Figure 1: Study selection process (CNKI: China National Knowledge Infrastructure)
Study |
Population | Country |
Name of vaccine |
Clinical |
Trial number |
Blinding |
NO of |
Controls |
Asano 2021 |
18-55;56-60;≥70y | Japan |
ChAdOx1 nCoV-19 |
Ⅰ/Ⅱ |
NCT04568031 |
Double |
192 |
65 |
Chappel 2021 |
18-55y | Australia |
Sclamp |
Ⅰ |
NCT04495933 |
Double |
98 |
22 |
Chu 2021 |
18-55y; | United States |
mRNA-1273 |
Ⅱ |
NCT04405076 |
Double |
400 |
200 |
Clemens 2021 |
18-55;56-60;≥70y | Brazil |
ChAdOx1 nCoV-19 |
Ⅲ |
ISRCTN89951424 |
Unblinded |
4772 |
4661 |
Emary 2021 |
>18y | United Kingdom |
ChAdOx1 nCoV-19 |
Ⅱ/Ⅲ |
NCT04400838 |
Single |
4244 |
4290 |
Fadlyana 2021 |
18-59y | Indonesia |
CoronaVac |
Ⅲ |
NCT04508075 |
Double |
810 |
810 |
Formica 2021 |
18-59y;60-84y | Australia; |
NVX-CoV2373 |
Ⅱ |
NCT04368988 |
Double |
1032 |
257 |
Frenck 2021 |
12-15/16-25y | United States |
BNT162b2 |
Ⅲ |
NCT04368728 |
Double |
3009 |
3043 |
Guo 2021 |
18-59y; ≥60y | China |
Vero Cell |
Ⅰ/Ⅱ |
ChiCTR2000031809 |
Double |
826 |
294 |
Han 2021 |
3-17y | China |
CoronaVac |
Ⅰ/Ⅱ |
NCT04551547 |
Double |
438 |
114 |
Hsieh 2021 |
20-64; ≥65 | Taiwan, China |
MVC-COV1901 |
Ⅱ |
NCT04695652 |
Double |
3304 |
550 |
Kaabi 2021 |
>18y | United Arab Emirates; Bahrain |
WIV04/HB02 |
Ⅲ |
NCT04510207 |
Double |
26936 |
13471 |
Kremsner |
18-60y; ≥61y | Germany; Belgium;Argentina,et al |
CVnCoV |
Ⅱ/Ⅲ |
NCT04652102 |
Single |
2003 |
1978 |
Li 2021 |
18-55;65-85 | China |
BNT162b1 |
Ⅰ |
NCT04523571 |
Double |
96 |
48 |
Logunov 2021 |
>18y | Russia |
rAd26/rAd5 |
Ⅲ |
NCT04530396 |
Double |
16501 |
5476 |
Madhi 2021 |
18-65y | South Africa |
ChAdOx1 nCoV-19 |
Ⅰ/Ⅱ |
NCT04444674 |
Double |
81 |
79 |
Meng 2021 |
18-55y; >56y | China |
Sf9 cells |
Ⅰ/Ⅱ |
NCT04640402 |
Double |
925 |
202 |
Munro 2021 |
≥30y | United Kingdom |
ChAdOx1 |
Ⅱ |
ISRCTN73765130 |
Single |
2215 |
668 |
Pan 2021 |
18-59y | China |
KCONVAC |
Ⅰ/Ⅱ |
ChiCTR2000038804 |
Double |
448 |
112 |
Ramasamy 2020 |
18-55;56-60;≥70y | United Kingdom |
ChAdOx1 nCoV-19 |
Ⅱ/Ⅲ |
NCT04400838 |
Single |
460 |
100 |
Richmond 2021 |
18-54y;55-75y | Australia |
SCB-2019 |
Ⅰ |
NCT04405908 |
Double |
121 |
30 |
Shu 2021 |
18-59y; | China |
V-01 |
Ⅱ |
ChiCTR2100045107 |
Double |
360 |
80 |
Tanriover 2021 |
18-59y | Turkey |
CoronaVac |
Ⅲ |
NCT04582344 |
Double |
6650 |
3568 |
Toback 2021 |
18-64y;≥65y | United Kingdom |
NVX-CoV2373 |
Ⅲ |
NCT04583995 |
Single |
7020 |
7019 |
Voysey 2021 |
≥18y | The United Kingdom, Brazil, South Africa |
ChAdOx1 nCoV-19 |
Ⅲ |
ISRCTN89951424;NCT04324606;NCT04400838;NCT04444674 |
Single/Double |
8597 |
8181 |
Wu 2021 |
≥60y | China |
CoronaVac |
Ⅰ/Ⅱ |
NCT04383574 |
Double |
348 |
74 |
Xia 2021 |
18-59y;≥60y | China |
BBIBP-CorV |
Ⅰ/Ⅱ |
ChiCTR2000032459 |
Double |
480 |
160 |
Yang 2021 |
18-59y | China |
ZF2001 |
Ⅰ/Ⅱ |
NCT04445194/NCT04466085 |
Double |
720 |
160 |
Zhang 2020 |
18-59y | China |
CoronaVac |
Ⅰ/Ⅱ |
NCT04352608 |
Double |
96 |
48 |
Zhu 2020 |
≥18y | China |
Ad5 |
Ⅱ |
NCT04341389 |
Double |
382 |
126 |
Table 1: Characteristics of included studies reporting the safety and efficacy of COVID-19 candidate vaccines in RCT studies
|
studies |
Reaction/total % |
RR (95%) |
I² |
|||
vaccinated |
unvaccinated |
||||||
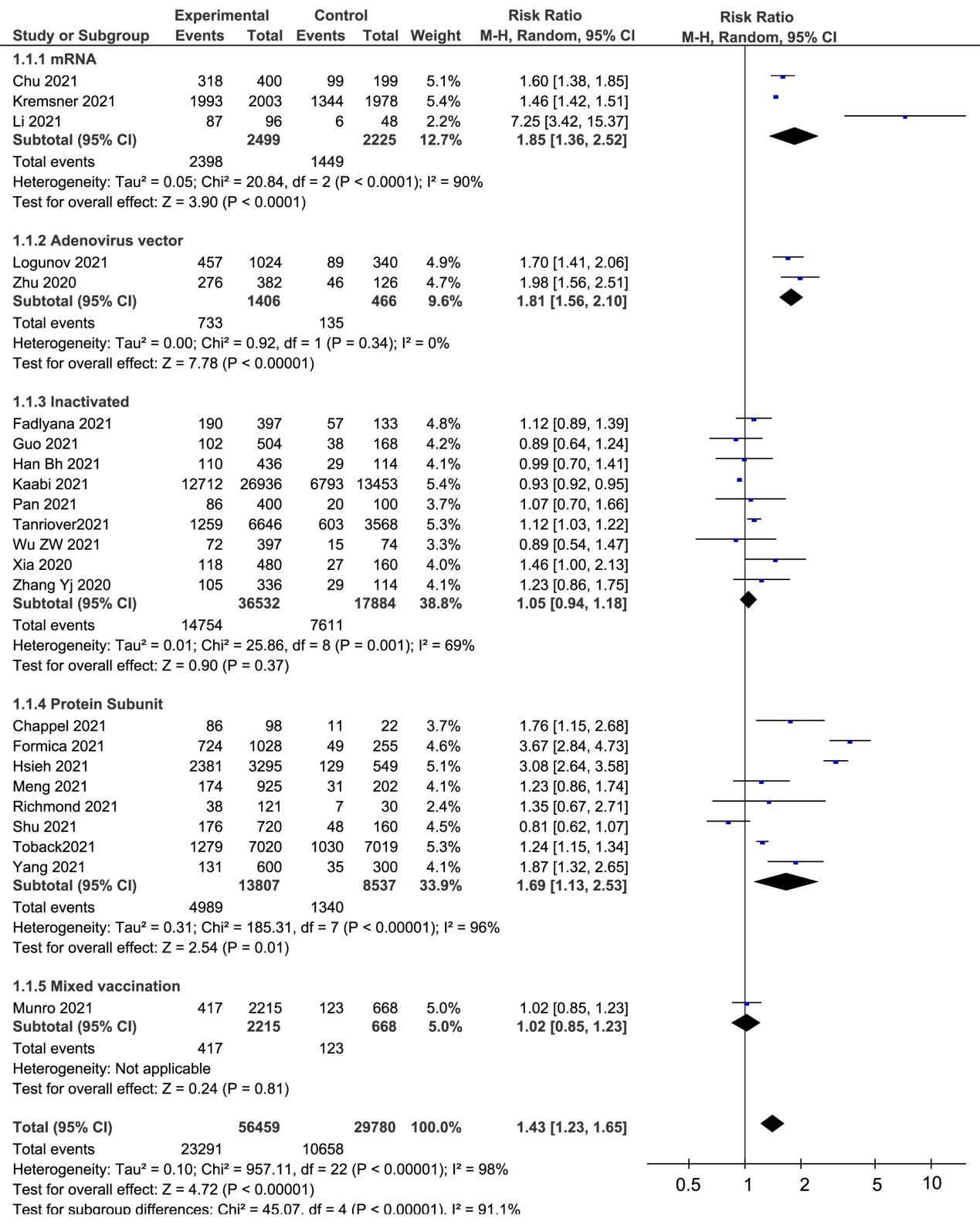
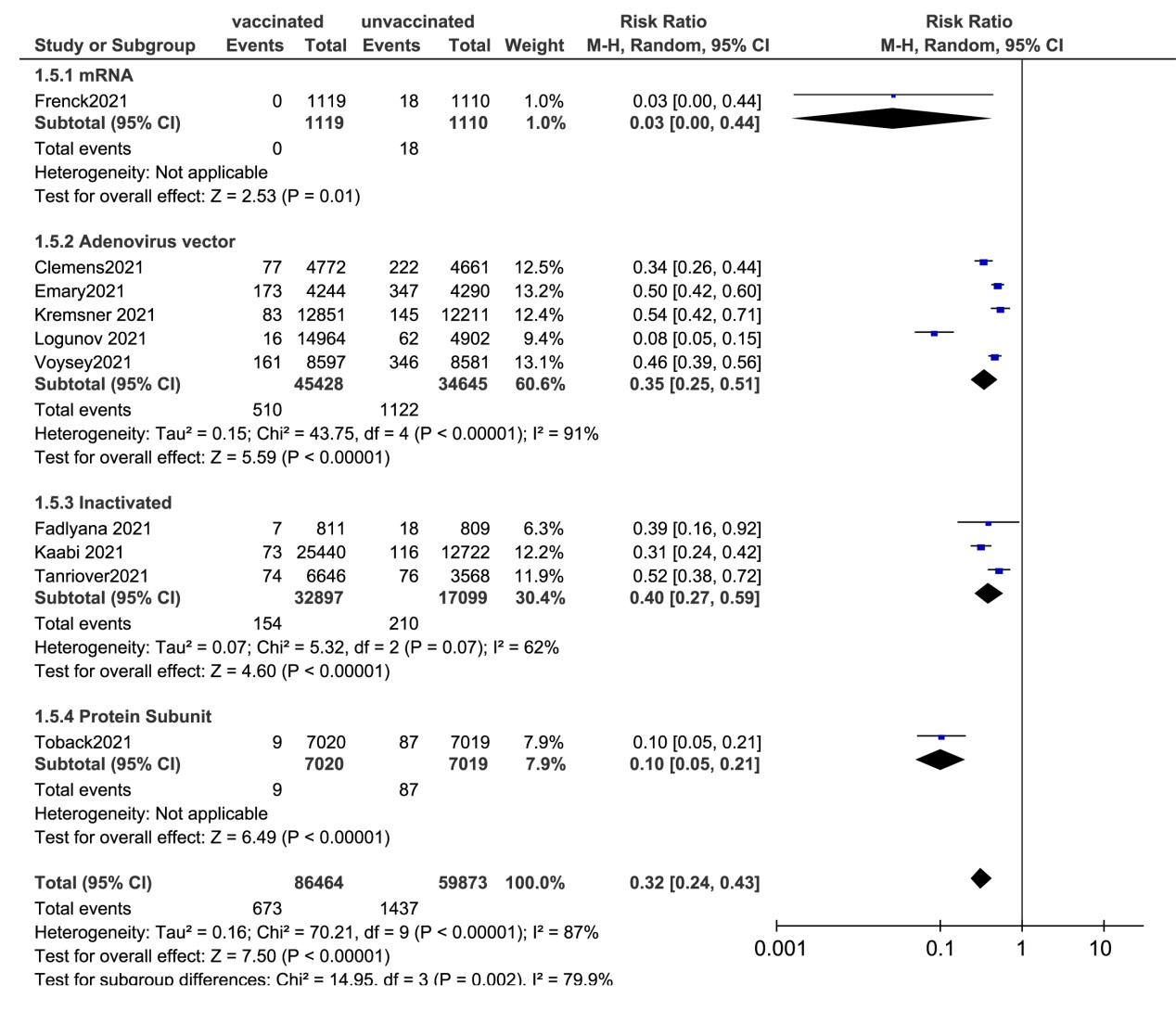
Total adverse reactions |
23 |
23231/56459 |
41.15% |
10658/29780 |
35.79% |
1.42 [1.23, 1.64] |
96% |
Systemic adverse reactions |
18 |
17519/45468 |
38.53% |
7000/21532 |
32.51% |
1.26 [1.08, 1.47] |
95% |
Local adverse reactions |
18 |
12851/45468 |
28.26% |
4758/21532 |
22.10% |
2.77 [1.68, 4.55] |
98% |
Total adverse reactions |
|||||||
mRNA vaccines |
3 |
2338/2499 |
93.56% |
1449/2225 |
65.12% |
1.85 [1.34, 2.55] |
91% |
Adenovirus vector vaccines |
2 |
733/1406 |
52.13% |
135/466 |
29.0% |
1.81 [1.56, 2.10] |
0% |
Inactivated vaccines |
9 |
14754/36532 |
40.39% |
7637/17884 |
42.70% |
1.05 [0.94, 1.18] |
69% |
Protein Subunit vaccines |
8 |
4959/13807 |
36.13% |
310/1518 |
20.42% |
1.77 [1.11, 2.83] |
94% |
Mixed vaccination |
1 |
417/2215 |
18.83% |
123/668 |
18.41% |
1.02 [0.85, 1.23] |
/ b |
Systemic adverse reactions |
|||||||
mRNA vaccines |
3 |
2012/2499 |
80.51% |
1297/2225 |
58.29% |
1.74 [1.20, 2.53] |
86% |
Adenovirus vector vaccines |
1 |
106/192 |
55.21% |
6/64 |
9.38% |
3.93 [2.11, 7.29] |
/ |
Inactivated vaccines |
8 |
11527/36085 |
31.94% |
5142/17745 |
28.95% |
1.02 [0.77, 1.36] |
97% |
Protein Subunit vaccines |
6 |
3766/6698 |
56.23% |
552/1498 |
36.85% |
1.18 [0.85, 1.63] |
87% |
Local adverse reactions |
|||||||
mRNA vaccines |
3 |
2082/2449 |
85.01% |
506/2225 |
22.74% |
5.30 [2.99, 9.38] |
85% |
Adenovirus vector vaccines |
1 |
116/192 |
60.42% |
6/64 |
9.38% |
6.44 [2.98, 13.92] |
/ |
Inactivated vaccines |
8 |
6836/36085 |
18.94% |
4017/17745 |
22.64% |
1.86 [1.13, 3.06] |
92% |
Protein Subunit vaccines |
6 |
3817/6698 |
56.99% |
229/1498 |
15.29% |
2.42 [1.21, 4.81] |
95% |
Pain |
|||||||
mRNA vaccines |
4 |
3326/4167 |
79.82% |
335/3915 |
8.56% |
14.07 [3.77, 52.53] |
98% |
Adenovirus vector vaccines |
2 |
317/574 |
55.23% |
15/190 |
7.89% |
6.94 [4.25,11.34] |
0% |
Inactivated vaccines |
9 |
6234/36532 |
17.06% |
3890/17884 |
21.75% |
1.66 [1.06, 2.57] |
91% |
Protein Subunit vaccines |
7 |
12230/46270 |
26.43% |
4571/21561 |
21.20% |
1.77 [1.11, 2.83] |
94% |
Redness |
|||||||
mRNA vaccines |
5 |
2608/6382 |
40.86% |
555/4583 |
12.11% |
4.19 [1.87, 9.41] |
98% |
Adenovirus vector vaccines |
4 |
776/1679 |
46.22% |
104/610 |
17.05% |
4.36 [1.42, 13.41] |
91% |
Inactivated vaccines |
9 |
322/36532 |
0.88% |
157/17884 |
0.88% |
0.96 [0.80, 1.17] |
0% |
Protein Subunit vaccines |
7 |
253/6787 |
3.73% |
6/1518 |
0.40% |
3.54 [0.99, 12.72] |
57% |
Swelling |
|||||||
mRNA vaccines |
4 |
248/4167 |
5.95% |
30/3915 |
0.77% |
7.56 [5.20, 11.00] |
0% |
Adenovirus vector vaccines |
3 |
18/655 |
2.75% |
1/270 |
0.37% |
3.51 [0.80, 15.46] |
0% |
Inactivated vaccines |
9 |
349/36532 |
0.96% |
175/17884 |
0.98% |
0.93 [0.78, 1.12] |
0% |
Protein Subunit vaccines |
7 |
472/6787 |
6.95% |
9/1518 |
0.59% |
7.86 [4.36, 14.14] |
0% |
Fever |
|||||||
mRNA vaccines |
4 |
606/4167 |
14.54% |
26/3915 |
0.66% |
21.98 [6.61, 73.02] |
81% |
Adenovirus vector vaccines |
3 |
128/655 |
19.54% |
12/270 |
4.44% |
3.54 [1.61, 7.79] |
9% |
Inactivated vaccines |
9 |
782/36532 |
2.14% |
371/17884 |
2.07% |
1.01 [0.90, 1.15] |
0% |
Protein Subunit vaccines |
6 |
134/6666 |
2.01% |
32/1488 |
2.15% |
1.17 [0.79, 1.72] |
0% |
Headache |
|||||||
mRNA vaccines |
4 |
2315/4167 |
55.56% |
1270/3915 |
32.44% |
1.75 [1.37, 2.23] |
91% |
Adenovirus vector vaccines |
3 |
182/655 |
27.79% |
87/270 |
32.22% |
1.69 [0.40, 7.13 |
94% |
Inactivated vaccines |
9 |
4148/36532 |
11.35% |
2000/17884 |
11.18% |
1.03 [0.98, 1.08] |
94% |
Protein Subunit vaccines |
7 |
1102/6787 |
16.24% |
192/1518 |
12.56% |
1.02 [0.72, 1.46] |
63% |
Fatigue |
|||||||
mRNA vaccines |
4 |
2477/4167 |
59.44% |
1368/3915 |
34.94% |
1.70 [1.31, 2.20] |
93% |
Adenovirus vector vaccines |
2 |
204/574 |
35.54% |
27/190 |
14.21% |
2.79 [1.59, 4.92] |
32% |
Inactivated vaccines |
8 |
665/9596 |
6.93% |
277/4431 |
6.25% |
1.19 [1.04, 1.36] |
0% |
Protein Subunit vaccines |
7 |
1561/6787 |
23.00% |
251/1518 |
16.53% |
1.12 [0.94, 1.33] |
18% |
Vomiting |
|||||||
mRNA vaccines |
4 |
370/4167 |
8.88% |
129/3915 |
3.30% |
2.86 [1.51, 5.41] |
70% |
Adenovirus vector vaccines |
2 |
8/574 |
1.39% |
1/190 |
0.53% |
1.87 [0.33, 10.53] |
0% |
Inactivated vaccines |
9 |
254/36532 |
0.70% |
56/17884 |
0.31% |
1.89 [1.43, 2.50] |
33% |
Protein Subunit vaccines |
7 |
353/6787 |
5.20% |
52/1518 |
3.43% |
1.26 [0.95, 1.66] |
0% |
Grade 3 |
|||||||
mRNA vaccines |
4 |
858/4167 |
20.59% |
83/3915 |
2.12% |
6.49 [2.64, 15.96] |
86% |
Adenovirus vector vaccines |
3 |
81/1598 |
5.07% |
26/530 |
4.91% |
1.81 [0.40, 8.20] |
77% |
Inactivated vaccines |
5 |
249/34919 |
0.71% |
121/17436 |
0.69% |
0.99 [0.80, 1.23] |
0% |
Protein Subunit vaccines |
5 |
167/5741 |
2.91% |
21/1286 |
1.63% |
1.60 [0.64, 4.02] |
66% |
Mixed vaccination |
1 |
71/2215 |
3.21% |
15/668 |
2.25% |
1.43 [0.82, 2.47] |
/ |
Table 2: Subgroup analysis of incidence rate of solicited adverse events and grade 3 adverse events
Tables at a glance
Figures at a glance