A Typical Pseudoprogression in A Case of NSCLC Neoadjuvant Immunochemotherapy: The Primary Tumor Regressed, But the Contralateral Hilar and Mediastinal Lymph Nodes Increased
Received Date: September 08, 2025 Accepted Date: October 04, 2025 Published Date: October 14, 2025
doi:10.17303/jcrto.2025.13.203
Citation: Guodong Zhang, Xiaohe Hao (2025) A Typical Pseudoprogression in A Case of NSCLC Neoadjuvant Immunochemotherapy: The Primary Tumor Regressed, But the Contralateral Hilar and Mediastinal Lymph Nodes Increased J Cancer Res Therap Oncol 13: 1-8
Abstract
Background: Neoadjuvant immune checkpoint inhibition combined with cytotoxic chemotherapy for resectable NSCLC have also obtained satisfactory treatment results. Although immunotherapy is associated with good outcomes, immunotherapy can be challenging to manage due to atypical response patterns that it triggers including pseudoprogression. We present a case of NSCLC neoadjuvant immunochemotherapy: the primary tumor regressed, but the hilar mediastinal lymph nodes increased, which proved to be atypical pseudoprogression.
Case presentation: A 67-year-old male who was diagnosed as right lung upper lobe adenocarcinoma with right hilar and mediastinal lymph node metastasis. After two cycles of neoadjuvant immunochemotherapy, reexamination by computed tomography showed the enlargement of ipsilateral hilar and mediastinal lymph nodes and new enlargement of contralateral hilar lymph nodes. Repeat imaging after one more cycle treatment demonstrated that decreasing of the primary lesion but stable right mediastinal and left hilar and mediastinal lymph node. Lymph node biopsy of group 4L lymph node by endobronchial ultrasound-guided transbronchial needle asspiration showed no cancer tissue was found. Finally the patient underwent right lung upper lobectomy and hilar and mediastinum lymph node dissection. Surgical pathology showed there was no residual cancer tissue, which achieved pathological complete remission, and the enlarged lymph nodes including 4L were presented as change after therapy, no visible tumor cells existed.
Conclusions: We report our case to provide further support for the awareness and diagnosis of atypical pseudoprogression occurring in the NSCLC immune treatment including neoadjuvant chemoimmunotherapy. We also note that the enlargement of ipsilateral hilar and mediastinal lymph nodes and new enlargement of contralateral hilar lymph nodes might be a manifestation of immune treatment, but more cases are needed to support this conclusion.
Keywords: non-small cell lung cancer; immune therapy; immune checkpoint inhibitors; pseudoprogression
List of abbreviations
SCLC: small cell lung cancer;
NSCLC: non-small cell lung cancer
ICIs: immune checkpoint inhibitors
PET-CT: positron emission tomography-computed tomography
CT: computed tomography
MDT: multidisciplinary team
Introduction
There were more than 2.2 million cases of newly diagnosed lung cancers worldwide and 1.9 million cases of deaths from cancer in 2020, making it one of the most prevalent malignant tumors [1]. Lung cancers were grouped into two types: small cell lung cancer (SCLC; ~15-20%), and non-small cell lung cancer (NSCLC; ~80–85%). NSCLC includes squamous cell carcinoma, adenocarcinoma, and large-cell carcinoma [2]. Though many advances in early diagnosis and treatment, approximately 40% of NSCLC cases have developed metastasis when they are first diagnosed, with poor prognosis [3]. With the development of immune checkpoint inhibitors (ICIs), treatment of advanced non-small cell lung cancer (NSCLC) has dramatically changed. Immune checkpoint inhibition alone, or combined with cytotoxic chemotherapy, has become the norm for treating advanced NSCLC [4]. Neoadjuvant immune checkpoint inhibition combined with cytotoxic chemotherapy for resectable NSCLC have also obtained satisfactory treatment results [5]. Although ICI are associated with good outcomes, immunotherapy can be challenging to manage due to atypical response patterns that it triggers including pseudoprogression. Imaging examinations remain an important method for diagnosis, efficacy evaluation, and prediction of lung cancer [6]. However, persisting challenges encompass heterogeneity in radiomics feature extraction across studies and the difficulty in differentiating immune-related phenomena (eg, inflammatory changes versus true progression) with single-modality assessments imaging examinations remain an important method for diagnosis, efficacy evaluation, and prediction of lung cancer [6]. We present a case of NSCLC neoadjuvant immunochemotherapy: the primary tumor regressed, but the hilar mediastinal lymph nodes increased, which proved to be pseudoprogression.
Case Presentation:
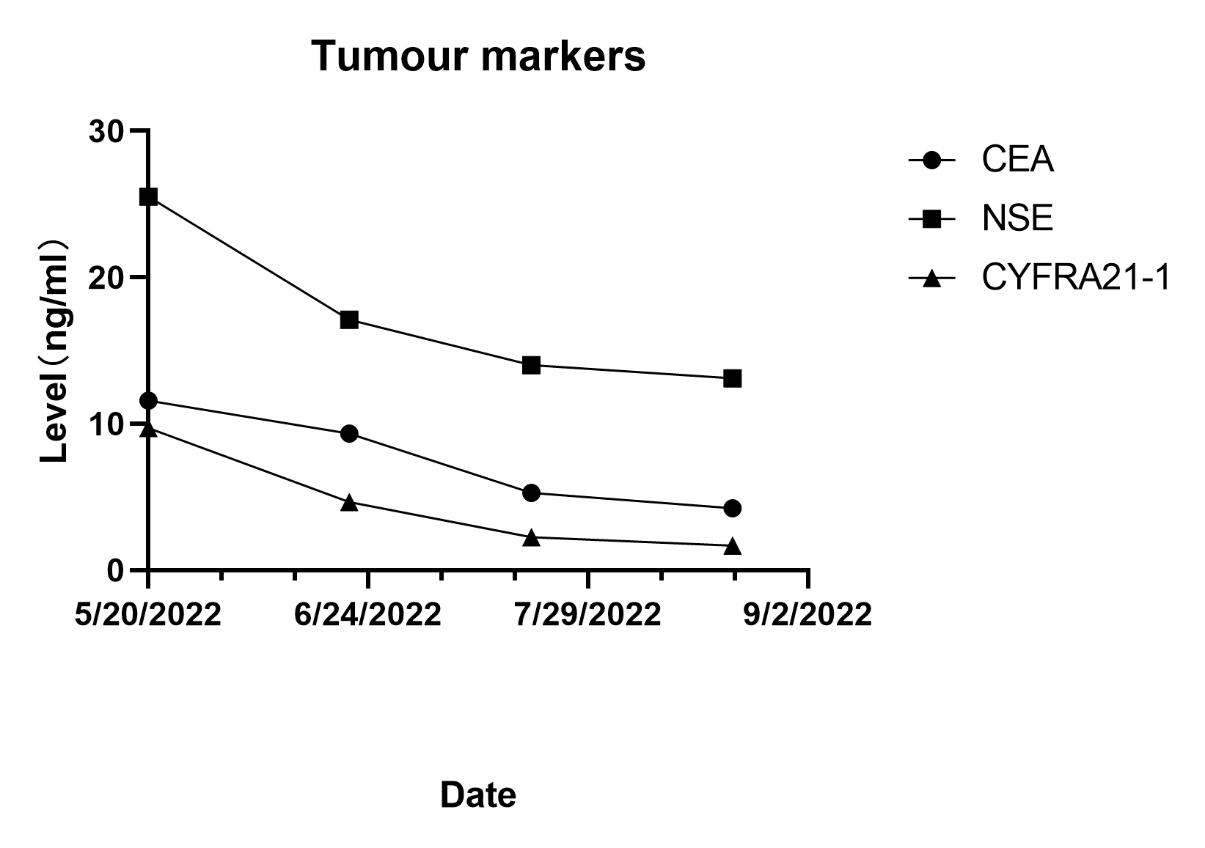
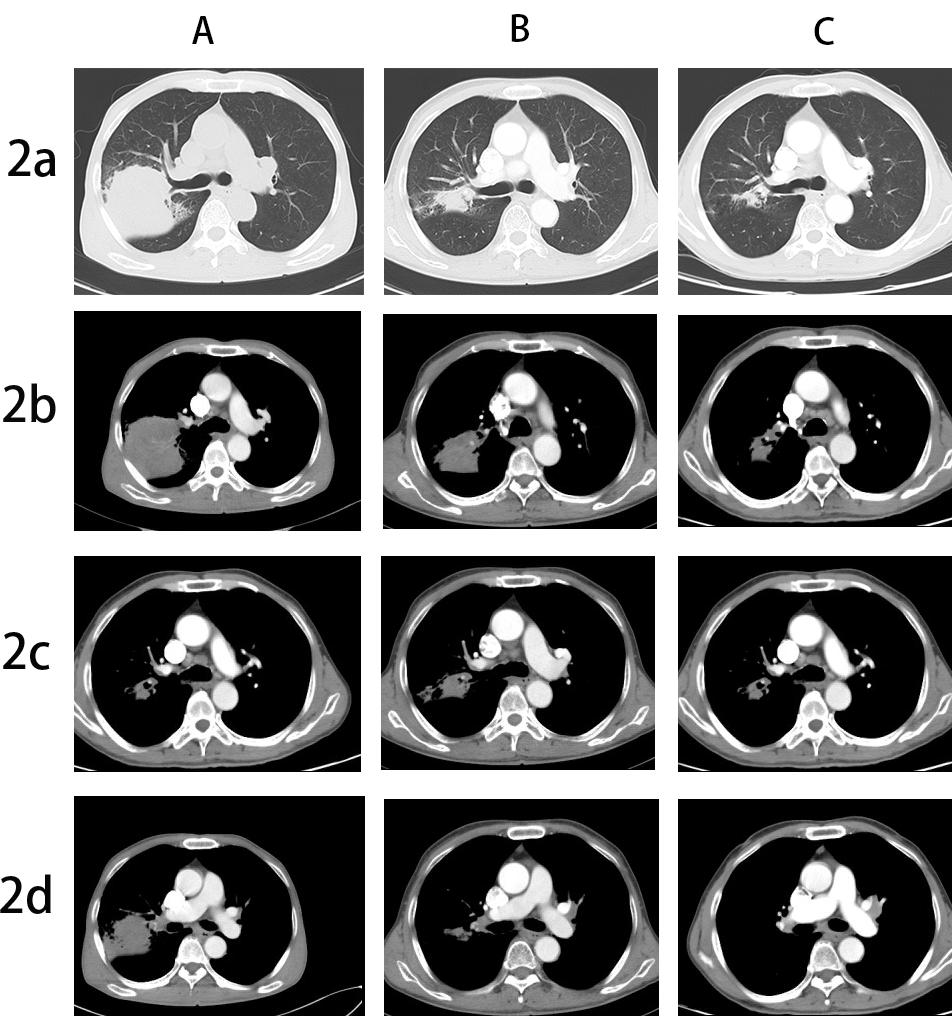
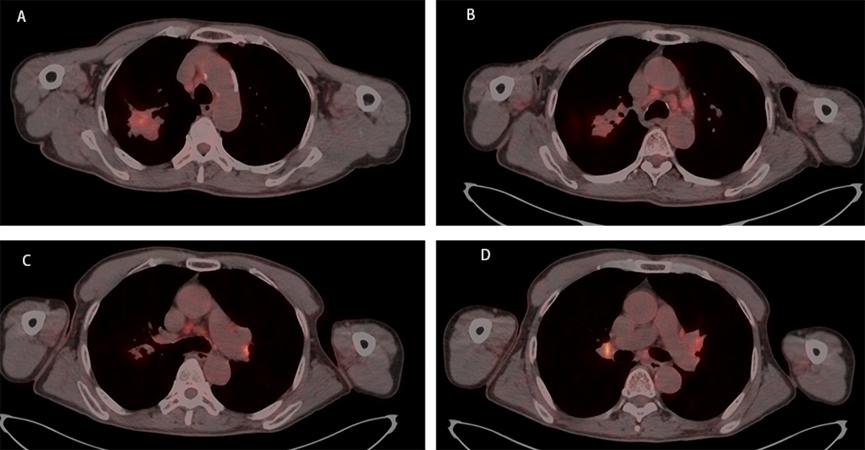
A 67-year-old male, with a continuous smoking history of over 30 years, was admitted to hospital for blood in phlegm half a month and was diagnosed as right lung upper lobe adenocarcinoma with right hilar and mediastinal lymph node metastasis examined by PET-CT. CT-guide needle biopsy was performed, and subsequent HE staining and immunohistochemistry confirmed adenocarcinoma with PD-L1(+,5%). Quantitative polymerase chain reaction (qPCR) assay of 10 genes in primary tumor tissue showed G12S\G12D mutation in exon 2 of KRAS gene. Neoadjuvant chemotherapy is administered to patients anticipated to benefit from subsequent adjuvant chemotherapy; however, it only modestly enhances 5-year recurrence-free and OS rates by about 5% to 6% [7]. The incorporation of immune checkpoint inhibitors (ICIs) into neoadjuvant chemotherapy demonstrates potential, having a minimal impact on the likelihood of significant perioperative complications or mortality, while presenting new opportunities for managing treatable NSCLC, with a major pathological response rate varying between 21% and 45%. Additionally, induction with ICIs does not increase toxicities that could delay surgical interventions [8]. Following the results of the CheckMate-816 study, immunochemotherapy is now suggested as a neoadjuvant approach [9]. After discussing in the multidisciplinary team (MDT), in view of the difficulty of operation, three cycles of neoadjuvant Camrelizumab(anti-PD-1 antibody) combined with pemetrexed and carboplatin were administered to the patient. However, repeat imaging after two cycles demonstrated concern for disease progression with right mediastinal lymph node increased and new left hilar and mediastinal(4L) lymph node metastasis. But the right upper lung lesions became significantly smaller with the tumor biomarker, CEA, NSE and Cyfra21-1 continued to decline (Figure 1). However, the side effects of immunochemothrapy are small, with no high-grade toxicities, the patient chose to continue to receive one more cycle treatment. Repeat imaging after one more cycle treatment demonstrated that decreasing of the primary lesion but stable right mediastinal and left hilar and mediastinal lymph node (Figure 2a-d). Positron emission tomography-computed tomography (PET-CT) was performed and showed that the local residual tumor with slightly high FDG metabolism, hilar and mediastinal lymph node enlargement with high FDG metabolism (Figure 3A-D). After the re-MDT, it was considered that the tumor shrank significantly and the tumor markers continued to decrease. The nature of the contralateral hilar lymph nodes was the same as that of the 4L group lymph nodes. It is recommended to complete the EBUS-tbna examination and puncture the 4L group lymph nodes. Lymph node biopsy of group 4L lymph node by Ebus-tbna showed no cancer tissue was found. In view of the possibility of pseudoprogression, after a sufficient communication, the patient underwent a right lung upper lobectomy and hilar and mediastinum lymph node dissection. Surgical pathology showed there was no residual cancer tissue, which achieved pathological complete remission, and the enlarged lymph nodes including 4L were presented as change after immunotherapy, no visible tumor cells existed. Now, the patient had finished all treatment and reexamination periodically for 13 months, no evidence of relapse or metastasis exist.
Discussion and Conclusion
Immune system-related pseudoprogression was first described in melanoma patients receiving ipilimumab[10]. Pseudoprogression is commonly defned as an increase in tumor size or appearance of new lesions, followed by tumor shrinkage or achievement of stable disease over the course of immunotherapy [11]. Since pseudoprogression and tumor recurrence are difficult to discriminate, it can greatly interfere with the therapeutic strategies, and even lead to erroneous treatment. In fact, among patients with advanced NSCLC, the rate of pseudoprogression after immunotherapy has been reported as 0.6–5.8% [12]. However, the rate of pseudoprogression after neoadjuvant chemoimmunotherapy has seldom been reported. Wenxiao Jia et al reported a case that a lung squamous cancer patient who was assessed to be “PD” on the basis of reexamination CT after four cycles of neoadjuvant immunochemotherapy; however, the surgical pathology indicated a partial response to immunochemotherapy [13]. To our knowledge, we first report a case of NSCLC neoadjuvant immunochemotherapy: the primary tumor regressed, but the contralateral hilar and mediastinal lymph nodes increased, which proved pathological complete remission after surgery.
Pseudoprogression manifestations include tumor enlargement or emergence of new lesions and pleural or pericardial effusion, the manifestation of pseudoprogression in our case is that the enlargement of ipsilateral hilar and mediastinal lymph nodes and new enlargement of contralateral hilar lymph nodes. Initial enlargement of primary tumor might be due to either transient immune cell infltration, necrosis, or edema or continued tumor growth that precedes a desired immune response. Similarly, the appearance of new lesions including new enlargement of hilar and mediastinal lymph nodes might be associated with lymphocyte infltration of lesions that are not radiologically detectable during baseline assessment.
It is difficult to discriminate pseudoprogression and tumor recurrence, which greatly interfere with the therapeutic strategies. Diagnosis of pseudoprogression is dependent on clinical factors and laboratory findings, such as size of tumors, peripheral blood counts, circulating tumor DNA, and cytokine levels [14, 15]. In our case, the primary lesions became significantly smaller with the tumor biomarker, CEA, NSE and Cyfra21-1 continued to decline. Repeat imaging after another cycle demonstrated that decreasing of the primary lesion but stable right mediastinal and left hilar and mediastinal lymph node. We further performed lymph node biopsy of group 4L lymph node by Ebus-tbna and the results showed no cancer tissue was found. Finally, the patient underwent a right lung upper lobectomy and hilar and mediastinum lymph node dissection. Surgical pathology showed there was no residual cancer tissue, which achieved pathological complete remission. We consulted the experts, and the possible explanation may be as follows:A rare side effect of "histiocytic necrotizing lymphadenitis, benign, malignant, or cystic, polypoid tumor of unknown nature" was mentioned. With the widespread application of pd1, this side effect was gradually exposed to practical application, which was not uncommon. However, we have not found relevant reports, but this may require our attention in future clinical work. The expression of PD-L1, as measured by IHC, is recommended as a predictive factor to identify patients who would benefit from ICIs, however, not all PD-L1–positive patients respond well[16, 17].In our case, the expression of PD-L1 on the surface of tumor cells of the patients is 5%. Dong ZY et al. reported that KRAS mutation in lung adenocarcinoma may be served as a potential predictive factor in guiding anti–PD-1/PD-L1 immunotherapy, especially co-occurred with TP53 [18]. In previous study, Wenxiao Jia etal reported a neoadjuvant immunotherapy pseudoprogression case proved by surgical pathology, scRNA-seq, and IMC. Through scRNA-seq, they described the tumor-infiltrating immune cell atlas after neoadjuvant immunochemotherapy, and also identified a specific cell subtype, mitotic CD8+ T cells, which represent rapidly proliferative CD8+ T cells. IMC revealed a transformation from cold to hot tumor after neoadjuvant immunochemotherapy, with obvious cell-cell contacts between CD8+ T cells and CD14+ monocytes, CD16+ monocytes, providing evidence of in situ antigen presentation in the tumor microenvironment [19]. In summary, our case study described the enlargement of ipsilateral hilar and mediastinal lymph nodes and new enlargement of contralateral hilar lymph nodes might be a manifestation of immune treatment.
We report our case to provide further support for the awareness and diagnosis of pseudoprogression occurring in the NSCLC immune treatment including neoadjuvant chemoimmunotherapy. We also note that the enlargement of ipsilateral hilar and mediastinal lymph nodes and new enlargement of contralateral hilar lymph nodes might be a manifestation of immune treatment, but more cases are needed to support this conclusion.
Declarations
Ethics Declarations
Ethics approval and consent to participate
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Availability of Data and Materials
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Clinical Trial Number
Not applicable
Competing Interests
The authors declare that they have no competing interests
Funding
None
Authors' contributions
Xiaohe Hao is the corresponding author. Guodong Zhang conceived of the study and participated in its design and coordination. All authors read and approved the final manuscript.
Acknowledgements
Not applicable
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021): Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. 71: 209-49.
- Zappa C, Mousa SA: Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016, 5: 288-300.
- Tamura T, Kurishima K, Nakazawa K, Kagohashi K, Ishikawa H, Satoh H, Hizawa N (2015): Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. 3: 217-21.
- Remon J, Passiglia F, Ahn MJ, Barlesi F, et al. (2020) Immune Checkpoint Inhibitors in Thoracic Malignancies: Review of the Existing Evidence by an IASLC Expert Panel and Recommendations. J Thorac Oncol. 15: 914-47.
- Forde PM, Spicer J, Lu S, Provencio M, et al. (2022) Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N Engl J Med. 386: 1973-85.
- Tárnoki ÁD, Tárnoki DL, Dąbrowska M., et al (2024) new developments in the imaging of lung cancer. Breathe (Sheff), 20: 230176.
- NSCLC Meta-analysis Collaborative Group (2014). Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. 383: 1561-71.
- Eichhorn F, Klotz LV, Kriegsmann M, et al (2021) Neoadjuvant anti-programmed death-1 immunotherapy by pembrolizumab in resectable non-small cell lung cancer: First clinical experience. 153: 150-7.
- Forde PM, Chaft JE, Smith KN, et al. (2018) Neoadjuvant PD-1 Blockade in Resectable Lung Cancer. 378: 1976-86.
- Saenger YM, Wolchok JD (2008): The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. 8:1.
- Di Giacomo AM, Danielli R, Guidoboni M, et al (2009): Therapeutic efficacy of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with metastatic melanoma unresponsive to prior systemic treatments: clinical and immunological evidence from three patient cases. Cancer Immunol Immunother. 58: 1297-1306.
- Nishino M, Ramaiya NH, Chambers ES, et al. (2016): Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. 4: 84.
- Jia W, Zhu H, Gao Q, Sun J, et al. (2021): Case Report: Transformation from Cold to Hot Tumor in a Case of NSCLC Neoadjuvant Immunochemotherapy Pseudoprogression. 12: 633534.
- Martin-Romano P, Castanon E, Ammari S, et al (2020): Evidence of pseudoprogression in patients treated with PD1/PDL1 antibodies across tumor types. 9: 2643-52.
- Failing JJ, Dudek OA, Marin Acevedo JA, et al. (2019): Biomarkers of hyperprogression and pseudoprogression with immune checkpoint inhibitor therapy. 15: 2645-56.
- Herbst RS, Soria JC, Kowanetz M, Fine GD, et al (2014): Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. 515: 563-67.
- Garon EB, Rizvi NA, Hui R, Leighl N, et al (2015) : Pembrolizumab for the treatment of non-small-cell lung cancer. 372: 2018-28.
- Dong ZY, Zhong WZ, Zhang XC, et al (2017): Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. 23: 3012-24.
- Jia W, Zhu H, Gao Q, et al. (2021) Case Report: Transformation from Cold to Hot Tumor in a Case of NSCLC Neoadjuvant Immunochemotherapy Pseudoprogression. 633534.

Figures at a glance