Clinical Outcome in Periampullary Carcinoma Patients Treated with Adjuvant Chemoradiation: A Retrospective Study
Received Date: December 28, 2024 Accepted Date: January 28, 2025 Published Date: January 31, 2025
doi: 10.17303/jcrto.2025.13.102
Citation: Dr Ritu R Menon, Dr Arun Sankar S, Dr Lijeesh AL, Dr Preethi Sara, Dr Sajeed A, et al. (2025) Clinical Outcome in Periampullary Carcinoma Patients Treated with Adjuvant Chemoradiation: A Retrospective Study. J Cancer Res Therap Oncol 13: 1-19
Abstract
Aim and Objectives: This study was done to assess the outcomes in Periampullary Carcinoma patients who received Adjuvant Chemoradiation after surgical resection. The primary objectives were to assess the overall survival and disease free survival. The secondary objectives were to assess the patterns of relapse and also to evaluate the prognostic factors for survival.
Methods: Using a retrospective review, the overall survival, disease free survival, locoregional recurrence rates and prognostic factors were assessed for patients with Periampullary Carcinoma treated with Adjuvant Chemoradiation at Regional Cancer Centre, Thiruvananthapuram from March 1st 2014 to March 31st 2020.
Results and Discussion: A total of 92 patients were included in this study. The 2 year disease free survival (DFS) was 52% and overall survival (OS) was 62.2% for the entire cohort. At a median follow up of 48.5 months, 45 (48.9%) patients relapsed. Only 7 patients (15.5%) developed isolated loco-regional recurrences and 16 patients (35.5%) had loco-regional recurrences associated with distant metastases. Higher clinical and pathological stage as well as presence of perineural invasion, poorly differentiated histology and Classical type of Surgery were associated with a poorer outcome. The results of this study as well as the prognostic factors on survival are consistent with that obtained in previous similar studies from literature.
Conclusion: The results of this retrospective study suggest that Adjuvant Chemoradiation after curative resection of periampullary carcinoma, especially for locally advanced disease had survival advantage even though the long term prognosis is poor.
Keywords: Outcome; Periampullary Carcinoma; Adjuvant Chemoradiation; Prognosis; Chemoradiation=
Introduction
Periampullary Carcinoma comprises heterogenous group of neoplasms arising from head of pancreas, ampulla of Vater, distal common bile duct and second part of duodenum [1]. True periampullary carcinomas include those arising from ampulla of Vater, distal common bile duct and second part of duodenum [1]. The incidence is about 0.5-2% of all gastrointestinal neoplasms and 20% of all tumors of the extrahepatic biliary tree [2-4] and also prognosis is generally poor.
The curative treatment for periampullary Carcinoma is complete surgical resection and a sophisticated approach for diagnosis and treatment is needed to make sure that patients with periampullary carcinomas are treated optimally [11,17]. Localized disease is usually treated by pancreaticuduodenectomy which is considered the standard surgical procedure for ampullary carcinoma.
The outcome of resected ampullary cancer depends on the extent of local invasion, status of the surgical margins, presence or absence of nodal metastases and pathological subtype [21]. Five-year survival rates following pancreaticoduodenectomy range from 64 to 80 percent for patients with node-negative disease, and from 17 to 50 percent for node-positive disease [22,23]. There is no exact agreement regarding the optimal management of patients after resection of periampullary Carcinoma. Various clinical trials have compared the efficacy of Adjuvant Chemotherapy and Adjuvant Chemoradiation.
In a meta-analysis of 10 retrospective studies in 2016, patients who received either Adjuvant Chemo radiation or no Adjuvant therapy after resection of an ampullary Carcinoma, concluded that adjuvant Chemoradiation significantly reduced the risk of death even though more patients had locally advanced disease or nodal metastases in the treated group [35]. But the use of chemotherapy alone or chemoradiation or both adjuvant therapies following pancreatectomy is still unclear and also no global consensus has emerged suggesting superiority of any.
In our hospital Regional Cancer Centre, Trivandrum, all patients with pathological stage pT2 and above, pathological Node positive disease, inadequate nodal sampling, or HPR showing aggressive features (margin positive disease, perineural invasion and lymphovascular invasion) are given Adjuvant Chemoradiation followed by Adjuvant Chemotherapy. Hence in this study we assess the clinical outcome, patterns of relapse as well as prognostic factors in periampullary carcinoma patients treated with adjuvant chemoradiation.
Aim
To assess the clinical outcome in Periampullary Carcinoma patients treated with Adjuvant Chemoradiation at Regional Cancer Centre, Trivandrum during the time period 01-03- 2014 to 31-03-2020.
Objectives
Primary
To assess the Overall Survival and Disease free survival
Secondary
To assess the patterns of relapse.
To evaluate the prognostic factors for survival.
Methodology
Study Design – Retrospective Study
Study Population-Patients registered in Regional Cancer Centre, Trivandrum with the diagnosis of Periampullary carcinoma between 01-03-2014 to 31-03- 2020 who were treated with Adjuvant Chemoradiation.
Inclusion Criteria
1. Age 18 to 70 Ys
2. ECOG PS ≤ 2
3. Histologically proven Periampullary Carcinoma.
4. Patients who received Adjuvant treatment at RCC after Radical Surgery
5. Location of primary tumour – Ampulla of vater and distal CBD
Exclusion criteria
i. Past history of malignancy
ii. Previous Chemotherapy and Radiotherapy
iii. Neuroendocrine tumours of the pancreas
Procedure
The study was started after obtaining approval from the institutional review board on 3/11/2020 (IRB No- 11/2020/01). The case sheets of the patients diagnosed with periampullary carcinoma, who received Adjuvant chemoradiation during the specified time period were retrieved from the medical records department. The demographic data of patients, baseline investigations, stage of the disease, nature of primary treatment, adjuvant treatments, follow up were collected from the case records and the same details were filled up in the structured proforma.
Periampullary carcinoma patients after surgical resection were the population considered for Adjuvant Chemoradiation after Multidisciplinary tumor board discussion. Those who received only Adjuvant Chemotherapy and Adjuvant Radiation alone were not included in the study and hence not analyzed. Also the patients who were kept on follow up only, post-surgery were not included and analyzed.
Patients diagnosed with periampullary carcinoma after undergoing radical surgery, depending upon their postoperative histopathology report were subjected to Adjuvant treatment. Adjuvant therapy usually starts 6-8 weeks after surgical resection. Patients planned for Adjuvant Chemoradiation were simulated in a CT Simulator in supine position with comfortable bladder filling, and immobilized using wing board after obtaining informed consent. Intravenous contrast and oral contrast were administered for simulation. Topographic images were taken from T4-L5 in 2.5mm cuts. The acquired images were then sent to 3D Treatment Planning system. The same were used for contouring as per RTOG contouring guidelines [54].
Majority of them were planned using IMRT technique, delivering a dose of 45Gy over 25fractions using 6MV photons. Almost all of them were given concurrent chemotherapy during the course of radiation treatment, most of them received Concurrent Tab Capecitabine 825mg/m2 twice daily per orally on all days of Radiation. After completion of the proposed treatment course of Concurrent chemoradiation they were asked to report after 1-2 months. The consideration of Adjuvant Chemotherapy was under the discretion of the treating physician. Most of the patients received Adjuvant Chemotherapy with Intravenous Gemcitabine 1gm/m2.
The patients were followed up after completion of treatment at serial intervals with clinical examination and monitoring of serum CA 19-9 levels along with imaging as and when indicated. They were followed up at 3 monthly interval for first 2 years, 6 monthly interval for next 5 years and yearly follow up thereafter. The followup data was updated until 31st August 2021. In case of clinical suspicion or raised tumour marker on follow up evaluation, they were subjected to Imaging to rule out recurrence or development of a new primary if any.
Overall survival was calculated from the date of diagnosis to the date of death or last follow up. Disease free survival was calculated from the date of surgery to the date of disease recurrence. Patterns of relapse were collected and recorded from the case sheets. Any relapse at the primary site (tumour bed and anastomotic site) or regional node were taken as loco regional, while relapse at any other site was considered as systemic relapse. The management of recurrent disease, if Radical or Palliative and mode of treatment was also collected from the case files.
Results
Patient Characteristics
Data of 92 were available for retrospective analysis with a median age of 56 years (30-70 years), 53 patients (57.6%) were males and 39 patients (42.4%) were females. Only 24 male patients (26%) were smokers.
Most of the patients had comorbidities, diabetes being the most common among them. While 19 patients (20.7%) had an ECOG performance status of 1, the remaining 73 patients (79.3%) had ECOG Performance status 2.
Sixty-three patients (68.5%) had primary symptoms as loss of appetite, followed by jaundice (67.4% ) and itching (52.2%).
Thirty-two patients (34.7%) had elevated CA 19-9 levels and only seven patients (7.6%) had associated gallstones.
Tumour Characteristics
The location of primary tumour was confirmed by histopathology report. Seventy two patients (78.26%) had tumour located in the ampulla of vater and distal common bile duct was the primary site of tumour in 20 patients (21.73%).
Majority of the patients (78.2%) had moderately differentiated grade of Adenocarcinoma. The histology of pancreaticobiliary and intestinal type was reported only in 5 patients each (5.4%), the histology status of remaining 82 patients were uncategorised.
Majority of the patients had pT3 disease (61 patients, 66.3%), and 30 patients (32.5%) had early stage (pT1 & T2) disease. Only 31 patients (33.6%) had twelve or more lymph nodes being removed during surgery which was taken as adequate nodal dissection. While most patients (49 patients, 53.2%) were lymph node negative, 31(33.6%) and 12(13%) patients had pN1 and pN2 disease respectively.
Forty three patients (46.7%) had composite stage III, while 21 (22.8%) and 28 (30.4%) patients had stage I & II disease respectively.
Post-operative histopathology report showed that 17 patients (18.5%) had lymphovascular invasion and 21 patients (22.8%) had perineural invasion.
The histopathology report of 46 patients (50%) confirmed infiltration of duodenal wall and that of 23 patients (25%) had pancreatic invasion.
Treatment Characteristics
Patients included in the study received Adjuvant treatment after curative surgery. The curative surgery was either Pylorus preserving Pancreaticoduodenectomy (82.6%) or classical surgery (17.4%). Only 1 patient had R1 resection. All patients underwent feeding jejunostomy along with the surgery.
The median interval between surgery and the start of Adjuvant treatment was 62 days (Range-38-74 days).
All 92 patients received adjuvant chemoradiation, this was followed by chemotherapy in 84 patients, 8 patients were unwilling and did not receive adjuvant chemotherapy.
The technique of Radiation delivery was IMRT or IGRT in 54 patients (58.6%), 3DCRT in 38 patients (41.3%).
85 patients received radiation to a dose of 45 Gy in 25 fractions, 6 patients received 45 Gy in 23 fractions and one patient 50 Gy in 25 fractions. Only one patient had interruption in the radiation, for a period of 17 days due to personal reasons, the patient received 2 additional fractions(3.6 Gy in 2 fractions) as gap correction.
The Concurrent Chemotherapy agent used in majority of the patients (N=86, 93.5%) was Oral Capecitabine at a dose of 825mg/m2 twice daily. Other regimen used was IV 5-Fluorouracil 500mg on the first three and last three days of Radiation.
After the completion of Adjuvant Chemoradiation, 84 patients (91.3%) received Adjuvant Chemotherapy with a median interval of 34 days. The most common Chemotherapy agent used was IV Gemcitabine 1 g/m2 D1D8 three weekly (82.6%) upto six cycles. Other agents used were Capecitabine, 5 Flurouracil and Calcium Leucovorin.
Treatment Outcome
After a median follow-up of 48.5 months, out of the 92 patients with periampullary carcinoma treated by radical intent with curative surgery followed by adjuvant chemoradiation and chemotherapy, 45 patients developed recurrence during the follow-up period.
8 patients had marker elevation during follow up and were evaluated for recurrence and relapse. Out of which only 2 of them had radiological and clinical evidence of recurrence. The remaining 6 patients had only transient elevation of CA 19-9 and the serum marker reverted back to normal on further follow-up.
Survival Outcomes
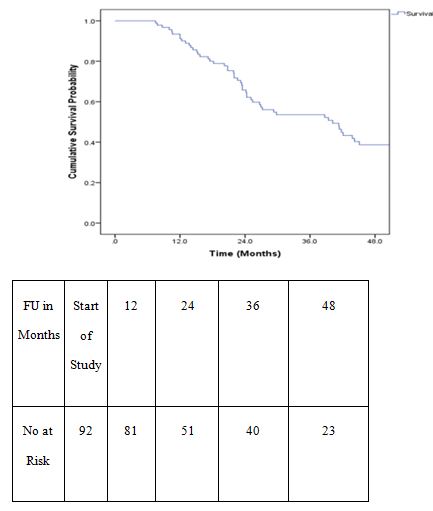
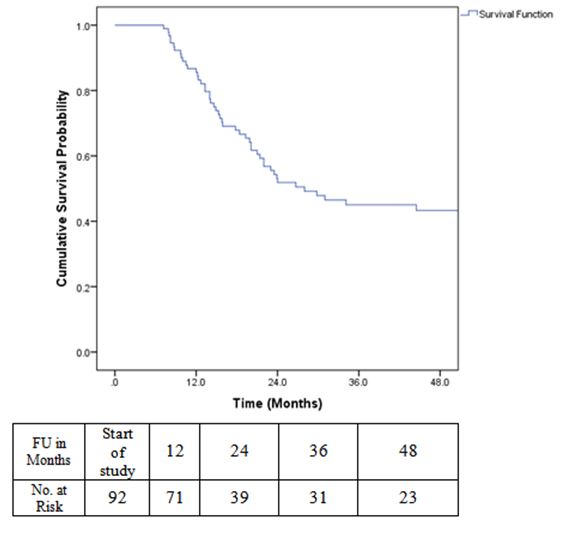
Overall survival time was calculated from the date of diagnosis to the date of death or last follow up. Survival was estimated using Kaplan Meier method. The 2 year overall survival for the entire group of patients analysed was 62.2%.
Disease free survival (DFS) was calculated from the date of surgery to the date of disease recurrence. DFS at 2 years was 52%.
Patterns of Relapse
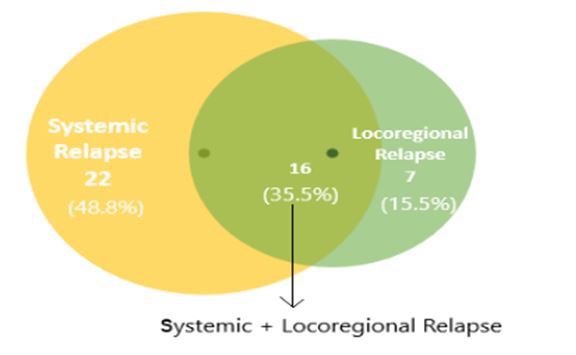
Any relapse at the primary site (tumour bed and anastomotic site) or regional node was taken as loco regional, while relapse at any other site was considered as systemic relapse. In this study out of 45 patients who relapsed, 22 patients (48.8%) had systemic relapse, 7 patients (15.5%) had locoregional relapse and remaining 16 patients (35.5%) had a combination of both.
Out of 45 patients who relapsed, 23 patients (51.1%) had stage III disease initially, whereas only 9 patients (20%) of Stage I had relapse.
In the study, total 38 patients had systemic relapse and the sites of relapse were liver being the most common (21 patients-55.2%) followed by lung (9 patients-23.6%) and bone (8 patients-21%).
On relapse, patients were treated with Palliative Chemotherapy and Radiotherapy. Best supportive care was the option in patients with poor performance status. Out of these, 39 patients died due to disease progression and only 6 patients were alive with disease at the time of data analysis.
Survival Analysis of Prognostic Factors
Patient Related Factors
The patient-related factors with potential prognostic value with respect to 2 years overall and disease-free survival were estimated using Cox-regression model. Age, gender and habits of the study population were analysed to evaluate the impact on survival. A p value of <0.05 was considered as significant. It was observed that none of the patient related factors had a significant influence on survival.
Tumour Related Factors
Various tumour related factors were recorded and analyzed. The tumour-related factors included location, pathological T and N stage, grade, lymphovascular invasion, perineural invasion and pancreatic invasion.
There was a significant difference in overall survival by pathological nodal stage (2 year OS for pN0 patients was 72.3% and that for pN1 and pN2 patients was 50.2%; p value-0.037). Also patients with poorly differentiated histology had a poorer disease free survival when compared to those with well and moderately differentiated histology (2 year DFS for patients with moderate and poorly differentiated histology was 47.4% and it was 72.8% for patients with well differentiated histology; p value-0.032).
Patients with perineural invasion had shorter Overall and Disease free Survival when compared to those who did not have perineural invasion (2 year OS for patients with PNI was 41.3% and for those without PNI was 67.8%; p value-0.011, 2 year DFS for patients with PNI was 34.4% and for those without PNI was 56.5%; p value-0.035).
Treatment Related Factors
Preoperative biliary drainage, type of surgery, technique of Radiation and Adjuvant Chemotherapy were the treatment related factors which were analysed.
It was observed preoperative biliary drainage did not have any impact on survival.
Patients who underwent Pylorus preserving pancreaticoduodenectomy had a better overall survival than those who underwent Classical surgery (2 year OS for patients who underwent PPPD was 65.9% and it was 43.1% for those who underwent Classical Surgery, p value-0.045).
Univariate Analysis
Univariate Cox regression for OS and DFS was done for various prognostic factors. It was observed that the risk of death was lower for patients who underwent PPPD, who did not have PNI and who had pathological N0 stage.
Cox regression analysis for DFS showed that risk of recurrence was higher for patients with perineural invasion and those with poorly differentiated histology.
Multivariate Analysis
Multivariate analysis showed significant difference in OS in terms of type of surgery and perineural invasion. Patients who underwent Classical Surgery had a hazard ratio of 2.169 (95% CI for HR 1.094 -4.298) as compared to those who underwent Pylorus preserving pancreaticoduodenectomy. The hazard ratio for patients with perineural invasion was 2.341 (95% CI for HR 1.256 -4.363) when compared to those without perineural invasion.
There was a significant difference in DFS with respect to grade of tumour in multivariate analysis. Patients who had moderate to poorly differentiated histology had a hazard ratio of 2.645 (95% CI for HR 1.047 -6.682) as compared to those who had well differentiated histology.
Discussion
Periampullary carcinoma contributes to only a small proportion of GI malignancies worldwide and is associated with a poor survival even in patients who had undergone a curative resection. Adjuvant treatment including Adjuvant chemoradiation and Chemotherapy are given after surgery in an attempt to improve survival even though these modalities have toxicities.
In our hospital Regional Cancer Centre, Trivandrum, all patients with pathological stage pT2 and above ,pathological Node positive disease, inadequate nodal sampling, or HPR showing aggressive features (margin positive disease, perineural invasion and lymphovascular invasion) are given Adjuvant Chemoradiation (45Gy/25# with Concurrent Capecitabine) followed by Adjuvant Chemotherapy (Inj Gemcitabine 1g/m2 IV D1 D8 Q 3 weekly x 4-6 cycles).This study was done to evaluate the survival outcomes in patients with resected periampullary carcinoma treated with Adjuvant Chemoradiation.
The first study showing an effect of Adjuvant Chemoradiation in pancreatic Cancer was the GITSG trial in which the efficacy of combined radiation and fluorouracil as adjuvant therapy for pancreatic cancer was evaluated, but they excluded patients with Periampullary Carcinoma [27]. The median survival for the treatment group (20 months) was significantly longer than that observed for the control group (11 months) and hence the study was closed prematurely.
In this study overall survival at 24 months was 62.2% and median follow up was 48.5 months. The landmark trial by Klinkenbijl and colleagues, EORTC Phase III trial evaluating the role of Adjuvant Radiotherapy and 5-Fluorouracil after curative resection of cancer of the pancreas and Periampullary Region [32] had an estimated 2 year OS of 70% for periampullary carcinoma patients treated with Adjuvant Chemoradiation. In the ESPAC3 trial [28] the 2 year OS for Adjuvant chemotherapy arm (Gemcitabine as well as Fluorouracil plus folinic acid group) was around 65% and the median survival was 35.2 months. The good survival outcome in our study could also be influenced by the fact that 22% of our patients had stage I disease.
45 patients relapsed in this study and as evident from literature sources [32,33] systemic relapses were more than loco regional recurrences. Liver was the most common site of relapse (55.2%) followed by lung and bone. This was similar to the collaborative study conducted at the John Hopkins hospital and Mayo Clinic where 24.7% had liver metastasis. In the EORTC Phase III trial [32] also liver was the most common site of relapse (34%).
From the available literature [20,21] the tumour related factors that predicts the survival were pathological stage, grade of the tumour, presence of lymphovascular invasion and perineural invasion. Patients with advanced pathological stage, poorly differentiated histology, LVSI and PNI have poor survival outcome [20].
In this study, patients with early pathological T stage had a better survival trend compared to those with locally advanced disease (2 year OS for pT1 and pT2 was 72% and that for pT3 and pT4 was 57.5%, p value 0.175) however it was not statistically significant. The study conducted at the Institute of Liver and Biliary Sciences, New Delhi by Baghmar & Agrawal in which the sample size was 95 (similar to this study) also showed poor overall survival for pathological T3 and T4 tumours when compared to early stage and this was statistically significant.
In this study, patients with lymph node involvement (pN1 and pN2) had shorter disease free and overall survival when compared to patients with no lymph node involvement (2 year OS for pN0 and pN1 pN2 was 72.3% and 50.2% with p value 0.037, 2 year DFS for pN0 and pN1 pN2 was 60.1% and 42.4% with p value 0.105). A similar finding was observed in the Indian study conducted by Baghmar, in which 59 pathological node positive patients had poorer survival when compared to node negative patients (pN+--HR 2.4 (1.17–5.1); p value-0.017 for OS and HR 3.5 (1.6–7.6); p value- 0.002 for PFS. Also in the French multicentric study conducted by Robert and colleagues (21) the median DFS as well as OS was found to be significantly shorter for patients with lymph node metastases than that for patients without lymph node involvement (Median DFS for pN0 was 21.1 months and for pN+ was 121 months ; p value= 0.0001, Median OS for pN0 was 124.5 months and for pN+ was 37 months; p value= 0.0002).The study conducted in Germany by Lemke and colleagues (20), where survival and prognostic factors in Pancreatic and Ampullary Cancer were analysed, it was observed that, absence of lymph node metastasis substantially improved survival in ampullary cancer, but not in pancreatic ductal adenocarcinoma. This could be because in that study majority of the patients with pancreatic cancer were diagnosed with a histopathologically-advanced primary tumor (pT3 and pT4) and existence of lymph node metastases (pN1).
This study had only 31 patients (33.6%) with adequate nodal dissection (twelve or more lymph nodes being removed during surgery). The study conducted by Falconi and colleagues published in the Annals of Surgical Oncology in 2008 [26] evaluating the prognostic relevance of number of lymph nodes being resected in Carcinoma of Ampulla of Vater concluded that patients with sixteen or more resected lymph nodes had a 5-year disease-specific survival (DSS) of 81% compared with 45% in those who had lower number of lymph nodes being resected with p value of 0.001. Also the role of extended retroperitoneal lymphadenectomy for Periampullary Carcinoma was evaluated in a single institutional series at The Johns Hopkins Medical Institutions, Baltimore, Maryland [18,25].
The study had 4 patients with pathological T1 stage and all of them relapsed, 2 patients had associated pathological N2 disease and the other two had inadequate lymph node sampling. This finding suggests that as mentioned in the previous trials lymph node involvement has more prognostic importance than tumor extent in determining survival of patients with Periampullary Carcinoma.
The study had eighteen patients with well differentiated histology and they had better survival outcome (2 year OS for well differentiated tumours vs moderately and poorly differentiated tumours was 69.9% and 60.5% with p value -0.225, 2 year DFS for well differentiated tumours vs moderately and poorly differentiated tumours was 72.8% and 47.4% with p value -0.032). The Indian study by Baghmar also showed that patients with well differentiated histology had a better survival trend when compared to those with moderate to poorly differentiated histology however no statistical significance was obtained and the study included only two patients with poorly differentiated histology (poorly differentiated histology–HR--1.7 (0.7–3.8); p value- 0.16 for PFS, HR– 1.55 (0.7–3.3); p value- 0.25). A significant influence on survival in terms of differentiation of tumour was observed in the study by Lemke and colleagues [20], where patients with poorly differentiated histology had a HR of 1.9 when compared to those with well to moderately differentiated histology with a p value of <0.01 in terms of overall survival.
Lymphovascular and perineural invasion also had influence on survival. In this study patients without LVSI and PNI had better disease free and overall survival trend, however statistical significance was obtained only in terms of perineural invasion both for overall survival as well as for disease free survival (2 year OS for patients with LVSI was 42.1% and for those without LVSI was 72.3% with p value 0.119, 2 year DFS for patients with LVSI was 41.3% and for those without LVSI was 54.3% with p value 0.33, 2 year OS for patients with PNI was 41.3% and for those without PNI was 67.8% with p value 0.011, 2 year DFS for patients with PNI was 34.4% and for those without PNI was 56.5% with p value 0.0.35). In the French multicentric study [21] also, median overall survival was much more for patients without LVSI, but it was not statistically significant.
Also absence of PNI was a factor for better overall survival as evident from literature [20,21]. In the study by Bettschart and colleagues [13], 88 patients with ampullary neoplasm were prospectively analysed to assess the predictors of survival. It was observed that patients without PNI had a significant median survival when compared to those with PNI (p value-0·001).The Indian study by Baghmar of 95 patients with Periampullary Carcinoma, 63 patients had PNI. Their 5 year PFS and OS were shorter, although not statistically significant, when compared to those without PNI (HR–1.2 (0.6–2.3); p value– 0.56 for PFS, HR–1.9 (0.9–4.0); p value- 0.06 for OS).
The study showed that ampullary cancers had a better survival trend when compared to cancers arising from distal common bile duct (2 year OS for patients with ampullary carcinoma was 67.5% and for patients with distal CBD 2 year OS was only 45% eventhough it was not statistically significant, 2 year DFS for ampullary tumours 52% whereas for distal CBD tumours it was 51.3%). The study conducted at the University of Chicago Medical Center [1], included 647 tumors of the duodenum, Ampulla, head of Pancreas, and distal CBD. It was observed that there was a significantly increased (p < 0.001) rate of 5 year survival for patients with tumors of the ampulla or duodenum as compared to patients with tumors of the bile duct or head of the pancreas (21% and 0.9%, respectively). Indian study by Baghmar & Agrawal [36] published in 2018 also showed that survival for ampullary carcinoma was better when compared to distal CBD tumours (estimated 5-year survival was 66% for ampullary carcinoma and for distal CBD tumours it was only 21%).
The influence on survival by certain other factors were also reported in the literature which included the requirement of preoperative biliary drainage, pancreatic invasion, type of resection and also the histological subtypes.
This study had 30 patients who underwent preoperative biliary drainage, their 2 year OS was 47.8% and DFS was 34.8%, the 2 year OS and DFS for those who did not undergo preoperative biliary drainage was 74.6% and 60.8% , this difference was however not statistically significant. In the study done at Department of Gastroenterology and Hepatology, Singapore General Hospital [19] it was observed that biliary drainage before surgery for ampullary cancer significantly reduced postoperative wound infection with no influence on overall survival. The study by Baghmar showed a poorer overall survival for those who underwent preoperative biliary drainage.
In this study pancreatic invasion was reported only in 23 patients and they had a 2 year OS of 60.1% and 2 year DFS of 57.9%. The difference in OS and DFS when compared to patients without pancreatic invasion was not statistically significant. The study by Bettschart and colleagues [13] reported a significant difference in median survival in terms of pancreatic invasion (Median survival of 46.7 months for those without pancreatic invasion vs 35.1 months for those with pancreatic invasion, p value-0·018).
Only 1 patient in this study had R1 resection, hence the type of resection was not considered for survival analysis. In the single institutional study by Baghmar, 8 patients had R1 resection and their survival trend was poor when compared to those with R0 resection (HR– 1.9 (0.89–4.3) 0.09 for OS, HR– 1.3 (0.55–3.1) 0.51 for PFS).
In this study the histological subtype of pancreaticobiliary and intestinal was reported only in five patients each, hence the same was not analysed for survival prognosis. This factor had an influence on survival as evident from literature. In the retrospective French Multicentric Study [21], patients with pancreaticobiliary type had poor survival outcome when compared to those with intestinal type. A notable finding in that study was that patients with pancreaticobiliary type also had other aggressive features like higher pathological stage, presence of LVSI and PNI. The study by Baghmar, also revealed poor survival outcome for pancreaticobiliary subtype.
Overall survival was better for patients who underwent pylorus preserving pancreaticoduodenectomy (2 year OS for PPPD was 65.9% and those who underwent Classical surgery was 43.1%, this difference was statistically significant with p value 0.045) in this study. This improved rates of overall survival could probably be attributed to the fact that majority of patients who underwent pylorus preserving pancreaticoduodenectomy had ampullary cancers. The trials that compared the type of surgery in periampullary cancers could not conclude the superiority of PPPD over classical approach [22].
The dose of Adjuvant Radiation followed at our centre, for pancreatic as well as periampullary tumours is 45Gy in 25 fractions and 92.3% of patients in this study received the same. This was comparable to the Radiation dose used in both the EORTC trials [32,33]. In the Indian study conducted at Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow by Sikora and colleagues Adjuvant Radiotherapy was delivered at a median dose of 50.4Gy. The Indian study by Baghmar evaluating the role of adjuvant treatment in Periampullary Carcinoma also had a Chemoradiation dose of 52 Gy/25 fractions. The concurrent Chemotherapy agent used in all these studies was 5-Fluorouracil or its pro drug Oral Capecitabine, similar to this study.
Following Concurrent Chemoradiation, 82.6 % of patients in this study received Adjuvant Chemotherapy with IV Gemcitabine, 8 patients did not receive any Adjuvant Chemotherapy, and remaining 8 patients received other chemotherapeutic agents (Capecitabine, 5FU and Calcium Leucovorin, FOLFIRINOX, CAPEOX and Cisplatin with Gemcitabine). In the study conducted by Regine and colleagues, published in JAMA Oncology [34] 221 patients with resected pancreatic cancer received Adjuvant Gemcitabine and also had a survival benefit, although this improvement was not statistically significant. In this study also, patients who received Adjuvant Chemotherapy had a better overall survival than those who did not receive Adjuvant Chemotherapy even though it was not statistically significant. The ESPAC 3 trial evaluated the role of Adjuvant chemotherapy in Periampullary Carcinoma [28], patients after curative resection were randomized to observation alone or Adjuvant Chemotherapy with either IV Folinic acid with 5 Fluorouracil or to IV Gemcitabine.In the observation group, the median survival was 35.2 months (95%% CI, 27.2-43.0 months) and was 43.1 (95%, CI, 34.0-56.0) in the 2 chemotherapy groups (hazard ratio, 0.86; (95% CI, 0.66-1.11; p value=0.25). After adjusting for independent prognostic variables of age, bile duct cancer, poor tumor differentiation, and positive lymph nodes and after conducting multiple regression analysis, the hazard ratio for chemotherapy compared with observation was 0.75 (95% CI, 0.57-0.98; p value=0.03), thus concluding that multivariable analysis demonstrated a statistically significant survival benefit associated with Adjuvant Chemotherapy.
Thus Adjuvant Chemoradiation may be considered in the treatment of periampullary carcinoma, especially for locally advanced disease, and the decision to recommend it to the patient must be based on proper pathological staging and adverse features in the postoperative histopathology report, also taking into account the possible risk factors for adverse effects of treatment.
Strengths and Limitations
The study has several strengths and limitations. 98.9% of patients completed the planned course of Radiation with Concurrent Chemotherapy without any interruption. Also 91.3% of patients reported for Adjuvant Chemotherapy and received the same without any interruption. Being a single institutional study all patients received treatment under a uniform protocol. The study results show the survival of patients is comparable to that of similar studies from literature.
The limitations included the retrospective nature of the study and a limited sample size of 92 patients. After surgery, only patients fit for adjuvant treatment receive the same, and this selection bias limits the value of this study and also impacts on survival results. A major limitation of the study is the lack of toxicity data. Being a retrospective study, follow-up bias can also influence the study results. There was no direct comparison between Adjuvant Chemotherapy and Chemoradiation in this study and the optimal treatment option is still unknown. Also there is a need for a longer follow up period to assess long term survival among the study population.
Conclusion
The results of this retrospective study suggest that Adjuvant Chemoradiation after curative resection of periampullary carcinoma, especially for locally advanced disease had survival advantage even though the long term prognosis is poor.
Acknowledgement
I express my sincere gratitude to my guide, Dr Arun Sankar S, Associate Professor, for his valuable guidance, timely advice and help in the completion of this thesis. I also express my sincere gratitude to my co-guide Dr Lijeesh A L, Assistant Professor, for his great help and support in the completion of this thesis.
I am greatly indebted to Dr Francis V James, Professor and HOD, Department of Radiation Oncology, for his valuable guidance and support from the inception to completion of this thesis.
I am grateful to Dr Aleyamma Mathew,Professor and HOD, Dr Preethi Sara George, Additional Professor in Statistics and Epidemiology for their help in the statistical analysis.
I also express my gratitude to all staff of the library, Medical records & information centre, for their contribution in compiling this work.
I am also extremely thankful to my family and colleagues for all the support and encouragement.
I would also like to thank Almighty for letting me through difficult times and helping me in successful completion of my thesis
Finally, I thank all the patients who are the substance of this work.
- Michelassi F, Erroi F, Dawson PJ, Pietrabissa A, Noda S, Handcock M, et al. (1989) Experience with 647 consecutive tumors of the duodenum, ampulla, head of the pancreas, and distal common bile duct. Ann Surg. 210: 544-54.
- Benhamiche AM, Jouve JL, Manfredi S, Prost P, Isambert N, Faivre J (2000) Cancer of the ampulla of Vater: results of a 20-year population-based study. Eur J Gastroenterol Hepatol. 12: 75-9.
- Goodman MT, Yamamoto J (2007) Descriptive study of gallbladder, extrahepatic bile duct, and ampullary cancers in the United States, 1997-2002. Cancer Causes Control. 18: 415-22.
- Poruk KE, Griffin JF, Wolfgang CL, Cameron JL (2019) Chapter 96 - Pancreatic and Periampullary Cancer. In: Yeo CJ, editor. Shackelford’s Surgery of the Alimentary Tract, 2 Volume Set (Eighth Edition). Philadelphia: Elsevier; 1136-48.
- Urbach DR, Swanstrom LL, Khajanchee YS, Hansen PD (2001) Incidence of cancer of the pancreas, extrahepatic bile duct and ampulla of Vater in the United States, before and after the introduction of laparoscopic cholecystectomy. Am J Surg. 181: 526-8.
- Chan C, Herrera MF, de la Garza L, Quintanilla-Martinez L, Vargas-Vorackova F, Richaud-Patin Y, et al. (1993) Clinical Behavior and Prognostic Factors of Periampullary Adenocarcinoma [Internet]. Vol. 222, Annals of Surgery. 632-7.
- Sikdar N, Saha G, Dutta A, Ghosh S, Shrikhande SV, Banerjee S (2018) Genetic Alterations of Periampullary and Pancreatic Ductal Adenocarcinoma: An Overview. Curr Genomics. 19: 444-63.
- Ray-Offor E (2019) Periampullary cancer and cancer in head of pancreas: What is the difference? [Internet]. Vol. 4, Gastroenterology, Hepatology and Endoscopy.
- Martin JA, Haber GB (2003) Ampullary adenoma: clinical manifestations, diagnosis, and treatment. Gastrointest Endosc Clin N Am. 13: 649-69.
- Kapural L, Grace PD (2017) Nausea and Vomiting Associated with Abdominal Wall Pain [Internet]. Nausea and Vomiting. 69-75.
- Bakkevold KE, Arnesjø B (1992) Carcinoma of the pancreas and papilla of Vater: Presenting symptoms, signs, and diagnosis related to stage and tumour site a prospective multicentre trial in 472.
- Adsay V, Ohike N, Tajiri T, Kim GE, Krasinskas A, Balci S, et al. (2012) Ampullary Region Carcinomas: Definition and Site Specific Classification With Delineation of Four Clinicopathologically and Prognostically Distinct Subsets in an Analysis of 249 Cases. Am J Surg Pathol. 36: 1592.
- Bettschart V, Rahman MQ, Engelken FJF, Madhavan KK, Parks RW, Garden OJ (2004) Presentation, treatment and outcome in patients with ampullary tumours. Br J Surg. 91: 1600-7.
- Tsuji T, Hiraoka T, Kanemitsu K, Takamori H, Tanabe D, Tashiro S (2001) Lymphatic spreading pattern of intrahepatic cholangiocarcinoma. Surgery. 129: 401-7.
- Ryan D, Mamon H, Fernandez-del Castillo C (2013) Ampullary carcinoma: treatment and prognosis.
- Shimizu T, Akita M, Sofue K, Toyama H, Itoh T, Fukumoto T, et al. (2019) Pancreatobiliary-type intraductal papillary mucinous neoplasm of the pancreas may have 2 subtypes with distinct clinicopathologic and genetic features [Internet]. Vol. 91, Human Pathology. 26-35.
- Ahn DH, Bekaii-Saab T (2014) Ampullary cancer: an overview. Am Soc Clin Oncol Educ Book. 112-5.
- Yeo CJ, Cameron JL, Lillemoe KD, Sohn TA, Campbell KA, Sauter PK, et al. (2002) Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 236: 355-66.
- Abdullah SA, Gupta T, Jaafar KA, Chung YFA, Ooi LLPJ, Mesenas SJ (2009) Ampullary carcinoma: effect of preoperative biliary drainage on surgical outcome. World J Gastroenterol. 15: 2908-12.
- Lemke J, Schäfer D, Sander S, Henne-Bruns D, Kornmann M (2014) Survival and prognostic factors in pancreatic and ampullary cancer. Anticancer Res. 34: 3011-20.
- Robert P-E, Leux C, Ouaissi M, Miguet M, Paye F, Merdrignac A, et al. (2014) Predictors of long-term survival following resection for ampullary carcinoma: a large retrospective French multicentric study. Pancreas. 43: 692-7.
- Diener MK, Knaebel HP, Heukaufer C, Antes G (2007) A systematic review and meta-analysis of pylorus-preserving versus classical pancreaticoduodenectomy for surgical treatment of periampullary.
- Roberts R, Krige JE, Bornman P, Terblanche J (1999) Pancreaticoduodenectomy for ampullary carcinoma. Atlanta. 65: 1043-8.
- Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA, et al. (2000) Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 4: 567-79.
- Riall TS, Cameron JL, Lillemoe KD, Campbell KA, Sauter PK, Coleman J, et al. (2005) Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma--part 3: update on 5-year survival. J Gastrointest Surg. 9: 1191-204.
- Falconi M, Crippa S, Domínguez I, Barugola G, Capelli P, Marcucci S, et al. (2008) Prognostic relevance of lymph node ratio and number of resected nodes after curative resection of ampulla of Vater carcinoma. Ann Surg Oncol. 15: 3178-86.
- Kalser MH, Ellenberg SS (1985) Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 120: 899-903.
- Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, et al. (2012) Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 308: 147-56.
- Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 350: 1200-10.
- Neoptolemos JP, Stocken DD, Tudur Smith C, Bassi C, Ghaneh P, Owen E, et al. (2009) Adjuvant 5-fluorouracil and folinic acid vs observation for pancreatic cancer: composite data from the ESPAC-1 and -3(v1) trials. Br J Cancer. 100: 246-50.
- Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. (2013) Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 310: 1473-81.
- Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, et al. (1999) Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 230: 776-82.
- Smeenk HG, van Eijck CHJ, Hop WC, Erdmann J, Tran KCK, Debois M, et al. (2007) Long-term Survival and Metastatic Pattern of Pancreatic and Periampullary Cancer After Adjuvant Chemoradiation or Observation: Long-term Results of EORTC Trial 40891. Ann Surg. 246: 734.
- Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, et al. (2008) Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 299: 1019-26.
- Kim BH, Kwon J, Chie EK, Kim K, Kim YH, Seo DW, et al. (2018) Adjuvant Chemoradiotherapy is Associated with Improved Survival for Patients with Resected Gallbladder Carcinoma: A Systematic Review and Meta-analysis. Ann Surg Oncol. 25: 255-64.

Tables at a glance
Figures at a glance