Non-gestational Choriocarcinoma in the Lung with Kidney Metastasis
Received Date: January 17, 2025 Accepted Date: February 17, 2025 Published Date: February 20, 2025
doi:10.17303/jcrto.2025.13.104
Citation: Zorán Belics, Antónia Fürich, Dorottya Rózsa, Mária Madarász, Levente Bogyó, et al. (2025) Non-gestational Choriocarcinoma in the Lung with Kidney Metastasis. J Cancer Res Therap Oncol 13: 1-9
Abstract
Choriocarcinoma is a highly aggressive malignant tumor primarily composed of cytotrophoblast and syncytiotrophoblast cells without villi, characterized by beta-human chorionic gonadotropin (beta-hCG) production. While most cases are of gestational origin, rare non-gestational choriocarcinomas can arise from pluripotent germ cells in extragenital locations, independent of pregnancy. Primary pulmonary choriocarcinoma is extremely rare, with kidney metastasis being particularly uncommon in the literature. We present a case of a 43-year-old patient with this unusual presentation, describing the diagnostic journey from an implantation of unknown location to the final histopathological diagnosis based on lobectomy and nephrectomy specimens, including molecular genetic identification of the pulmonary tumor. Following thoracic and renal surgery, the patient received adjuvant chemotherapy and immunotherapy (anti-PD-1 blocking monoclonal antibodies). Ongoing monitoring includes regular imaging studies and beta-hCG testing.
Keywords: Gestational Trophoblastic Disease; beta-hCG; Primary Pulmonary Choriocarcinoma; Renal Metastasis; Chemotherapy; Immunotherapy
Introduction
Choriocarcinoma is a highly vascular and invasive tumor composed of anaplastic trophoblasts, specifically cytotrophoblasts and syncytiotrophoblasts without villi. The tumor can be classified as either gestational or non-gestational based on its origin. Non-gestational choriocarcinoma may arise from germ cells or develop in association with high-grade somatic malignancies [1]. Both types produce beta-human chorionic gonadotropin hormone (beta-hCG).
The incidence of choriocarcinoma varies geographically, occurring in 1/30,000 births in Europe and 1/1,000 births in Asia. Among gestational choriocarcinomas, 80% develop from molar pregnancies, 15% follow miscarriage or childbirth, and approximately 5% originate in the ovaries. Following a molar pregnancy, malignant transformation of residual trophoblast cells can occur after a variable latency period ranging from weeks to years. The tumor frequently metastasizes to highly vascularized organs, including the brain, lungs, and liver.
Non-gestational choriocarcinoma, a pluripotent germ cell tumor, can affect both sexes. In women, it typically occurs post-menopause, with the primary tumor developing in extragenital organs such as the bladder, lung, mediastinum, or liver. In men, it usually originates from testicular germ cells and commonly presents in extragenital locations [2].
Primary pulmonary choriocarcinoma is particularly rare, with fewer than 40 cases reported in the medical literature [3-8]. This case report details the diagnostic process of an implantation of unknown location that led to the discovery of this rare diagnosis, managed through a multidisciplinary approach.
Case Report
A 43-year-old woman presented to our hospital with a 53-day amenorrhea and positive pregnancy test, despite regular menstrual cycles. Her medical history was notable for one previous uncomplicated pregnancy resulting in delivery via cesarean section. Transvaginal ultrasound revealed a normal-sized uterus with regular 10 mm endometrial thickness and bilaterally normal adnexa. Despite the missed period and positive pregnancy test, we could not confirm either intrauterine or extrauterine implantation, necessitating further investigation.
Initial laboratory testing revealed markedly elevated serum beta-hCG levels of 3,189 IU/L, which continued to rise to 3,481 IU/L after two days. Diagnostic curettage was performed, and histopathological examination of the endometrial tissue showed proliferative endometrium without evidence of chorionic or decidual tissue, and no cellular atypia.
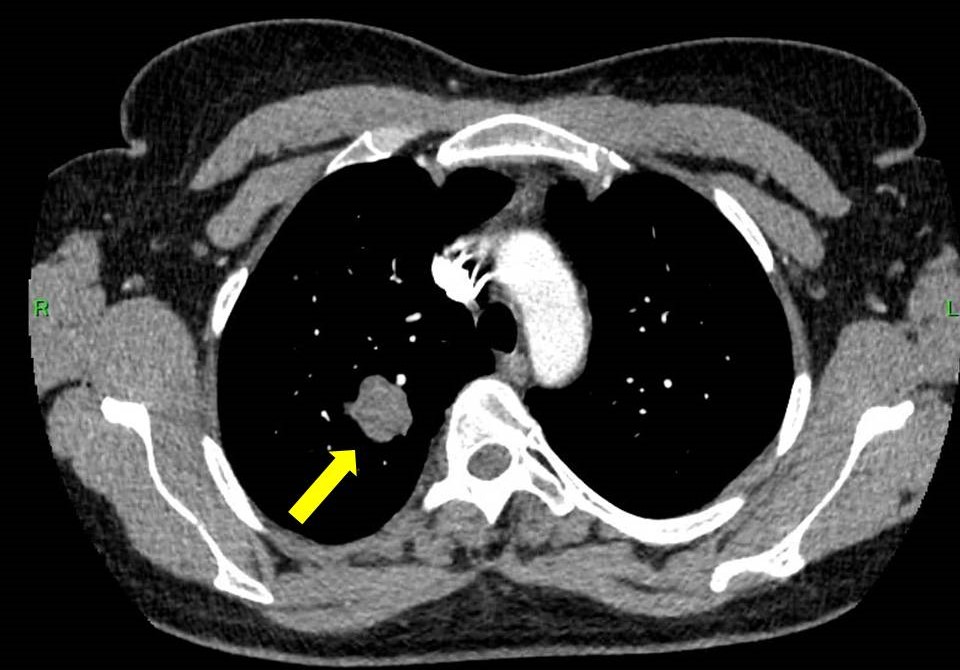
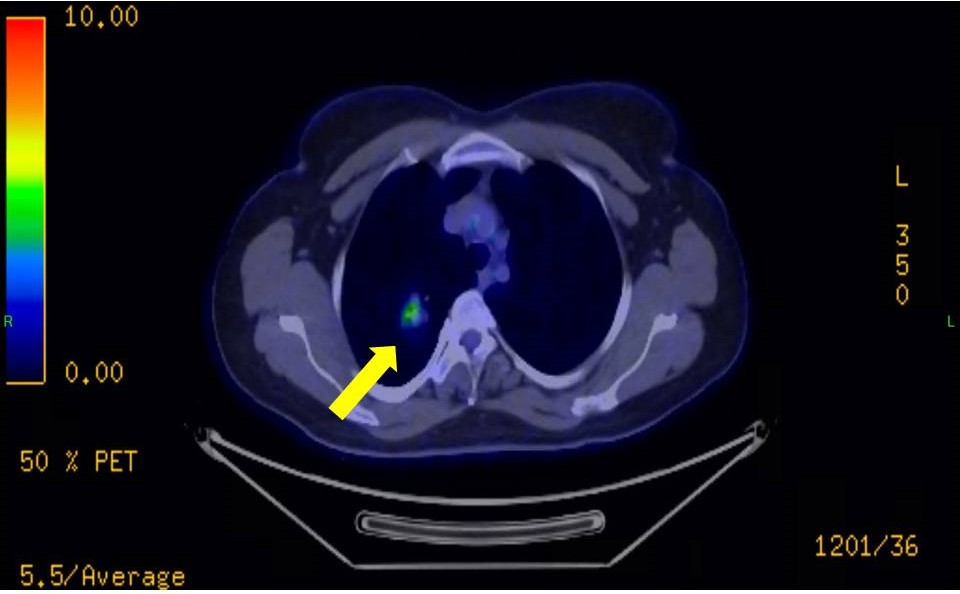
Prior to planned diagnostic laparoscopy, the patient developed new symptoms including hemoptysis and right lower back pain. CT angiography and PET-CT imaging of the chest and abdominal CT revealed a malignant tumor in the right upper lobe (figure 1,2) and hemorrhagic cysts in the right kidney. During this period, serum beta-hCG levels continued to rise, reaching 4,273 IU/L.
Surgical management proceeded in two stages. First, right nephrectomy was performed, with histopathological examination confirming choriocarcinoma metastasis. Subsequently, video-assisted thoracoscopic surgery was undertaken to remove the right upper lobe along with regional lymphadenectomy. Post-operatively, serum beta-hCG levels decreased to 827 IU/L.
Molecular genetic testing was performed (DNA isolation method: Promega, Maxwell RSC DNA FFPE kit, DNA concentration, quality (ng/ml): I.: 27,9; II.: 39 ng/μl, Short tandem repeat (STR) assay Qiagen ESSPlex SEQS kit: Samples I and II are identical) to differentiate between choriocarcinoma of gestational and germ cell origin. The absence of identifiable paternal alleles in the tumor tissue suggested non-gestational origin. The patient then received four cycles of combination chemotherapy with bleomycin, etoposide, and cisplatin, resulting in a reduction of serum beta-hCG to 3 IU/L.
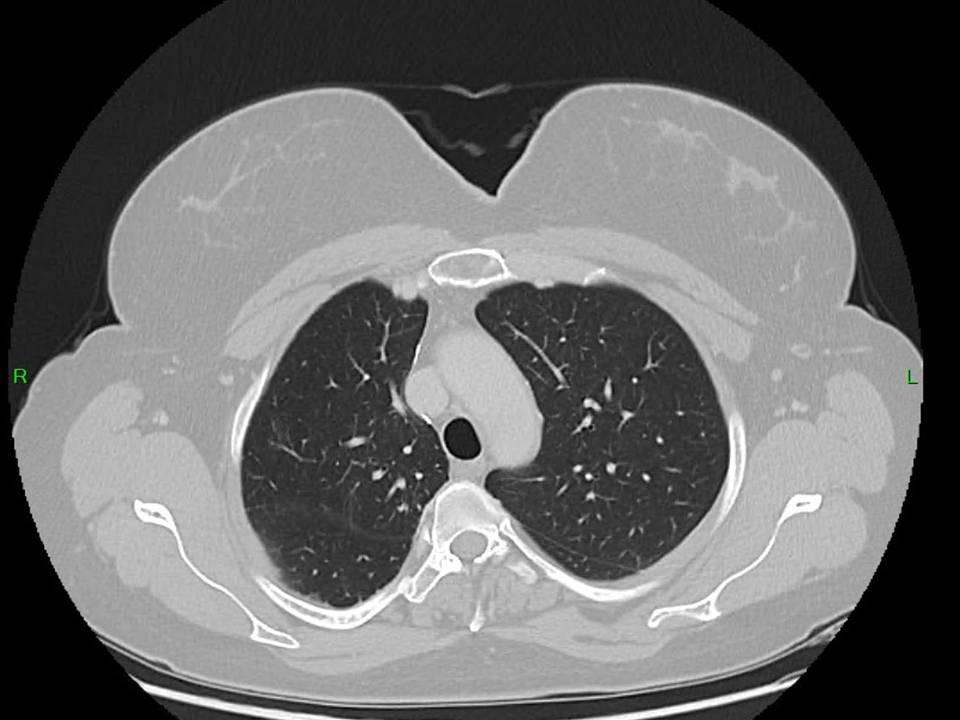
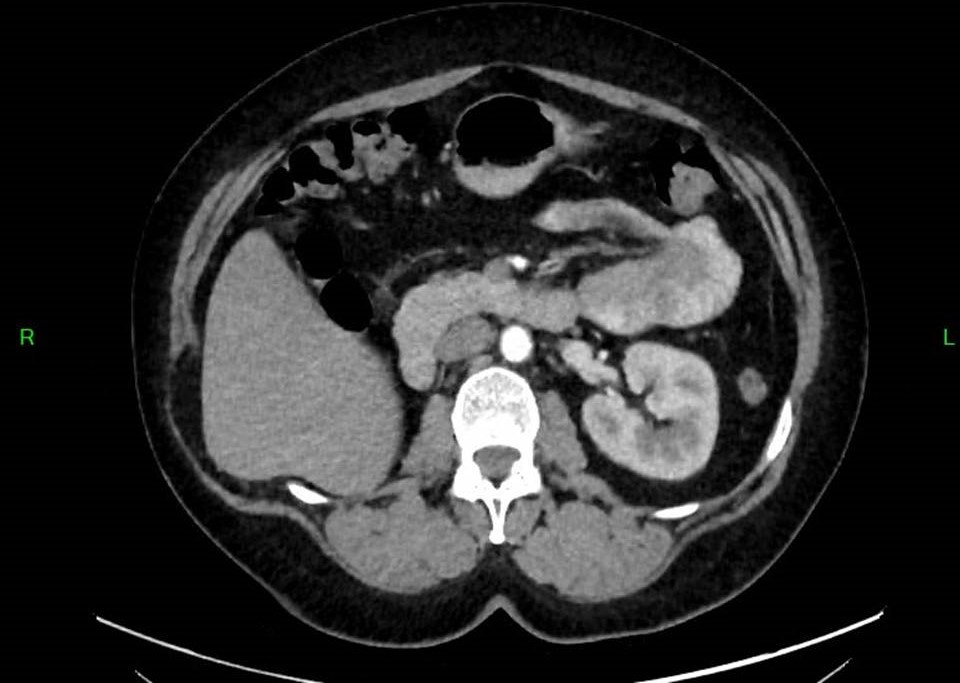
Regular monitoring with CT imaging (figure 3,4) and laboratory testing continued. After one year, following a gradual increase in hormone levels to 64 IU/L, pembrolizumab immunotherapy was initiated. This intervention successfully reduced serum beta-hCG to 30 IU/L. Following 11 cycles of immunotherapy, the patient maintains good general condition with stable low serum beta-hCG levels (10-20 IU/L). Follow-up bimanual gynecological examination and imaging studies one year post-treatment completion showed no evidence of disease. The table 1 provides a clear, chronological overview of the patient’s beta-hCG levels, imaging findings, and treatment modalities at each stage of patient diagnosis and management.
Discussion
The combination of missed menstruation and positive pregnancy test typically suggests pregnancy, with transvaginal ultrasound serving as the primary tool for confirming implantation location. In cases where implantation cannot be visualized either intrauterine or extrauterine (pregnancy of unknown location), protocol dictates serial ultrasound examinations and hormone level monitoring. The diagnostic algorithm may include surgical procedures such as endometrial ablation and laparoscopy. In our case, histological examination of the endometrium excluded both intrauterine and classical ectopic implantation.
In case of elevated hCG, it is important to provide an analysis of rival diagnoses, including ectopic pregnancy and pregnancy of unknown location, gestational trophoblastic disease (GTD), and germ cell tumors. Ectopic pregnancy and pregnancy of unknown location, a common differential diagnosis in cases of elevated beta-hCG levels without intrauterine pregnancy, was ruled out in our patient due to the absence of chorionic or decidual tissue on histopathological examination of the diagnostic curettage and the lack of adnexal abnormalities on transvaginal ultrasound imaging. Gestational trophoblastic disease, including molar pregnancy and gestational choriocarcinoma, was excluded based on the absence of paternal alleles in molecular genetic testing, confirming a non-gestational origin. Germ cell tumors, which can also present with elevated beta-hCG levels and metastatic disease, were considered unlikely due to the absence of primary gonadal lesions and the distinct histopathological features of choriocarcinoma in the lung and kidney. By systematically addressing these differential diagnoses and providing a clear rationale for their exclusion, the diagnostic process is strengthened, and the validity of the final diagnosis is reinforced.
When these investigations prove inconclusive, clinicians should consider non-gestational choriocarcinoma of ectopic origin [9-12].
Planned laparoscopy was deferred due to the patient's unexpected symptoms suggesting extragenital origin, leading to the utilization of advanced imaging techniques including CT angiography and PET-CT [13].
Non-gestational choriocarcinomas typically carry a worse prognosis than their gestational counterparts, characterized by rapid progression and frequent presence of distant metastases at diagnosis [14]. In our patient, the presenting symptoms of hemoptysis and progressive lower back pain led to the discovery of concurrent lung and kidney involvement. While renal metastasis is uncommon even in gestational choriocarcinoma [15], our literature review found no previously reported cases of renal metastasis from primary pulmonary choriocarcinoma.
Current evidence, including our experience, suggests that optimal treatment consists of surgical resection followed by adjuvant chemo- and immunotherapy [16]. Our patient received sequential chemotherapy protocols (BEP and EMA-CO). As with other malignancies, early diagnosis significantly impacts treatment efficacy and survival rates [17]. In our case, the interval from initial presentation to diagnosis and treatment of extragenital choriocarcinoma was relatively brief.
Immunotherapy has emerged as an increasingly important treatment modality for malignant tumors. Checkpoint inhibitors, such as pembrolizumab, can effectively restore host immunity by targeting the interaction between programmed cell death protein 1 (PD-1) on T-cells and its ligand (PD-L1) on tumor cells. The successful use of pembrolizumab in our patient demonstrates the potential value of immunotherapy in managing this rare malignancy [18].
While the patient is currently in a stable condition with low beta-hCG levels (10–20 IU/L) and no evidence of disease on imaging, it is important to discuss predicted long-term outcomes and compare them to potentially similar cases in the literature. Non-gestational choriocarcinoma is associated with a high risk of relapse and poor long-term survival due to its aggressive nature and frequent metastatic spread at diagnosis. The available studies suggest that the 5-year survival rate for non-gestational choriocarcinoma is approximately 30–50%, significantly lower than that of gestational choriocarcinoma [19,20]. Relapse rates are also high, with up to 40–60% of patients experiencing recurrence, often within the first two years post-treatment [21]. In our patient, the use of multimodal therapy, including surgery, chemotherapy, and immunotherapy, may improve long-term outcomes compared to cases reported in the available literature. However, close monitoring of beta-hCG levels and imaging studies is essential to detect early signs of relapse. Further studies are needed to establish standardized follow-up protocols and evaluate the impact of immunotherapy on long-term survival in non-gestational choriocarcinoma.
Conclusion
When elevated serum beta-hCG levels are present without ultrasonographic evidence of intrauterine or extrauterine implantation, clinicians should consider non-gestational choriocarcinoma with extragenital localization. While primary pulmonary choriocarcinoma remains rare, our case demonstrates that modern imaging techniques can facilitate diagnosis, and contemporary treatment approaches combining surgery with targeted immunotherapy can achieve favorable outcomes. This case contributes to the limited literature on this unusual presentation and highlights the importance of considering extragenital choriocarcinoma in the differential diagnosis of elevated beta-hCG with pregnancy of unknown location.
- Mangla M, Palo S, Kanikaram P, Kaur H (2024) Non-gestational choriocarcinoma: an raveling the similarities and distinctions from its gestational counterpart. Int J Gynecol Cancer, 34: 926-34.
- Zhang X, Ding B, Chen L, et al. (2022) Primary pulmonary choriocarcinoma in male: report a case with genetic testing and review of the literature. Transl Cancer Res. 11: 1844-9.
- Umemori Y, Hiraki A, Aoe K, Murakami T, Maeda T, Matsuda E, et al. (2004) Primary choriocarcinoma of the lung. Anticancer Res. 24: 1905-10.
- Serno J, Zeppernick F, Jäkel J, Schrading S, Maass N, Meinhold-Heerlein I, et al. (2012) Primary pulmonary choriocarcinoma: case report and review of the literature. Gynecol Obstet Investig. 74: 171-6.
- Snoj Z, Kocijancic I, Skof E (2017) Primary pulmonary choriocarcinoma. Radiol Oncol. 51: 1-7.
- Wu PS (2020) Primary choriocarcinoma of the lung: a case report and literature review. Int J Clin Exp Pathol. 13: 2352-5.
- Bishop BN, Edemekong PF (2023) Choriocarcinoma. Availabe from: https://www.ncbi.nlm.nih.gov/books/NBK535434/
- Kaushik H, Deshmukh M, Gupte S, Kanchankar N, Dongre A (2024) A case report on primary pulmonary choriocarcinoma. Cureus, 16: e63466.
- Kirk E, Condous G, Bourne T (2009) Pregnancies of unknown location. Best Pract Res Clin Obstet Gynaecol. 23: 493-9.
- Barnhart K, van Mello NM, Bourne T, Kirk E, Van Calster B, Bottomley C, et al. (2011) Pregnancy of unknown location: a consensus statement of nomenclature, definitions, and outcome. Fertil Steril. 95: 857-66.
- van Mello NM, Mol F, Opmeer BC, Ankum WM, Barnhart K, Coomarasamy A, et al. (2012) Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update, 18: 603-17.
- Boyraz G, Bozdağ G (2013) Pregnancy of unknown location. J Turk Ger Gynecol Assoc. 14: 104-8.
- Zhang J, Dong A, Wang Y (2024) FDG PET/CT findings of primary pulmonary choriocarcinoma in a postmenopausal woman. Clin Nucl Med. 49: 698-700.
- Stockton L, Green E, Kaur B, De Winton E (2018) Non-gestational choriocarcinoma with widespread metastases presenting with type 1 respiratory failure in a 39-year-old female: case report and review of the literature. Case Rep Oncol. 11: 151-8.
- Pahwa S, Sharma A, Kamboj M, Gupta G, Pasricha S.et al. (2023) Metastatic choriocarcinoma of the kidney in the absence of existing primary uterine tumor. A rare presentation. J Cancer Res Ther. 19: 819-22.
- Goldfarb JA, Dinoi G, Mariani A, Langstraat CL (2020) A case of multi-agent drug resistant choriocarcinoma treated with Pembrolizumab. Gynecol Oncol Rep. 32: 100574.
- Cheung AN, Zhang HJ, Xue WC, Siu MK (2009) Pathogenesis of choriocarcinoma: clinical, genetic and stem cell perspectives. Future Oncol. 5: 217-31.
- Niu M, Yi M, Wu Y, Lyu L, He Q, Yang R, et al. (2023) Synergistic efficacy of simultaneous anti-TGF-beta/VEGF bispecific antibody and PD-1 blockade in cancer therapy. J Hematol Oncol. 16: 94.
- Smith HO, Hilgers RD, Bedrick EJ, et al. (2003) Ethnic differences in risk for gestational trophoblastic disease in New Mexico: A 25-year population-based study. Am J Obstet Gynecol. 188: 357-66.
- Ngan HYS, Seckl MJ, Berkowitz RS, et al. (2018) Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet. 2: 79-85.
- Lurain JR (2011) Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. Am J Obstet Gynecol. 204: 11-8.

Tables at a glance
Figures at a glance