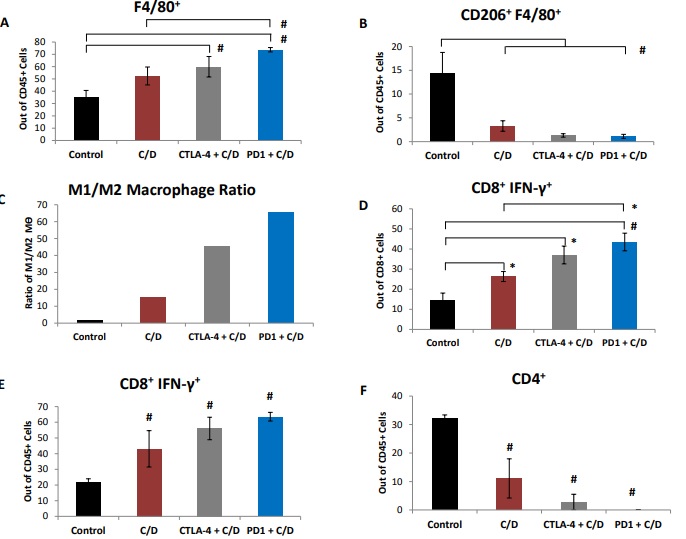
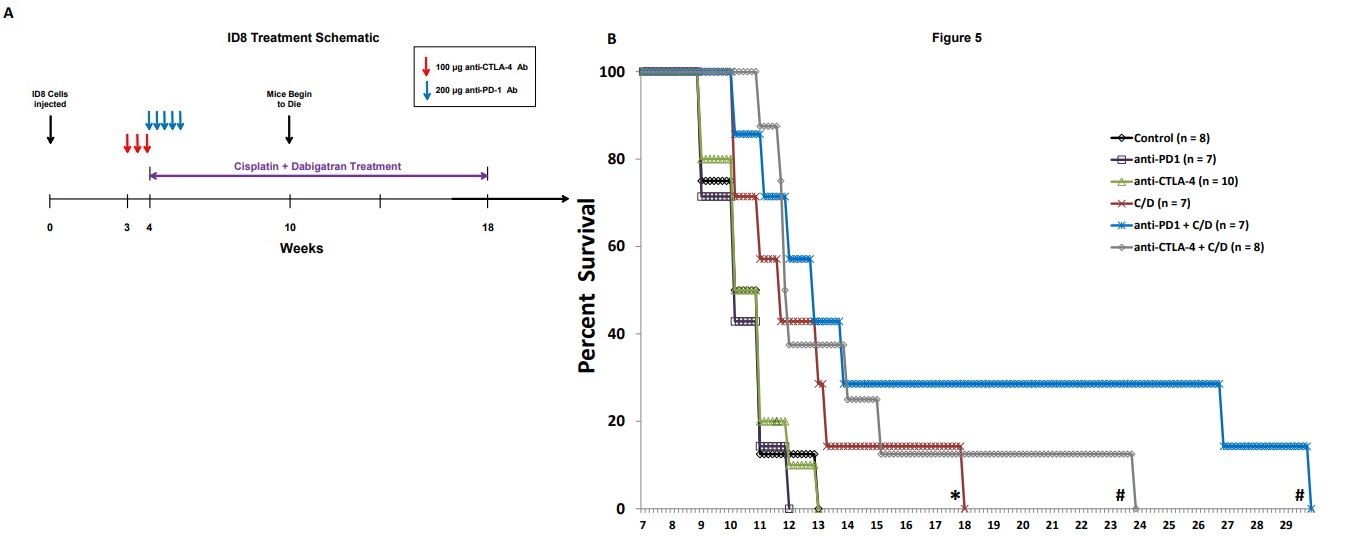
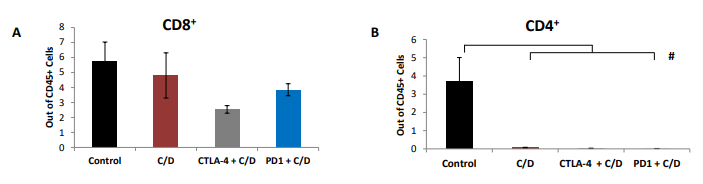
Figure 1 Co-treatment with cisplatin and dabigatran etexilate reduces tumor burden early during tumor progression. Four weeks after mice were i.p. injected with 2.0 x 106 ID8-luc cells, all treatments were initiated. Anti-PD-1 antibodies (200 μg every two days, five doses total) were i.p. injected. Mice were injected i.p. with cisplatin (1 mg/kg) once weekly. Dabigatran etexilate was administered by oral gavage twice daily (80 mg/kg) Monday through Friday, and mice were placed on chow supplemented with dabigatran etexilate (10 mg/g chow) over the weekends. (A) Final tumor loads of mice assessed by bioluminescence imaging of the opened peritoneal cavity six weeks after tumor injection (2 weeks of treatment with cisplatin and dabigatran). (B) Cells (2 x106) isolated from peritoneal lavages were treated for 4 h at 37ºC with ionomycin (500 ng/ml) and PMA (50 ng/ml) to stimulate activated T cells to produce IFN-γ in the presence of Brefeldin A to block cytokine secretion. Ascites cells were stained with anti- CD8α and anti-CD45 antibodies in the presence of Brefeldin A. Cells were then fixed, permeabilized, and intracellularly stained with an anti-IFN-γ antibody and analyzed by flow cytometry. n = 5 mice per group ± SEM; * = p< 0.05 and # = p<0.01 compared to control vehicle-treated tumor-bearing mice.