximum inhibitory concentration, which gives credence to previous studies [98-101]. Moreover, apoptotic cancer cell death has been regarded as one of the ultimate goals of anticancer therapy to cause cell death, considering the blockage of the apoptotic pathway leading to apoptosis resistance in CRPC cells has been responsible for ineffective oncological treatment (therapy resistance) [99,102-104]. Therefore, clinicians and drug developers seek improved chemotherapy agents that can induce cancer cells to be susceptible to apoptotic cell death, because apoptotic inducement is of integral importance in the studies of conventional drugs to use as anticancer therapy [98-104]. Meanwhile, JOMD-1307 cellular uptake and heterogenous distribution in PC cells with high far-red auto-fluorescence presence was predominately localized in the cytoplasm of the PC cells (Figure. 3), which suggests JOMD-1307 mitochondrial targeting capacity and PDT presence of far-red emission that can cause cellular stress (ROS), gives its multifactorial therapeutic ability to delay CRPC cells growth [50,53,55,60]. In addition, survival proteins mostly Hsp27 via the alternative signaling pathway are involved in cellular survival signaling pathways (P13K/AKT/mTOR and MAPK) associated with TRPC and CRPC cell invasiveness. The increased c-Myc and HSF-1transcriptional amplification and upregulation of Hsp27 stress-induced signaling pathways (cytoprotecting and interaction with the partner proteins from undergoing proteasome degradation), thus hinders apoptotic cell death and induce cellular survival in CRPC (PC) cells progression [23,25,30,32,105,106]. Interestingly, JOMD-1307 could inhibit the expressions and interactions of Hsp27 with the c-Myc transcription function, and the Hsp27 transcription protein HSF-1 in PC cells (Figure. 4). Moreso, the inhibition of increased transcriptional regulation, expression, and interactions of Hsp27 stress-induced signaling in PC cells, affects the cellular survival of the partner proteins [15,16,28,33,48,107-109]. While, the effective inhibition of the Hsp27 stress-induced signaling pathways dysregulates the cellular downstream signaling pathways involved in the stimulation of TRPC cell's survival, therefore bringing about treatment sensitivity of CRPC progression [109-111]. In addition, JOMD-1307 could therapeutically and efficiently delay tumor growth in an in vivo experiment on PC preclinical tumor model (Figure. 5A), there was significant induction of apoptotic proteins (Figure, 5B) and from the ex vivo experiment with the xenografts, there was a significant decrease of proliferating cells in treated tumor model (Figure. 5C). This shows the antitumor efficiency of JOMD-1307 impairing the angiogenetic-mediated pathways involved in prostate tumor growth [16,109,112-114].
Therefore, these results elucidated the therapeutic effectiveness of JOMD-1307, as a prospective anticancer agent that can target and distort the mitochondria functions, disrupt survival protein Hsp27 oncogenic functions and the downstream signaling pathways, to disrupt PC cell division, and induce apoptotic cell death to inhibit CRPC cell and tumor growth (Figure. 6). In addition, the structural composition, therapeutic properties and efficacy of JOMD-1307 to mitigate CRPC cell invasiveness, propose its use as a single drug, thus can also be used for combination therapy with FDA-approved drugs used for PC treatment: e.g. Niraparib, Apalutamide, Cabazitaxel, Enzalutamide, etc for increased therapeutic effectiveness, provided synergistic compatibility is considered and possible multidrug resistance (MDR) is controlled.
Conclusion
JOMD-1307 compound’s high affinity to PC cells, cytoplasmic cellular permeability, and distribution increased its therapeutic ability to elicit anticancer effects on CRPC cells. These findings suggest that JOMD-1307 could be considered an improved promising chemotherapy agent to prevent TRPC cell progression and restore CRPC cell sensitivity to cancer treatment. However, recommended clinical trials would be considered for future elucidation of JOMD-1307 therapeutic effectiveness to mitigate CRPC invasiveness.
Interest Declaration
No conflict of interest
Contribution of Author
J.O. conceived this project; experiments were performed with collaborating institutes acknowledged; The manuscript was critically read by all authors before submission.
Acknowledgments
I acknowledge the support from Agoro, E.S., and George, G. Omietimi, J, FMC-Yenagoa, FU- Otuoke-Nigeria, NDU-Bayelsa State, INSERM-France, and CRCM-AMU-France.
Funded
FMC-Yenagoa.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 136: E359-86.
- Lin TT, Chen YH, Wu YP, Chen SZ, Li XD, Lin YZ, et al. ()2019 Risk factors for progression to castration- resistant prostate cancer in metastatic prostate cancer patients. J Cancer. 10: 5608-13.
- Anderson J, Van Poppel H, Bellmunt J, Miller K, Droz JP, Fitzpatrick JM (2007) Chemotherapy for older patients with prostate cancer. BJU Int. 99: 269-73.
- Langlais CS, Graff RE, Van Blarigan EL, Neuhaus JM, Cowan JE, Broering JM, et al. (2023) Post-diagnostic health behaviour scores and risk of prostate cancer progression and mortality. Br J Cancer. 129: 346-55.
- Fusi A, Procopio G, Della Torre S, Ricotta R, Bianchini G, Salvioni R, et al. (2004) Treatment options in hormone-refractory metastatic prostate carcinoma. Tumori. 90: 535-46.
- Verry C, Vincendeau S, Massetti M, Blachier M, Vimont A, Bazil ML, et al. (2022) Pattern of Clinical Progression Until Metastatic Castration-Resistant Prostate Cancer: An Epidemiological Study from the European Prostate Cancer Registry. Targ Oncol. 17: 441-51.
- Petrylak DP, Tangen CM, Hussain MHA, Lara PN, Jones JA, Taplin ME, et al. (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 351: 1513-20.
- Boyd RA, Majumder S, Stiban J, Mavodza G, Straus AJ, Kempelingaiah SK, et al. (2023) The heat shock protein Hsp27 controls mitochondrial function by modulating ceramide generation. Cell Reports. 42: 113081.
- Shorning BY, Dass MS, Smalley MJ, Pearson HB (2020) The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int J Mol Sci. 21: 4507
- Jamaspishvili T, Berman DM, Ross AE, Scher HI, De Marzo AM, Squire JA, et al. (2018) Clinical implications of PTEN loss in prostate cancer. Nat Rev Urol. 15: 222-34.
- Saraon P, Drabovich AP, Jarvi KA, Diamandis EP (2014) Mechanisms of Androgen-Independent Prostate Cancer. EJIFCC. 25: 42-54.
- Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. (2013) Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 155: 1309-22.
- Puhr M, Hoefer J, Eigentler A, Ploner C, Handle F, Schaefer G, et al. The Glucocorticoid Receptor Is a Key Player for Prostate Cancer Cell Survival and a Target for Improved Antiandrogen Therapy. Clin Cancer Res. 2018 Feb 15;24(4):927–38.
- Li F, Ling X, Chakraborty S, Fountzilas C, Wang J, Jamroze A, et al. (2023) Role of the DEAD-box RNA helicase DDX5 (p68) in cancer DNA repair, immune suppression, cancer metabolic control, virus infection promotion, and human microbiome (microbiota) negative influence. J Exp Clin Cancer Res. 42: 213.
- Liu AB, Liu J, Wang S, Ma L, Zhang JF (2024) Biological role and expression of translationally controlled tumor protein (TCTP) in tumorigenesis and development and its potential for targeted tumor therapy. Cancer Cell International. 24: 198.
- Ręka P, Grolik J, Stadnicka KM, Kołton-Wróż M, Wołkow P (2023) Synthesis of Nonsymmetrically Substituted 2,3-Dialkoxyphenazine Derivatives and Preliminary Examination of Their Cytotoxicity. J Org Chem. 88: 1339-51.
- Gibert B, Eckel B, Fasquelle L, Moulin M, Bouhallier F, Gonin V, et al. (2012) Knock down of heat shock protein 27 (HspB1) induces degradation of several putative client proteins. PLoS One. 7: e29719
- Freire PR, Cutler JA, Armstrong SA (2024) Therapeutic Targeting of the Menin–KMT2A Interaction. Annual Review of Cancer Biology. 8: 291-307.
- Katsogiannou M, Ziouziou H, Karaki S, Andrieu C, Henry de Villeneuve M, Rocchi P (2015) The hallmarks of castration-resistant prostate cancers. Cancer Treat Rev. 41: 588-97.
- Elbadawy M, Usui T, Yamawaki H, Sasaki K (2019) Emerging Roles of C-Myc in Cancer Stem Cell- Related Signaling and Resistance to Cancer Chemotherapy: A Potential Therapeutic Target Against Colorectal Cancer. International Journal of Molecular Sciences. 20: 2340.
- Miller DM, Thomas SD, Islam A, Muench D, Sedoris K (2012) c-Myc and Cancer Metabolism. Clin Cancer Res. 18: 5546-53.
- Hu C, Yang J, Qi Z, Wu H, Wang B, Zou F, et al. (2022) Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm. 3: e161.
- Jia G, Wu W, Chen L, Yu Y, Tang Q, Liu H, et al. (2023) HSF1 is a novel prognostic biomarker in high-risk prostate cancer that correlates with ferroptosis. Discov Oncol. 14: 107.
- Shiota M, Yokomizo A, Naito S (2011) Increased androgen receptor transcription: a cause of castration-resistant prostate cancer and a possible therapeutic target. J Mol Endocrinol. 47: R25-41.
- Jha RK, Kouzine F, Levens D (2023) MYC function and regulation in physiological perspective. Front Cell Dev Biol. 11: 1268275.
- Zhou M, Boulos JC, Omer EA, Klauck SM, Efferth T (2023) Modes of Action of a Novel c-MYC Inhibiting 1,2,4-Oxadiazole Derivative in Leukemia and Breast Cancer Cells. Molecules. 28: 5658.
- Medda A, Compagnoni M, Spini G, Citro S, Croci O, Campaner S, et al. (2023) c-MYC-dependent transcriptional inhibition of autophagy is implicated in cisplatin sensitivity in HPV-positive head and neck cancer. Cell Death Dis. 14: 1-14.
- Madden SK, de Araujo AD, Gerhardt M, Fairlie DP, Mason JM (2021) Taking the Myc out of cancer: toward therapeutic strategies to directly inhibit c-Myc. Molecular Cancer. 20: 3.
- Yoo H, Choi SK, Lee J, Park SH, Park YN, Hwang SY, et al. (2021) Drug-Like Small Molecule HSP27 Functional Inhibitor Sensitizes Lung Cancer Cells to Gefitinib or Cisplatin by Inducing Altered Cross-Linked Hsp27 Dimers. Pharmaceutics. 13.
- Chi KN, Yu EY, Jacobs C, Bazov J, Kollmannsberger C, Higano CS, et al. (2016) A phase I dose- escalation study of apatorsen (OGX-427), an antisense inhibitor targeting heat shock protein 27 (Hsp27), in patients with castration-resistant prostate cancer and other advanced cancers. Ann Oncol Off J Eur Soc Med Oncol.27: 1116-22.
- Whitfield JR, Soucek L (2021) The long journey to bring a Myc inhibitor to the clinic. Journal of Cell Biology.220: e202103090.
- Gumilar KE, Chin Y, Ibrahim IH, Tjokroprawiro BA, Yang JY, Zhou M, et al. (2023) Heat Shock Factor 1 Inhibition: A Novel Anti-Cancer Strategy with Promise for Precision Oncology. Cancers. 15: 5167.
- Chin Y, Gumilar KE, Li XG, Tjokroprawiro BA, Lu CH, Lu J, et al. (2023) Targeting HSF1 for cancer treatment: mechanisms and inhibitor development. Theranostics. 13: 2281-300.
- Chen X, Zhang T, Su W, Dou Z, Zhao D, Jin X, et al. (2022) Mutant p53 in cancer: from molecular mechanism to therapeutic modulation. Cell Death Dis. 13: 1-14.
- Lampros M, Vlachos N, Voulgaris S, Alexiou GA (2022) The Role of Hsp27 in Chemotherapy Resistance. Biomedicines. 10: 897.
- Chasov V, Davletshin D, Gilyazova E, Mirgayazova R, Kudriaeva A, Khadiullina R, et al. (2024) Anticancer therapeutic strategies for targeting mutant p53-Y220C. J Biomed Res. 38: 222-32.
- Chi KN, Yu EY, Jacobs C, Bazov J, Kollmannsberger C, Higano CS, et al. (2016) A phase I dose- escalation study of apatorsen (OGX-427), an antisense inhibitor targeting heat shock protein 27 (Hsp27), in patients with castration-resistant prostate cancer and other advanced cancers. Ann Oncol Off J Eur Soc Med Oncol.27: 1116-22.
- Suárez-García S, Solórzano R, Alibés R, Busqué F, Novio F, Ruiz-Molina D (2021) Antitumour activity of coordination polymer nanoparticles. Coordination Chemistry Reviews. 441: 213977.
- Ganguli M, Jayachandran KN, Maiti S (2004) Nanoparticles from cationic copolymer and DNA that are soluble and stable in common organic solvents. J Am Chem Soc. 126: 26-7.
- Dervan PB (2001) Molecular recognition of DNA by small molecules. Bioorg Med Chem. 9: 2215-35.
- Maiti M, Kumar GS (2007) Molecular aspects on the interaction of protoberberine, benzophenanthridine, and aristolochia group of alkaloids with nucleic acid structures and biological perspectives. Med Res Rev.27: 649-95.
- Kelkar SS, Reineke TM (2011) Theranostics: combining imaging and therapy. Bioconjug Chem. 22: 1879-903.
- Yoon HY, Jeon S, You DG, Park JH, Kwon IC, Koo H, et al. (2017) Inorganic Nanoparticles for Image- Guided Therapy. Bioconjug Chem. 28: 124-34.
- Zhang J, Ning L, Huang J, Zhang C, Pu K. Activatable molecular agents for cancer theranostics. Chem Sci. 11:618-30.
- O’Dwyer E, Bodei L, Morris MJ (2021) The Role of Theranostics in Prostate Cancer. Semin Radiat Oncol. 31: 71-82.
- Marcu L, Bezak E, Allen BJ (2018) Global comparison of targeted alpha vs targeted beta therapy for cancer: In vitro, in vivo and clinical trials. Crit Rev Oncol Hematol. 123: 7-20.
- Allen S, Osorio O, Liu YG, Scott E (2017) Facile assembly and loading of theranostic polymersomes via multi- -impingement flash nanoprecipitation. J Control Release. 262: 91-103.
- Llombart V, Mansour MR (2022) Therapeutic targeting of “undruggable” MYC. eBioMedicine. 75.
- Sarkar D, Das P, Basak S, Chattopadhyay N (2008) Binding interaction of cationic phenazinium dyes with calf thymus DNA: a comparative study. J Phys Chem B. 112: 9243-9.
- Pradhan AB, Haque L, Roy S, Das S (2014) Binding of phenazinium dye safranin T to polyriboadenylic acid: spectroscopic and thermodynamic study. PLoS One. 9: e87992.
- Wang C, Zhang J, Yin J, Gan Y, Xu S, Gu Y, et al. (2021) Alternative approaches to target Myc for cancer treatment. Sig Transduct Target Ther. 6: 1-14.
- Karuppiah V, Alagappan K, Sivakumar K, Kannan L (2016) Phenazine-1-carboxylic acid-induced programmed cell death in human prostate cancer cells is mediated by reactive oxygen species generation and mitochondrial-related apoptotic pathway. Journal of Applied Biomedicine. 14: 199-209.
- Junior R, Campanholi K, Morais F, Moraes Pinto LAD, Rando F, Pozza M, et al. (2023) Phenazines and Photoactive Formulations: Promising Photodrugs for Photodynamic Therapy.
- Zhang Z, Wang Q, Zhang X, Mei D, Mei J (2023) Modulating the Luminescence, Photosensitizing Properties, and Mitochondria-Targeting Ability of D-π-A-Structured Dihydrodibenzo[a,c]phenazines. Molecules. 28: 6392.
- Lin Y, Yang B, Huang Y, Zhang Y, Jiang Y, Ma L, et al. (2023) Mitochondrial DNA-targeted therapy: A novel approach to combat cancer. Cell Insight. 2: 100113.
- Yan J, Liu W, Cai J, Wang Y, Li D, Hua H, et al. (2021) Advances in Phenazines over the Past Decade: Review of Their Pharmacological Activities, Mechanisms of Action, Biosynthetic Pathways and Synthetic Strategies. Mar Drugs. 19: 610.
- Wang L, Evans JC, Ahmed L, Allen C (2023) Folate receptor targeted nanoparticles containing niraparib and doxorubicin as a potential candidate for the treatment of high grade serous ovarian cancer. Sci Rep. 13: 3226.
- Song Y, Lu M, Qiu H, Yin J, Luo K, Zhang Z, et al. (2018) Activation of FOXO3a reverses 5- Fluorouracil resistance in human breast cancer cells. Exp Mol Pathol. 105: 57-62.
- Zhang C, He LJ, Ye HZ, Liu DF, Zhu YB, Miao DD, et al. (2018) Nrf2 is a key factor in the reversal effect of curcumin on multidrug resistance in the HCT-8/5-Fu human colorectal cancer cell line. Mol Med Rep. 18: 5409-16.
- Zong WX, Rabinowitz JD, White E (2016) Mitochondria and Cancer. Mol Cell. 61: 667-76.
- Lama D, Vosselman T, Sahin C, Liaño-Pons J, Cerrato CP, Nilsson L, et al. (2024) A druggable conformational switch in the c-MYC transactivation domain. Nat Commun. 15: 1865.
- Umar H, Mukerjee N, Bello R, Alshehri M, Chukwuemeka P, Awolaja O, et al. (2022) Discovery of Novel HSP27 Inhibitors as Prospective Anti-Cancer Agents Utilizing Computer-Assisted Therapeutic Discovery Approaches. Cells.
- Nappi L, Aguda AH, Nakouzi NA, Lelj-Garolla B, Beraldi E, Lallous N, et al. (2020) Ivermectin inhibits HSP27 and potentiates efficacy of oncogene targeting in tumor models. J Clin Invest. 130: 699-714.
- Bouvard C, Lim S, Ludka J, Yazdani N, Woods A, Chatterjee A, et al. (2017) Small molecule selectively suppresses MYC transcription in cancer cells. Proceedings of the National Academy of Sciences. 114: 201702663.
- Llombart V, Mansour MR (2022) Therapeutic targeting of “undruggable” MYC. eBioMedicine. 75: 103756.
- Shiota M, Yokomizo A, Naito S (2011) Increased androgen receptor transcription: a cause of castration-resistant prostate cancer and a possible therapeutic target. J Mol Endocrinol. 47: R25-41.
- Zhou M, Boulos JC, Omer EA, Klauck SM, Efferth T (2023) Modes of Action of a Novel c-MYC Inhibiting 1,2,4-Oxadiazole Derivative in Leukemia and Breast Cancer Cells. Molecules. 28: 5658.
- Singh A, Kumar P, Sarvagalla S, Bharadwaj T, Nayak N, Coumar MS, et al. (2022) Functional inhibition of c-Myc using novel inhibitors identified through “hot spot” targeting. Journal of Biological Chemistry. 298.
- Mrouj K, Andrés-Sánchez N, Dubra G, Singh P, Sobecki M, Chahar D, et al. (2021) Ki-67 regulates global gene expression and promotes sequential stages of carcinogenesis. Proc Natl Acad Sci U S A. 118: e2026507118.
- Pratheeshkumar P, Budhraja A, Son YO, Wang X, Zhang Z, Ding S, et al. (2012) Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR- 2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS One. 7: e47516.
- Lv J jia, Song W ting, Li X min, Gao J mei, Yuan Z li (2020) Synthesis of a New Phenyl Chlormethine- Quinazoline Derivative, a Potential Anti-Cancer Agent, Induced Apoptosis in Hepatocellular Carcinoma Through Mediating Sirt1/Caspase 3 Signaling Pathway. Front Pharmacol. 11: 911.
- Vellky JE, Ricke WA (2020) Development and prevalence of castration-resistant prostate cancer subtypes. Neoplasia. 22: 566-75.
- Thurin NH, Rouyer M, Gross-Goupil M, Rebillard X, Soulié M, Haaser T, et al. (2020) Epidemiology of metastatic castration-resistant prostate cancer: A first estimate of incidence and prevalence using the French nationwide healthcare database. Cancer Epidemiology. 69: 101833.
- Lucas A, Petrylak DP (2006) The case for early chemotherapy for the treatment of metastatic disease. J Urol. 176: S72-75.
- Perks C, Takahashi SI (2022) The Role of the IGF/Insulin-IGFBP Axis in Normal Physiology and Disease. Frontiers Media SA; 98 p.
- Miyake H, Nelson C, Rennie PS, Gleave ME (2000) Overexpression of insulin-like growth factor binding protein-5 helps accelerate progression to androgen-independence in the human prostate LNCaP tumor model through activation of phosphatidylinositol 3’-kinase pathway. Endocrinology. 141: 2257-65.
- Kiyama S, Morrison K, Zellweger T, Akbari M, Cox M, Yu D, et al. (2003) Castration-induced increases in insulin-like growth factor-binding protein 2 promotes proliferation of androgen- independent human prostate LNCaP tumors. Cancer Res. 63: 3575-84.
- Kotta S, Aldawsari HM, Badr-Eldin SM, Nair AB, YT K (2022) Progress in Polymeric Micelles for Drug Delivery Applications. Pharmaceutics. 14: 1636.
- Torchilin VP (2007) Nanocarriers. Pharm Res. 24:2333-4.
- Oh JK, Siegwart DJ, Lee H il, Sherwood G, Peteanu L, Hollinger JO, et al. (2007) Biodegradable nanogels prepared by atom transfer radical polymerization as potential drug delivery carriers: synthesis, biodegradation, in vitro release, and bioconjugation. J Am Chem Soc. 129: 5939-5.
- Keskin D, Zu G, Forson AM, Tromp L, Sjollema J, van Rijn P (2021) Nanogels: A novel approach in antimicrobial delivery systems and antimicrobial coatings. Bioactive Materials. 6: 3634-57.
- Fulton MD, Najahi-Missaoui W (2023) Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int J Mol Sci. 24: 6615.
- Milani S, Berti D, Dante S, Hauss T, Baglioni P (2009) Intercalation of single-strand oligonucleotides between nucleolipid anionic membranes: a neutron diffraction study. Langmuir. 25: 4084-92.
- Bildstein L, Dubernet C, Marsaud V, Chacun H, Nicolas V, Gueutin C, et al. (2010) Transmembrane diffusion of gemcitabine by a nanoparticulate squalenoyl prodrug: an original drug delivery pathway. J Control Release. 147: 163-70.
- Baroud M, Lepeltier E, Thepot S, El-Makhour Y, Duval O (2021) The evolution of nucleosidic analogues: self-assembly of prodrugs into nanoparticles for cancer drug delivery. Nanoscale Adv. 3: 2157-79.
- Moreau L, Barthélémy P, Li Y, Luo D, Prata CAH, Grinstaff MW (2005) Nucleoside phosphocholine amphiphile for in vitro DNA transfection. Mol Biosyst. 1: 260-4.
- Zhou X, Wang S, Zhu Y, Pan Y, Zhang L, Yang Z (2020) Overcoming the delivery barrier of oligonucleotide drugs and enhancing nucleoside drug efficiency: The use of nucleolipids. Med Res Rev. 40: 1178-99.
- Dutta K, Das R, Medeiros J, Kanjilal P, Thayumanavan S (2021) Charge-Conversion Strategies for Nucleic Acid Delivery. Adv Funct Mater. 31: 2011103.
- Ceballos C, Khiati S, Prata CAH, Zhang XX, Giorgio S, Marsal P, et al. (2010) Cationic nucleoside lipids derived from universal bases: A rational approach for siRNA transfection. Bioconjug Chem. 21: 1062-9.
- Khiati S, Pierre N, Andriamanarivo S, Grinstaff MW, Arazam N, Nallet F, et al. (2009) Anionic nucleotide--lipids for in vitro DNA transfection. Bioconjug Chem. 20: 1765-72.
- Rochford G, Molphy Z, Kavanagh K, McCann M, Devereux M, Kellett A, et al. (2020) Cu(ii) phenanthroline-phenazine complexes dysregulate mitochondrial function and stimulate apoptosis. Metallomics. 12: 65-78.
- Ajith S, Almomani F, Elhissi A, Husseini GA (2023) Nanoparticle-based materials in anticancer drug delivery: Current and future prospects. Heliyon. 9: e21227.
- Deshmukh R, Singh V, Harwansh RK, Agrawal R, Garg A, Singh S, et al. (2024) Emerging Trends of Nanomedicines in the Management of Prostate Cancer: Perspectives and Potential Applications. Pharmaceutics. 16: 297.
- Manogue C, Fleming W, Ledet E, Jaeger E, Layton J, Barata P, et al. (2022) Continuous IV Infusion of 5-Flourouracil in Heavily Pretreated Metastatic Castrate-Resistant Prostate Cancer. Clin Genitourin Cancer. 20: 586-90.
- Guzmán Rodríguez A, Sablón Carrazana M, Rodríguez Tanty C, Malessy MJA, Fuentes G, Cruz LJ (2022) Smart Polymeric Micelles for Anticancer Hydrophobic Drugs. Cancers (Basel). 15: 4.
- Dyshlovoy SA, Pelageev DN, Hauschild J, Borisova KL, Kaune M, Krisp C, et al. (2019) Successful Targeting of the Warburg Effect in Prostate Cancer by Glucose-Conjugated 1,4- Naphthoquinones. Cancers (Basel). 11: 1690.
- Fontana F, Anselmi M, Limonta P (2023) Unraveling the Peculiar Features of Mitochondrial Metabolism and Dynamics in Prostate Cancer. Cancers (Basel). 15: 1192.
- Ocker M, Höpfner M (2012) Apoptosis-modulating drugs for improved cancer therapy. Eur Surg Res. 48: 111-20.
- Singh V, Khurana A, Navik U, Allawadhi P, Bharani KK, Weiskirchen R (2022) Apoptosis and Pharmacological Therapies for Targeting Thereof for Cancer Therapeutics. Sci. 4: 15.
- Njangiru IK, Bózsity-Faragó N, Resch VE, Paragi G,Frank É, Balogh GT, et al. (2024) A Novel 2- Methoxyestradiol Derivative: Disrupting Mitosis Inhibiting Cell Motility and Inducing Apoptosis in HeLa Cells In Vitro. Pharmaceutics. 16: 622.
- Kim Y, Yoo S, Lim B, Hong JH, Kwak C, You D, et al. (2023) A novel biguanide derivative, IM176, induces prostate cancer cell death by modulating the AMPK-mTOR and androgen receptor signaling pathways. Prostate Int. 11: 83-90.
- Sato A, Hiramoto A, Kim HS, Wataya Y (2020) Anticancer Strategy Targeting Cell Death Regulators: Switching the Mechanism of Anticancer Floxuridine-Induced Cell Death from Necrosis to Apoptosis. International Journal of Molecular Sciences. 21: 5876.
- Kim R, Kin T, Beck WT (2024) Impact of Complex Apoptotic Signaling Pathways on Cancer Cell Sensitivity to Therapy. Cancers. 16: 984.
- Diepstraten ST, Anderson MA, Czabotar PE, Lessene G, Strasser A, Kelly GL (2022) The manipulation of apoptosis for cancer therapy using BH3-mimetic drugs. Nat Rev Cancer. 22: 45-64.
- Qiu X, Boufaied N, Hallal T, Feit A, de Polo A, Luoma AM, et al. (2022) MYC drives aggressive prostate cancer by disrupting transcriptional pause release at androgen receptor targets. Nat Commun. 13: 2559.
- Hu C, Yang J, Qi Z, Wu H, Wang B, Zou F, et al. (2022) Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm. 3: e161.
- Weber LI, Hartl M (2023) Strategies to target the cancer driver MYC in tumor cells. Front Oncol. 13: 1142111.
- Hung CL, Liu HH, Fu CW, Yeh HH, Hu TL, Kuo ZK, et al. (2023) Targeting androgen receptor and the variants by an orally bioavailable Proteolysis Targeting Chimeras compound in castration resistant prostate cancer. EBioMedicine. 90: 104500.
- He Y, Xu W, Xiao YT, Huang H, Gu D, Ren S (2022) Targeting signaling pathways in prostate cancer: mechanisms and clinical trials. Sig Transduct Target Ther. 7: 1-31.
- Zhang Y, Ming A, Wang J, Chen W, Fang Z (2024) PROTACs targeting androgen receptor signaling: Potential therapeutic agents for castration-resistant prostate cancer. Pharmacological Research. 205: 107234.
- Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, et al. (2021) Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Sig Transduct Target Ther. 6: 1-48.
- Poirier D, Roy J, Maltais R, Weidmann C, Audet-Walsh É (2023) An Aminosteroid Derivative Shows Higher In Vitro and In Vivo Potencies than Gold Standard Drugs in Androgen-Dependent Prostate Cancer Models. Cancers. 15: 3033.
- Hao Q, Wu Y, Vadgama JV, Wang P (2022) Phytochemicals in Inhibition of Prostate Cancer: Evidence from Molecular Mechanisms Studies. Biomolecules. 12: 1306.
- Pratheeshkumar P, Budhraja A, Son YO, Wang X, Zhang Z, Ding S, et al. (2012) Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR- 2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS One. 7: e47516.

Figures at a glance