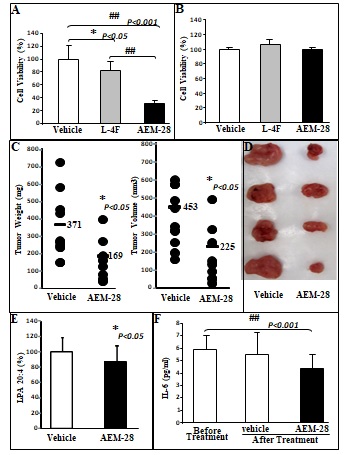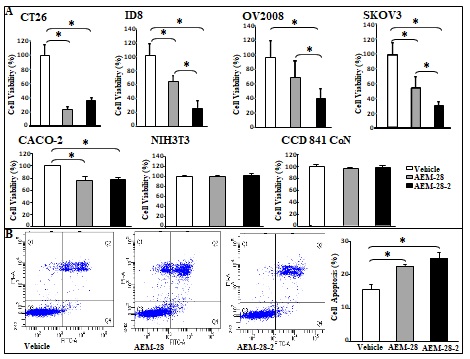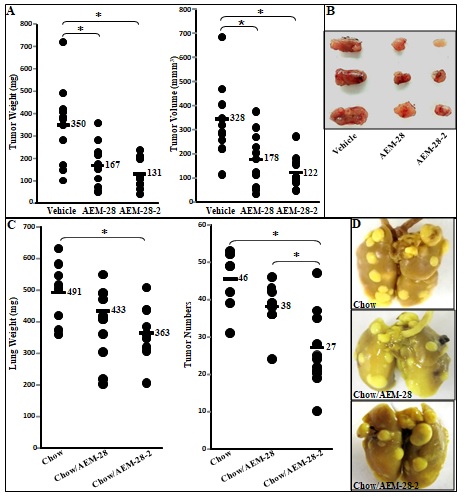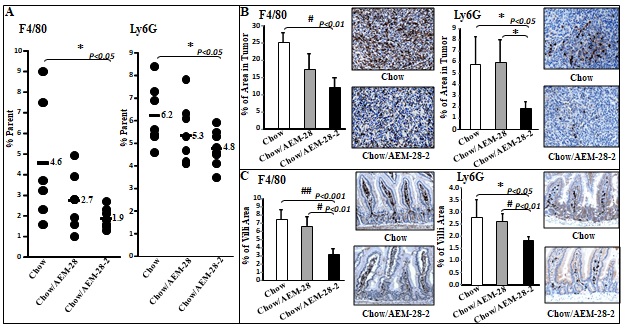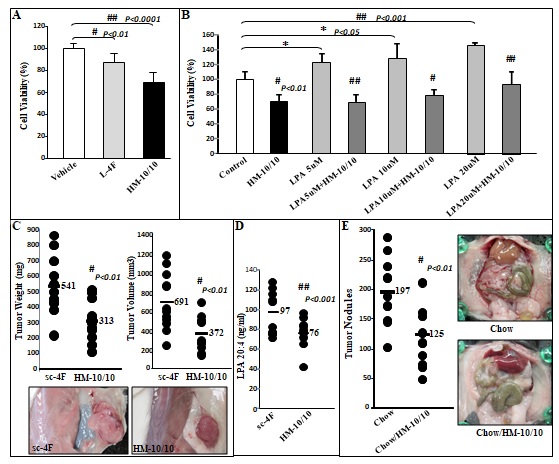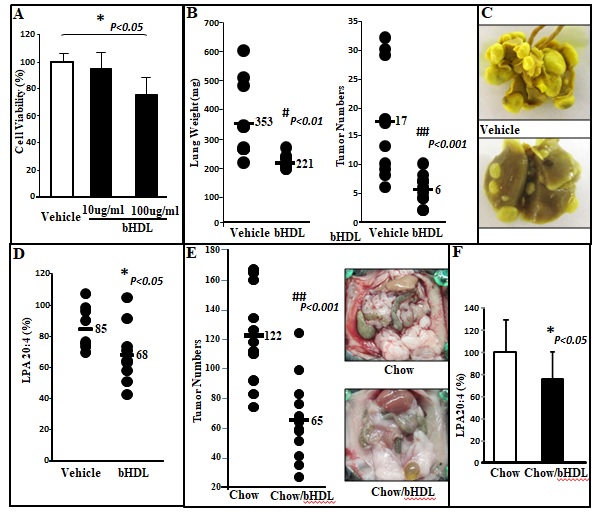
Figure 1 CT26 cell-mediated lung tumors in BALB/c mice and ID8 cell-mediated ovarian cancer burden in C57BL/6J mice are significantly decreased in mice administered bHDL. (A) CT26 cells were cultured as described in Materials and Methods and incubated with either vehicle (control) or bHDL at a concentration of 10ug/ml or 100ug.ml. CT26 cells were assayed for viability using MTS assay. Data are represented as the mean ± SD of the percent of control cells. All experiments were conducted in triplicate and each assay was carried out in quadruplicates. (B-D) Lung tumors were established in BALB/c mice (n=11 per group, 6 weeks of age) as described in Materials and Methods. Mice were sacrificed 3 weeks after CT26 cells were administered by tail vein injection. Lungs were harvested and weighed. Lung tumors were counted. (B) Left panel: lung weights of mice that were administered vehicle alone or bHDL (10mg/kg/day) subcutaneously (p< 0.01); Right panel: the number of tumors counted on the lung surface from the 2 groups of mice (p< 0.001). (C) Representative tumors from the two groups of mice showing tumor nodules on their lung surface. (D) Plasma LPA 20:4 levels were measured from (B) as described under Materials and Methods. (E-F) Wild-type C57BL/6J mice (n = 11 per group, 8 weeks of age) were injected with ID8 cells by intraperitoneal injection (8 × 106 cells per mouse) and tumor burden was analyzed after 10 weeks treated with a regular chow or a chow with bHDL. (E) Left panel: The total number of tumors nodules for each mouse was counted in each group; Right panel: representative images of mice from the two groups showing the tumor nodules on the peritoneal membranes. (F) Plasma LPA 20:4 levels in the two groups of mice from (E).