Transfemoral Carotid Artery Stenting has Very Low Stroke, Death and TIA Incidence in Hands of Experienced Interventional Cardiologists as Noted in the VQI Registry Data
Received Date: August 02, 2024 Accepted Date: September 02, 2024 Published Date: September 05, 2024
doi: 10.17303/jcvm.2024.10.102
Citation: Kevin Kang, Robert Maholic, Gurjaipal Kang, John Wilson (2024) Transfemoral Carotid Artery Stenting has Very Low Stroke, Death and TIA Incidence in Hands of Experienced Interventional Cardiologists as Noted in the VQI Registry Data. J Cardio Vasc Med 10: 1-12
Abstract
Introduction: Transfemoral carotid artery stenting (tfCAS) is a United States Food and Drug Administration (FDA) approved procedure in patients with symptomatic and asymptomatic carotid artery stenosis. Recently, Centers for Medicare and Medicaid Services (CMS) expanded the coverage for tfCAS. However, tfCAS’s stroke incidence compared to carotid endarterectomy (CEA) remains a concern. The acceptable threshold for tfCAS is a 30-day stroke and death risk of less than 6% for symptomatic and less than 3% for asymptomatic patients. Given that adverse events continue after discharge, the threshold for in-hospital events is less than 4% for symptomatic patients and 2% for asymptomatic patients. We studied the in-hospital stroke/death incidence and transient ischemic attack (TIA) incidence of tfCAS in our community hospital. We hypothesize that at our institution, stroke/death/TIA risk will be low and numerically lower than previously published data because each of our operators is an experienced interventional cardiologist (IC) as defined below while the operator volume was more mixed in a some of the prior studies. We also hypothesize that tfCAS when done by experienced ICs is safe despite the concern of increased stroke risk in crossing the aortic arch with equipment during tfCAS. We acknowledge that this concern can be avoided in transcarotid carotid artery revascularization (TCAR). However, ICs do all their coronary procedures by going across the aortic arch and may be experienced and skilled enough that going across the aortic arch is not a significant risk factor in tfCAS performed by experienced ICs. Additionally, our hospital has long history of doing carotid stents since the original tfCAS randomized trials and the greater institutional experience may also improve outcomes.
Methods: Between January 1, 2015, and February 2, 2024, 636 patients underwent tfCAS procedure. All patients that underwent tfCAS were included in our analysis. Stroke data was prospectively collected throughout as our program participated in Carotid VQI (Vascular Quality Initiative) registry. Hence, this was a retrospective analysis of prospectively collected data entered in the VQI.Carotid VQI registry is a database that collects and analyzes demographic, clinical, procedural and outcomes data using standardized format. VQI registry uses dedicated independent staff to collect accurate data and report it to NCDR (National Cardiovascular Data registry). For all patients, carotid stenosis must be more than 50% for symptomatic and more than 70% for asymptomatic patients to qualify for tfCAS.Primary endpoint in our study was defined as in-hospital major adverse outcomes that is stroke, death, and TIA. All cases were done by 3 ICs who met our definition of experienced IC. Experienced IC was defined for this study as having a lifetime experience of doing tfCAS independently for at least five years before the start of this study in 2015 and meeting a volume requirement of performing over 100 tfCAS cases over the duration of the study.
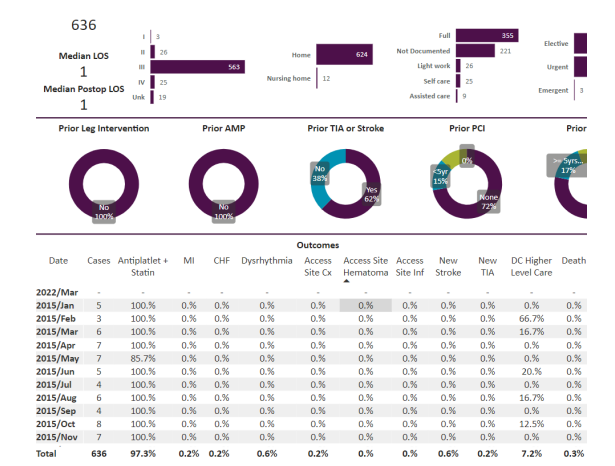
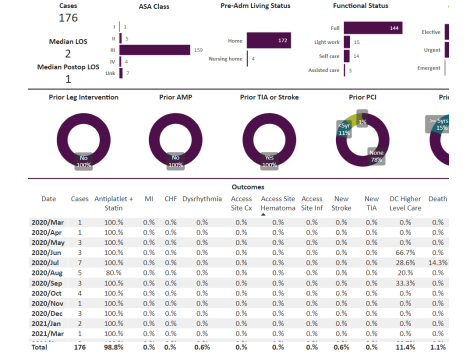
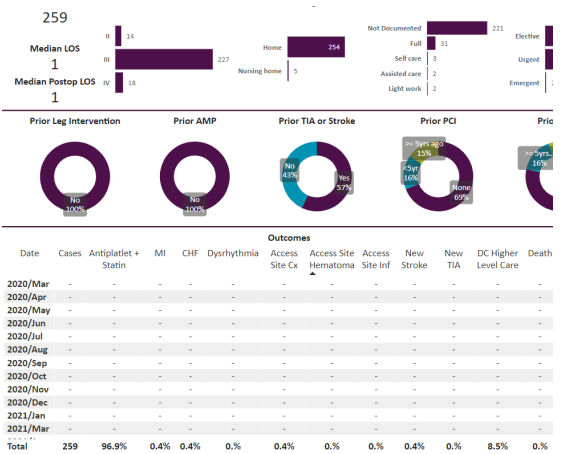
Results: tfCAS procedures were performed in 636 patients. The in-hospital stroke, death and TIA were 0.6%, 0.3% and 0.2% respectively for the overall population. The 176 symptomatic patients were separately analyzed, and the stroke, death and TIA were 0.6%, 1.1% and 0% respectively. Among the 201 asymptomatic patients, the stroke, death, and TIA were 1%, 0% and 0.5% respectively. Out of the total of 636 patients, 259 lacked sufficient data to be symptomatic or asymptomatic and were called “other”. Symptomatic is defined by a history of stroke or TIA within 180 days of the tfCAS. Regardless, the “other” patients got tfCAS by the same ICs and had stroke, death and TIA of 0.4%, 0% and 0% respectively. The tfCAS were almost all done with distal protection except for less than 5 out of 636 done with proximal protection. The tfCAS were also almost all done with transfemoral access with less than 5 patients done via transradial/transulnar access. None of the patients in the study got mechanical reperfusion therapy for acute stroke and all patients were done for primary or secondary prevention.
Conclusion: tfCAS for severe carotid stenosis in symptomatic or asymptomatic patients is safe in the hands of experienced ICs in a real-world patient population. The in-hospital stroke, death and TIA rates are numerically lower than the previously reported rates. Our in-hospital combined stroke and death rates in symptomatic and asymptomatic patients are 1.7% and 1% respectively and numerically less than the acceptable safety thresholds of 2% and 4% respectively for symptomatic and asymptomatic patient populations (1). Results may not be the same with less experienced operators, however (1). Our hospital has been doing tfCAS by the 3 ICs since 2001 and hence institutional experience may be a factor as well in our excellent outcomes.
Keywords: Carotid Stent; Experience; Stroke; Asymptomatic Patients; Transfemoral Carotid Artery Stenting
AbbreviationsASA=American Society of Anesthesiologists, Pre-Adm Living Status=preadmission living status, AMP-amputation, PCI=percutaneous intervention, CABG=coronary artery bypass grafting, CEA=carotid endarterectomy, CAS=Carotid artery stenting, all transfemoral less than 5 transradial or transulnar, Hx=history, CHF=congestive heart failure, MI=myocardial infarction, Inf=infection, DC=discharge
Introduction
Severe Carotid stenosis is an important cause of stroke that is a leading cause of death and disability [1]. About 10-15% of strokes are caused by severe stenosis of internal carotid artery [2]. Prior studies have shown that carotid stenosis treated by revascularization using Carotid endarterectomy (CEA) reduces the risk of stroke in patients with recent cerebrovascular symptoms [3]. CEA is beneficial in not just severe but also moderate symptomatic carotid stenosis [4]. CEA has reduced the stroke risk in asymptomatic carotid stenosis also [5,6]. Transfemoral Carotid stenting (tfCAS) is an alternative to carotid endarterectomy and in the initial studies was not as safe as CEA with large, randomized trials finding a higher risk of stroke in tfCAS patient groups [7,8]. More recent studies have shown that tfCAS and carotid endarterectomy have similar short- and long-term outcomes [9,10]. However, despite the recent results, major medical and surgical society guidelines still favor CEA over tfCAS often because of large meta-analyses or older randomized trials [11-13]. Recently Transcarotid artery revascularization (TCAR) has become rapidly adopted and even favored as the preferred form for carotid revascularization [14-16].
For our discussion, we are focused exclusively on transfemoral stenting (tfCAS) done by interventional cardiologists (ICs) who do not perform TCAR (Transcarotid artery revascularization). The purpose of this study was to evaluate the stroke, death, and TIA risk as a primary outcome and other major periprocedural and postprocedural risks like cardiovascular risk and access site complications as secondary outcome of tfCAS. All patients in our study were treated in a community hospital with all procedures performed by high volume ICs. It is likely that just like carotid endarterectomy and complex coronary procedures, carotid stenting has very low complication risk when performed by high volume operators in this case ICs [17,18] in a single institution. The previous studies have been criticized for lower volume operators performing tfCAS procedures [19].
tfCAS is a United States Food and Drug Administration (FDA) approved procedure in patients with symptomatic and asymptomatic carotid artery stenosis. More recently, Centers for Medicare and Medicaid Services (CMS) expanded the coverage for tfCAS [19]. However, its stroke risk compared to carotid endarterectomy (CEA) remains a concern [11,19]. The acceptable threshold for safety standards for tfCAS is a stroke and death risk of less than 6% for symptomatic and less than 3% for asymptomatic patients but these are 30-day event rates [11,19]. Given that some of the events occur after discharge, the in-hospital events have recently been suggested to be less than 4% for symptomatic patients and 2% for asymptomatic patients after tfCAS [19].
We hypothesize that with each of our operators being a very high-volume IC, our stroke risk will be very low and numerically lower than prior randomized studies as well as the prior observational data where the operator volume was more mixed [11,20]. So, we hypothesized that should be able to meet the safety standards of less than 2% and less than 4% death and stroke combined rates respectively for asymptomatic and symptomatic for tfCAS at our institution. We also hypothesize that tfCAS in hands of experienced ICs is safe despite concerns about going across the aortic arch with wires and catheters as opposed to TCAR [15]. Experienced IC was defined for this study as having a lifetime experience of doing tfCAS independently for at least five years before the start of this study in 2015 and meeting a volume requirement of performing over 100 tfCAS cases over the duration of the study. We hypothesize that ICs do all their coronary procedures by going across the aortic arch and hence are experienced and skilled enough that going across the aortic arch is not a significant risk factor for stroke when tfCAS procedures are done by experienced ICs
Materials and Methods
Between January 1, 2015, and February 2, 2024, 636 patients underwent tfCAS procedure. All patients that underwent tfCAS were included in our analysis. Stroke data was prospectively collected throughout as our program participated in Carotid VQI (Vascular Quality Initiative) registry. Hence, this was a retrospective analysis of prospectively collected data entered in the VQI.Carotid VQI registry is a database that collects and analyzes demographic, clinical, procedural and outcomes data using standardized format. VQI is a collaborative network of vascular specialists, hospitals, and researchers aiming to get quality data with the eventual goal of an improved patient care looking at various program’s outcomes. VQI registry uses dedicated independent staff to collect accurate data and report it to NCDR (National Cardiovascular Data registry). For all patients, carotid stenosis has to be more than 50% for symptomatic and more than 70% for asymptomatic patients to qualify for tfCAS [10,11].All cases were done by 3 ICs who met our definition of experienced IC.
All cases were done by 3 interventional cardiologists, each with a volume of over 100 carotid stent cases each over the study’s duration and lifetime experience of doing carotid stents independently of over five years at the start of this follow up in 2015. The hospital also has embodied dedicated carotid program meetings where the carotid stent outcomes are discussed by interventional cardiologists, vascular surgeons, neurosurgeons, neurointerventional and neurologists who all have chance to evaluate the data for its validity and accuracy. The data in the VQI is entered by independent group of people who are not employed by the interventional cardiologists and should not be affected by the operators’ biases.
Symptomatic patients were defined as those who had a history of cerebral infarction (CVA) or transient ischemic attack (TIA) symptoms within six months. Asymptomatic patients were those who had no stroke or TIA symptoms within the last 6 months.
Symptomatic patients with stenosis of more than 50% according to the North American Symptomatic Carotid Endarterectomy Trial (NASCET) criteria and asymptomatic patients with more than 70% stenosis with perfusion defect according to NASCET criteria were included as tfCAS indications [10,11].
Periprocedural major outcome was defined as a stroke/death/TIA that occurred after tfCAS. Periprocedural stroke was classified into TIA (transient ischemic attack) and CVA (cerebrovascular accident) according to standard neurological definitions [11]. Procedure-related death was defined as inhospital mortality.
Secondary outcomes were in-hospital MI, CHF, and vascular complications.
None of our patients had age less than 18 years. None of our patients had simultaneous carotid stenting with mechanical thrombectomy or carotid stenting during coil embolization since our procedures were all performed by adult ICs.
Preoperative imaging studies included carotid ultrasound and carotid computed tomography angiography (CTA). It is our practice to always get carotid Ultrasound prior to carotid angiography even if CTA is available.
Dual antiplatelets of aspirin 81 mg and clopidogrel 75 mg (rarely ticagrelor 90 mg BID) were routinely taken for at least a month after the procedure.
Stent types were first generation, and no micromesh stents were used [21-24]. The micromesh stents have numerically lower stroke rates than first generation stents. The two types of stents used over the course of our study were both first generation stents and not micromesh stents that is Precise (Cordis, Miami Lakes, FL, USA) and Xact (Abbott Laboratories, Chicago, IL, United States).
Carotid angioplasty and stenting procedures
All procedures were performed under local anesthesia via transfemoral routes with very rare transradial or transulnar route. During the procedure, oxygen saturation, electrocardiogram, and blood pressure were monitored.
After the placement of a 6F or 7F short arterial introducer sheath on the femoral artery, either bivalirudin or intravenous bolus injection of 50 IU to 60 IU/kg heparin was done depending on ACT level that was targeted at 250-300 in all cases. A distal embolic protection device was employed in all cases except very rarely a proximal protection device was used instead.
Pre dilation with a balloon usually 4 mm in diameter was done in all cases. Before balloon dilatation of stenotic carotid artery, the vital signs were closely monitored. The goal was angiographic less than 20% residual stenosis that was achieved in all our cases. After the procedure, patients were admitted to a monitored unit for overnight stay as a routine. Ultrasound of the carotid artery stent was taken within 24 hr. after tfCAS. NIH score was routinely monitored overnight by NIH stroke scale trained professionals overnight at least every 4 hours.
Symptomatic patients were referred usually by the in-hospital neurology service over the years. The neurology service at our hospital routinely consulted us on patients with symptomatic carotid stenosis and we responded with early carotid stent interventions that we had observed to be safe at our institution versus the published data [11].
Asymptomatic patients were referred from anywhere around the region given our hospital’s status as a referral hospital.
Over the last few decades, our ICs have participated in many randomized nationwide clinical trials involving tfCAS like SAPPHIRE, CREST 2 and PERFORMANCE 2 [20,23,24]. Also, we were able to take part in the carotid stent registries like CREST 2 explaining our high tfCAS volumes [20].
The carotid stents were almost all with distal protection except for less than 5 patients out of 636 total done with proximal protection with lack of protection in some earlier studies leading to poorer tfCAS results [25].
The carotid stents were also almost all done with transfemoral access with less than 5 patients done transradial or transulnar access and yet, the stroke rates were extremely low. This is despite the perception that TCAR may be better due to avoidance of the arch by the devices [14,16]. The procedures were all done by ICs who have done all their procedures across the aortic arch and hence have experience in that vascular territory.
Follow-up protocol
Patients underwent follow-up carotid ultrasound every year. Dual antiplatelet medication was continued for at least one month and preferentially 6 months after tfCAS. Clopidogrel was discontinued after that, and aspirin was maintained lifelong.
Results
A total of 636 patients underwent carotid stenting procedures. This was between between January 1, 2015 and February 2, 2024. Out of these, 201 were asymptomatic, 176 were symptomatic and the data was not clear in 259 and these remainder of the patients were not called symptomatic or asymptomatic but “other”. The total data is below in Figure 1.
The remainder of the patients out of the total of 636 that were not classified into clearly symptomatic or symptomatic but nonetheless were treated by the three inter ventional cardiologists at our institution between the same time period between 1/1/2015 to 12/24/2023 were also separately evaluated as the “other” patients. The data from these “other patients is shown in Figure 4.
Discussion
In this study, following CAS, the 30-day stroke and TIA rates were numerically very low compared to historical randomized trials or registries. In symptomatic patients, periprocedural stroke risk was extremely low (0%) as opposed to prior studies or as mentioned in major guidelines [11].
Carotid Revascularization Endarterectomy versus Stenting Trial (CREST) trial has one of the best data for showing equipoise of tfCAS with CEA without using micromesh second generation stents [11,20,21]. Our study with experienced interventional cardiologists has numerically a lower rate of stroke than CREST. In our study, the risk of other vascular complications and myocardial infarction are also numerically very low as can be seen from the figures 1 to 4.
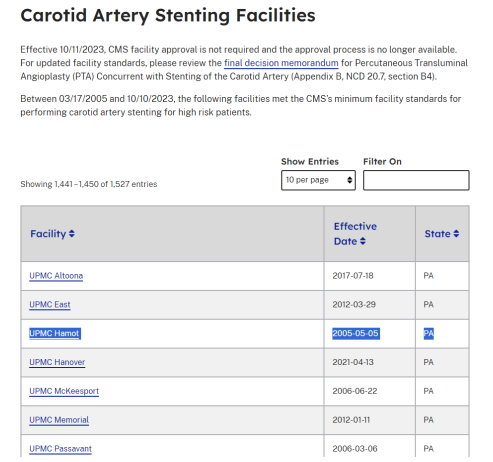
Our hospital, University of Pittsburgh Medical Center (UPMC) Hamot hospital’s long history as an accredited hospital by CMS (Figure 5) for carotid stenting attests to our hospital’s experienced operators and staff that led to the good outcomes that we saw. As noted in the figure, Hamot has been a CMS approved facility since 2005 while other hospitals in the figure were approved much later.
Among the UPMC hospitals, again our hospital has a unique high experience in doing tfCAS as noted in Table 1 below. In the figure, all the listed hospitals belong to UPMC system, but Hamot has largest tfCAS numbers.
Regarding our center’s participation in tfCAS clinical trials as outlined above, Table 2 below from a nationwide Carotid stent trial shows the good performance of our center in adhering to the protocol compared to the overall study indicating experience of our center in doing tfCAS nationwide trials.
So, not only is our IC volume high and our ICs doing tfCAS are experienced, but our facility is also experienced in doing tfCAS presenting an overall high level of experience leading to the excellent outcomes noted.
There is a debate on stent type in reducing stroke risk with newest stents having lower risk [21]. In our study, the stent type was left up to the discretion of the operator and first-generation stents were used with excellent results, testament to the role of operator experience once again.
Hyperperfusion syndrome or intracranial hemorrhage were not separately recorded per se but would fall under TIA or CVA in our institution’s tracking of neurological deficits as a lower NIH score [11].
The carotid stents were almost all with distal protection except for less than 5 patients out of 636 total done with proximal protection [11]. Again, the very low stroke rates suggest that distal protection if done properly by experienced operators may be enough for protection from strokes. Some of the prior trials showing worse outcomes for tfCAS have been criticized for relatively lower distal or proximal protection usage [25]. Again, the very low stroke rates suggest that distal protection if done properly by experience operators may be enough for protection from strokes.
None of the patients in the study got mechanical reperfusion therapy for acute stroke and all patients were done for primary or secondary prevention [26,27].
The carotid stents were almost all done with transfemoral access with less than 5 patients done transradial or transulnar access [21] and yet, the stroke rates were extremely low despite the perception that TCAR may be better due to avoidance of the aortic arch. The procedures were all done by interventional cardiologists who do all their procedures across the aortic arch though and hence have vast experience in that vascular territory
Limitation
It is a single center study, but it is supposed to show the special value of operator experience in better outcomes and hence it may be a strength of the study as well. The study was done with retrospective analysis of data, but the data had been collected prospectively as part of the VQI registry.
The other limitations include that 259 patients in the data were not defined as symptomatic or asymptomatic and were treated before such criteria became part of the VQI registry data. There is also lack of a control group in the study and the generalizability of the study is limited to centers similar to ours with trained ICs performing the procedures.
Conclusions
tfCAS for severe carotid stenosis in symptomatic or asymptomatic patient population is very safe in the hands of experienced ICs in a real-world patient population. The in-hospital stroke, death and TIA rates are numerically lower than the rates reported in previously published data [11]. Our in-hospital stroke/death rates in symptomatic and asymptomatic patients are less than 2% and 4% respectively and meet the acceptable safety thresholds [11,20]. Results may not be the same with less experienced operators, however [20].
Funding
None
Disclosures
None
Acknowledgements
None
- Murray CJ, Lopez AD (1997) Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 349: 1269-76.
- Bates ER, Babb JD, Casey DE, Cates CU, Feldman TE, et al. (2007) ACCF/SCAI/SVMB/SIR/ASITN 2007 clinical expert consensus document on carotid stenting. J Am Coll Cardiol. 49: 126-70.
- Randomised trial of endarterectomy for recently symptomatic carotid stenosis (1998) final results of the MRC European Carotid Surgery Trial (ECST). Lancet, 351: 1379-87.
- Barnett HJ, Taylor DW, Eliasziw M (1998) Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 339: 1415-25.
- Rothwell PM, Goldstein LB (2004) Carotid endarterectomy for asymptomatic carotid stenosis: asymptomatic carotid surgery trial. Stroke. 35: 2425-7.
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study Endarterectomy for asymptomatic carotid artery stenosis. JAMA, 273: (1995) 1421-8.
- SPACE Collaborators (2006) Stent Protected Angioplasty versus Carotid Endarterectomy in symptomatic patients: 30 days results from the SPACE Trial. Lancet, 368: 1239-47.
- JL Mas, G Chatellier, B Beyssen, A Branchereau, T Moulin, JP Becquemin, et al. (2006) Endarterectomy versus stenting in patients with severe symptomatic stenosis. N Engl J Med, 355: 1660-71.
- Bonati LH, Dobson J, Featherstone RL, Ederle J, van der Worp HB, de Borst GJ, et al. (2015) Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet. 385: 529-38.
- Brott TG, Howard G, Roubin GS, Meschia JF, Mackey A, Brooks W, et al. (2016) Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 374: 1021-31.
- Naylor R, Rantner B, Ancetti S, de Borst GJ, De Carlo M, Halliday A, Kakkos SK, et al. (2023) Editor's Choice - European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur J Vasc Endovasc Surg. 65: 7-111.
- Ricotta JJ, Malgor RD (2008) A review of the trials comparing carotid endarterectomy and carotid angioplasty and stenting. Perspect Vasc Surg Endovasc Ther. 20: 299-310.
- Meta-analysis of randomized trials comparing carotid endarterectomy and endovascular treatment. Eur J Vasc Endovasc Surg. 34: 470-9.
- Columbo JA, Martinez-Camblor P, Stone DH, Goodney PP, O'Malley AJ (2022) Procedural Safety Comparison Between Transcarotid Artery Revascularization, Carotid Endarterectomy, and Carotid Stenting: Perioperative and 1- Year Rates of Stroke or Death. J Am Heart Assoc. 11: e024964.
- Loufopoulos G, Manaki V, Tasoudis P, Karela NR, Sénéchaud C, Giannopoulos A, Ktenidis K, Spanos K (2024) Trans-Carotid Artery Revascularization Versus Carotid Endarterectomy in Patients With Carotid Artery Disease: Systematic Review and Meta-analysis of 30-day Outcomes. Angiology. 33197241241788.
- Stonko DP, Goldsborough E 3rd, Kibrik P, Zhang G, Holscher CM, Hicks CW (2022) Use of Transcarotid Artery Revascularization, Transfemoral Carotid Artery Stenting, and Carotid Endarterectomy in the US From 2015 to 2019. JAMA Netw Open. 5: e2231944.
- Kumamaru H, Jalbert JJ, Nguyen LL, Gerhard-Herman MD, Williams LA, Chen CY, et al. (2015) Surgeon case volume and 30-day mortality after carotid endarterectomy among contemporary medicare beneficiaries: before and after national coverage determination for carotid artery stenting. Stroke. 46: 1288-94.
- Choi KH, Lee SY, Song YB, Park TK, Lee JM, Yang JH, Choi JH, Choi SH, Gwon HC, Hahn JY (2023) Prognostic Impact of Operator Experience and IVUS Guidance on Long-Term Clinical Outcomes After Complex PCI. JACC Cardiovasc Interv. 16: 1746-58.
- Jabbour G, Yadavalli SD, Straus S, Sanders AP, Rastogi V, Eldrup-Jorgensen J, et al. (2024) Learning curve of Transfemoral Carotid Artery Stenting in the Vascular Quality Initiative Registry. J Vasc Surg. 28: S0741-5214.
- White CJ, Brott TG, Gray WA, Heck D, Jovin T, Lyden SP, Metzger DC, et al. (2022) Carotid Artery Stenting: JACC State-of-the-Art Review. J Am Coll Cardiol. 80: 155-70.
- Petkoska D, Zafirovska B, Vasilev I, Saylors E, Sachar R, Kedev S (2024) Transradial carotid artery stenting using double layer micromesh stent and novel post-dilation balloon with integrated embolic protection. Cardiovasc Revasc Med. 12: S1553-8389(24)00007-1.
- Mazurek A, Malinowski K, Sirignano P, Kolvenbach R, Capoccia L, et al. (2023) CArotid Revascularization system atic reviews and MEta-aNalyses (CARMEN) Collaborators. Carotid artery revascularization using second generation stents versus surgery: a meta-analysis of clinical outcomes. J Cardiovasc Surg (Torino) 64: 570-82.
- Langhoff R, Petrov I, Kedev S, Milosevic Z, Schmidt A, Scheinert D, et al. (2022) PERFORMANCE 1 study: Novel carotid stent system with integrated post-dilation balloon and embolic protection device. Catheter Cardiovasc Interv. 100: 1090-9.
- Bryn Mawr Communications. (2023, November 1). Contego’s Neuroguard Evaluated in 30-Day and 1-Year Outcomes in PERFORMANCE II. Endovascular Today. https://evtoday.com/news/contegos-neuroguard-evaluated-in -30-day-and-1-year-outcomes-in-performance-ii.
- Jansen O, Fiehler J, Hartmann M, Bruckmann H (2009) Protection or nonprotection in carotid stent angioplasty: the influence of interventional techniques on outcome data from the SPACE Trial. Stroke. 40: 841-6.
- Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. (2021) Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke. 52: e364-467.
- Bhaskar S, Stanwell P, Cordato D, Attia J, Levi C (2018) Reperfusion therapy in acute ischemic stroke: dawn of a new era? BMC Neurol. 18: 8.


Tables at a glance
Figures at a glance