
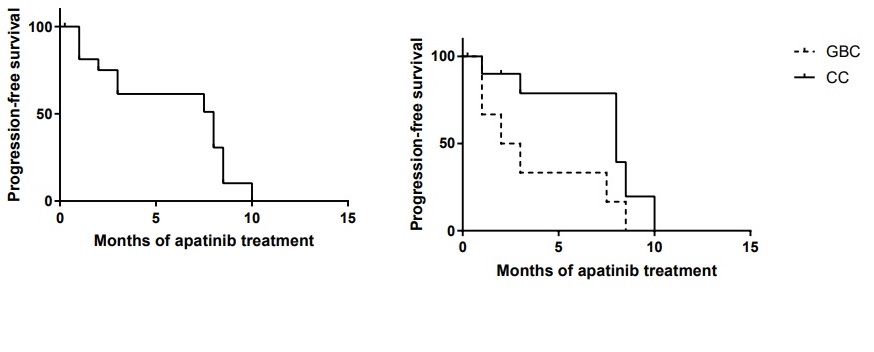
Figure 2 Figure 2A: Overall progression-free survival (PFS) for patients with advanced biliary tract cancer treated with apatinib monotherapy as a second-line regimen. The median PFS was 8 months.
Figure 2B: Progression-free survival (PFS) for patients with advanced cholangiocarcinoma (CC) and gallbladder carcinoma (GBC) treated with apatinib monotherapy as a second-line regimen. The median PFS in GBC group was 2.5 months and in CC group was 8 months, p=0.052
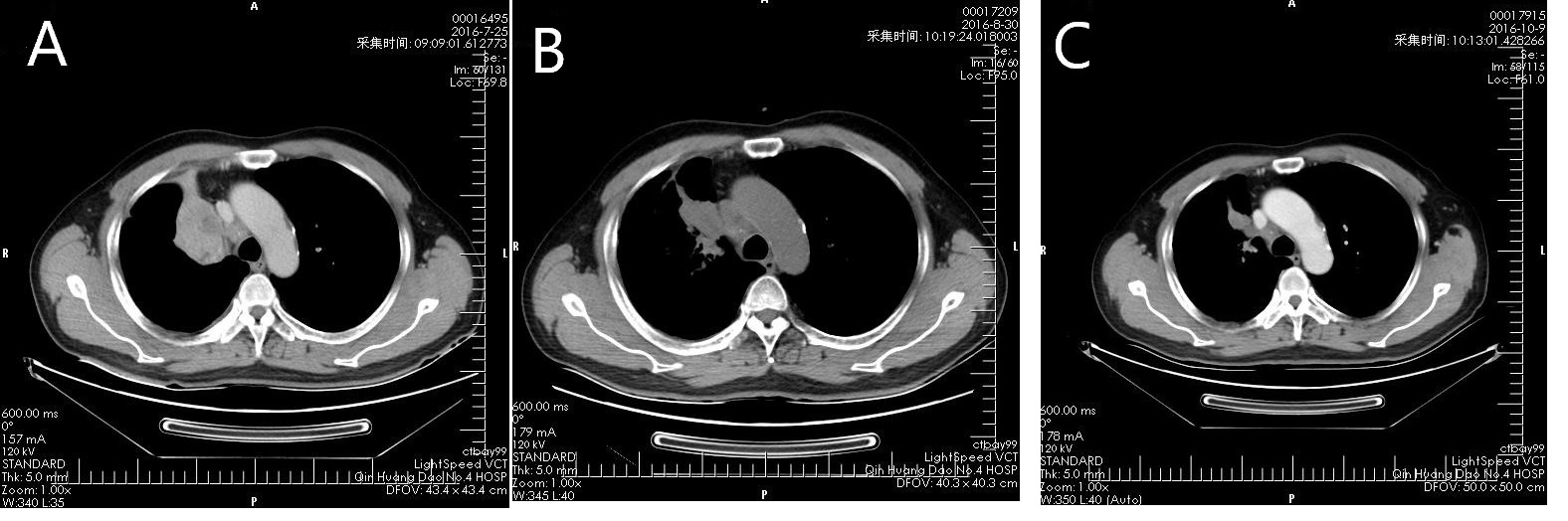
Figure 3 Images of a 65-year-old male patient with cholangiocarcinoma after treatment with apatinib monotherapy. Contrast-enhanced CT at baseline (A) showed an approximately 6.5*4 cm cholangiocarcinoma. CT images at 1 month (B) and 2.5 months (C) after baseline showed the tumor shrunk to 4.6*3.5 cm and 3.2*2 cm, respectively.
Category |
Definition |
Complete response (CR) |
Complete disappearance of all target lesions |
Partial response (PR) |
≥30 % decrease in tumor diameter from baseline |
Stable disease (SD) |
Small changes that do not meet the above criteria |
Progressive disease (PD) |
>20% increase in tumor diameter from baseline |
Table 1: Four response categories.
Characteristics |
Number of patients |
|
Sex |
Male |
14 |
Female |
4 |
|
Age (years) |
Median |
55 |
40-50 |
4 |
|
50-60 |
9 |
|
60-70 |
5 |
|
Metastatic site |
||
From cholangiocarcino ma |
Liver |
2 |
Retroperitoneal node |
5 |
|
Lungs |
3 |
|
Supraclavicular lymph node |
2 |
|
From gallbladder cancer |
Liver |
2 |
Gallbladder (locally advanced) |
4 |
|
Initial treatment/ recurrence |
||
Initial treatment |
10 |
|
Post-surgery Recurrence |
8 |
|
Gemcitabine (first-line medicine) therapy |
||
Monotherapy |
3 |
|
Gemcitabine-cisplatin |
15 |
|
Initial dosage of apatinib (mg) |
||
500 |
12 |
|
250 (dosage reduced from 500 within the first 10 days) |
6 |
|
Table 2: Patient characteristics
Adverse events |
Total, n% |
Grade |
|
G1-G2 |
G3 |
||
Hypertension |
11 (61%) |
7 |
4 |
Diarrhea |
6 (33%) |
5 |
1 |
Hand-foot syndrome |
5 (28%) |
4 |
1 |
Proteinuria |
8 (44%) |
7 |
1 |
Fatigue |
13 (72%) |
11 |
2 |
Liver dysfunction |
2 (11%) |
2 |
- |
Anemia |
2 (11%) |
3 |
- |
Neutropenia |
2 (11%) |
2 |
- |
Thrombocytopenia |
1(6%) |
1 |
- |
Table 3: Treatment-related adverse events: