Effectiveness and Safety of Multiple In-Situ Stent Balloon Inflations for Stent Deployment Optimization; An IVUS Guided Evaluation (ESSBISO Trial)
Received Date: July 05, 2025 Accepted Date: August 04, 2025 Published Date: August 07, 2025
doi:10.17303/jcvm.2025.11.104
Citation: Ahmed M. Abdel-Salam, Basem Abdel-Hamed, Tamer Elwasify, Ahmed Mandour, Ahmed Salah (2025) Effectiveness and Safety of Mult iple In-Situ Stent Balloon Inflations for Stent Deployment Optimization; An IVUS Guided Evaluation(ESSBISO Trial) J Cardio Vasc Med 11: 1-12
Abstract
Background: Treating long coronary artery lesions effectively requires meticulous lesion preparation and precise stent deployment. Although non-compliant balloons are often favored for optimal stent apposition, their use increase the procedural costs. Intravascular ultrasound (IVUS) provides high-resolution, real-time imaging, improving stent optimization and overall treatment outcomes.
Aim: This proof – of - concept study evaluates whether multiple in-situ stent balloon inflations can achieve successful stent deployment, as measured by strict IVUS criteria, while maintaining procedural safety.
Method: Using IVUS, we prospectively assessed the impact of repeated post-deployment inflations with the same stent balloon in 91 patients with ischemic heart disease (IHD) underwent elective PCI. Participants were randomized into three groups: Group A (single inflation), Group B (two inflations), and Group C (three inflations).
Results: Baseline demographics, lesion characteristics, and calcification severity were comparable across groups. Procedural data showed no significant differences in stent length, diameter, or inflation pressure, with all cases completed using a single-stent approach. IVUS analysis showed Group C had the highest rate of successful stent deployment, with full expansion, no malapposition, and minimal residual stenosis — outperforming Groups B and A. Multivariate analysis linked the number of inflations and inflation pressure to deployment success, with no procedural complications observed.
Conclusion: For type A lesions, three high-pressure inflations (10 sec/16 bars each) were highly effective, though complex lesions required additional strategies. This technique was less effective for complex lesions. The technique proved safe across all groups, supporting its use in select cases.
Keywords: Intravascular ultrasound; Stent Deployment; Stent; Cardiology; Real-Time Imaging
Introduction
Atherosclerosis remains a major global cause of death, even with decreasing incidence in some regions [1]. Although atherosclerotic plaques may develop silently throughout an individual's lifetime, only a minority progress to cause the majority of cardiovascular events [2]. Clinical evidence robustly supports the efficacy and safety of percutaneous coronary intervention (PCI) using current-generation drug-eluting stents (DES) for treating long, diffuse coronary lesions [3–8].
Initially, the strategy of using DES to cover the entire lesion length was adopted due to their perceived effectiveness [5]. However, this approach was later abandoned due to the increased risks of stent thrombosis (ST) and target lesion revascularization (TLR) associated with first-generation DES [9]. The development of second-generation DES, with thinner struts, advanced polymers, and optimized drug potency, has significantly improved PCI outcomes, reducing both TLR and ST rates [10–12].
In-stent restenosis and subacute ST remain critical concerns even in the era of DES. Clinically, ST can present dramatically as acute coronary syndrome (ACS) or sudden death, significantly contributing to post-PCI morbidity and mortality, with an incidence of 0.5% to 3% and a mortality rate exceeding 45%. The multifactorial nature of in-stent restenosis and subacute ST includes procedural factors, particularly the adequacy of stent deployment. Utilizing intravascular imaging to fine-tune procedural results offers a promising approach to reducing the risk of ST and improving patient outcomes [13–15].
Studies indicate that 20% to 30% of stents are under-expanded on intravascular ultrasound (IVUS) after standard deployment both with BMS [16,17] as well as DES [18]. Based on supporting evidence and in the absence of large prospective randomised outcome-based trials, Post dilatation with a non-compliant (NC) balloon to achieve optimal stent expansion and maximal luminal area is a logical technical recommendation, especially for complex lesions [19]. However, this approach increases both procedural time and cost.
Method
Trial Design
To our knowledge, this trial represents the first use of intravascular ultrasound (IVUS) to guide the assessment of efficacy and safety in employing in-situ stent balloon for multiple post-stent deployment inflations to achieve optimal stent deployment. The trial adopted a prospective, observational, single-center, proof-of-concept approach. IVUS served as the primary tool to confirm and evaluate the adequacy of stent deployment in patients presenting with long coronary artery lesions.
Study Duration
From February 2023 to February 2024. The study was approved by the relevant regulatory authorities, by the Ethics Committee of the Armed Force College of Medicine (AFCM), Cairo, Egypt. The academic leadership of the trial designed the protocol and supervised its implementation.
Studied Population
Ninety-one patients diagnosed with ischemic heart disease (IHD) admitted to the cardiology department of Kobri El-Quoba Military Complex hospitals who met the inclusion criteria and required coronary angioplasty based on their clinical conditions as guided by evidence-based guidelines.
Inclusion Criteria
Patients diagnosed with unstable angina or chronic coronary syndrome with sizable viable myocardium. Patients with long coronary artery lesions (> 20 mm) suitable for stent placement were included, provided they could provide informed consent.
Exclusion Criteria
(1) Patients with acute STEMI. (2) Patients with hemodynamic instability. (3) Presence of chronic total occlusions, left main coronary lesions, or previous coronary artery bypass grafting. (4) Contraindications to coronary angioplasty (e.g., active bleeding disorders). (5) Lesions unsuitable for stent placement. (6) Contraindications to intravascular ultrasound (IVUS) use. (7) Inability or unwillingness to undergo multiple post-stent deployment balloon inflations. (8) Unwillingness or inability to participate in the study protocol. (9) Pregnancy or breastfeeding. (10) History of severe contrast dye allergy. (11) Inability to provide informed consent due to cognitive impairment or language barriers.
Patients’ Stratification
Patients were stratified into three groups based on the number of balloon inflations post-stent deployment using the in-situ stent balloon.
Group A
Included 30 patients who underwent a single inflation, 30 seconds after stent deployment, followed by IVUS examination.
Group B
Consisted of 30 patients who received two inflations: one 30 seconds after stent deployment and another 10 seconds later, using the in-situ stent balloon, followed by IVUS examination.
Group C
Comprised 31 patients who underwent three inflations: one 30 seconds after stent deployment, followed by two additional inflations separated by 10-second intervals, also using the in-situ stent balloon, followed by IVUS examination.
Outcome Measures
Efficacy endpoint: The determination of procedural success relied on stringent criteria established for defining successful stent deployment through IVUS, aimed at maximizing procedural efficacy and patient outcomes. These criteria were:
Full Stent Expansion. (2) Appropriate Stent Apposition. (3) Minimal Residual Stenosis. (4) Adequate Stent Expansion.
Safety endpoint: Absence of complications especially: (1) Flow limiting edge dissection. (2) Stent fracture. (3) Perforation.
The patient was deemed successful if it meets all efficacy criteria & safety endpoint.
Statistical Analysis
Data were analyzed using SPSS version 25. Qualitative data were presented as frequency and percentage, while continuous quantitative data were expressed as median and interquartile range (IQR). The median is the middle value in an ordered data set, and the IQR is the range between the 25th and 75th percentiles. Statistical significance was determined with a P-value < 0.05 considered significant, < 0.001 highly significant, and > 0.05 insignificant. The Chi-square test was used for comparing non-parametric categorical data, and the Kruskal-Walli’s test was applied for comparisons among more than two groups with abnormally distributed data.
Results
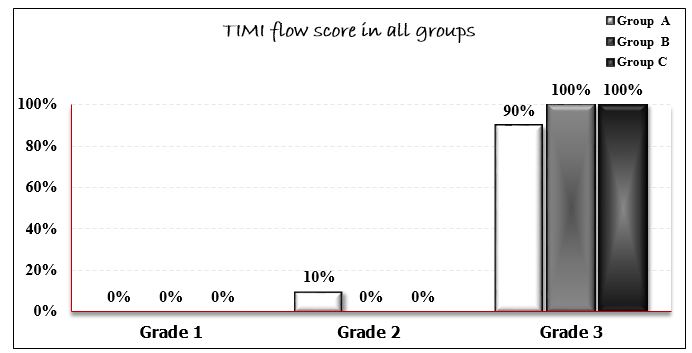
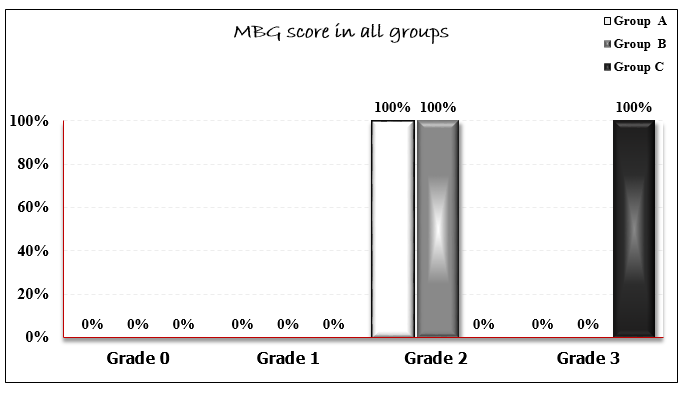
The study enrolled 91 male patients with ischemic heart disease who underwent elective PCI with one stent deployment in all cases. Their age was ranging from 42 to 65 years (mean 55.6 ± 9.1 years). Age distribution was comparable across all groups. Hypertension was present in 65.9% of participants, while 50.5% had diabetes mellitus. Active smoking and dyslipidemia were documented in 52.7% and 50.5% of cases, respectively. No statistically significant differences were found in the prevalence of these risk factors among the three study groups (Table 1). Lesion characteristics were similar across groups, with no significant difference in morphology or calcification severity (mild: 59.3%; moderate: 40.6%; severe: 11.1%). The right femoral approach was used in 77% of cases, with the left anterior descending artery (LAD) being the most frequent target site. Procedural data analysis revealed comparable stent lengths (P=0.43), diameters (P=0.36), and inflation pressures (P=0.06) among groups. A single stent strategy was performed in all procedures. TIMI flow scores differed significantly (P=0.04): Group A had 3 patients (10%) with TIMI 2 flow versus 27 (90%) with TIMI 3, whereas Groups B and C achieved TIMI 3 flow in all cases. Myocardial blush grade (MBG) showed even greater divergence (P<0.001), with Groups A and B predominantly scoring 2, while Group C uniformly attained grade 3 (Figures 1–2). No procedural complications including edge dissection, stent fracture, or perforation were observed.
Regarding IVUS finding, Group C exhibited optimal results, with 67.6% achieving full stent expansion and 0% malapposition, versus 56.7% and 0% in Group B, and 0% and 40% in Group A. Residual stenosis was least prevalent in Group C (32.3%), intermediate in Group B (46.7%), and universal in Group A (100%).
Quantitative measures including proximal reference lumen area (median 9.6 mm²), distal reference EEM area (10.3 mm²), and minimum lumen diameter consistently favored Group C (Table 2).
Successful deployment defined by composite IVUS criteria (full expansion, < 20% residual stenosis, complete apposition, and absence of complications) was achieved in 0%, 56.7%, and 67.6% of Groups A, B, and C, respectively. Overall, 43% of patients (n=39) met all success criteria (Table 3). Multivariate analysis identified key failure predictors as to be lesion related factors (Lesion type & angiographic calcification), Procedural variables (Number of inflation& inflation pressure) as well as IVUS parameters (Proximal/distal lumen areas, MLD, MLA). (Table 4. Operator-dependent success factors included triple balloon inflations and higher inflation pressures, which correlated with improved MBG scores (Table 5). However, these benefits were confined to type A lesions with mild calcification. Efficacy diminished markedly in complex, heavily calcified lesions, where neither inflation strategy substantially improved outcomes (Table 6).
Discussion
The evolution of PCI has dramatically transformed coronary artery disease management, offering patients a minimally invasive alternative to traditional bypass surgery [20]. While stent implantation remains central to restoring vascular patency, achieving optimal deployment in complex lesions - particularly lengthy segments - continues to challenge interventional cardiologists. These technical hurdles carry significant clinical consequences, as suboptimal stent placement may precipitate restenosis or thrombosis [21].
Modern catheterization laboratories increasingly rely on intravascular ultrasound (IVUS) for its unparalleled intraluminal visualization capabilities [22]. Where conventional angiography falls short by providing only silhouette images, IVUS delivers comprehensive cross-sectional assessment revealing not just lumen dimensions but also plaque morphology and vessel architecture [23]. This proves particularly valuable when treating diffuse lesions, where accurate length measurement and vessel sizing directly inform stent selection and positioning strategies.
Recent years have witnessed growing evidence supporting IVUS guided optimization of post-stent dilation protocols [24]. Our study builds upon this foundation by systematically evaluating how multiple balloon inflations influence stent expansion - a question previous investigations have addressed only peripherally. Where prior research focused predominantly on inflation pressure or duration, this study represents the first systematic investigation – up to our knowledge - into the effects of repeated stent balloon inflation post-stent deployment, using IVUS to predict the impact on stent expansion in patients with long coronary artery lesions. This study stands out by exploring multiple balloon inflation strategies under IVUS guidance, contrasting with previous research focused primarily on high-pressure or extended-duration inflations.
The demographic profile of our cohort with hypertension (65.9%), diabetes (50.5%), smoking (52.7%), and dyslipidemia (50.5%) prevalence mirroring typical CAD populations [25-28] enhances the generalizability of our findings. Similarly, the observed calcification distribution (59.3% mild, 40.6% moderate) aligns with Fujii et al.'s reports regarding contemporary PCI cohorts [29].
Several key findings emerged from our IVUS analysis: Group C (three inflations) demonstrated superior expansion (67.6% full expansion) compared to Group B (56.7%) and Group A (0%), Complete stent apposition was achieved in all Group C and B cases, versus 40% malapposition in Group A and residual stenosis showed stepwise improvement across groups (32.3% vs 46.7% vs 100%). These results dovetail with Skowroński et al.'s demonstration that sequential inflation improves stent dimensions [30], while extending their observations to include myocardial perfusion benefits (MBG 3 in 100% of Group C vs MBG 2 in others).
Contrary to conventional wisdom favoring prolonged (>60s) inflations [31-33], our data suggest multiple brief (10s) high-pressure (16atm) inflations may offer comparable - if not superior - results. This approach proved particularly effective for: achieving optimal stent-vessel wall contact, minimizing residual stenosis and preserving microvascular function (as reflected in MBG scores). The 71% success rate in Group C, alongside zero procedural complications, underscores both the efficacy and safety of this strategy. Notably, these benefits appear most pronounced in type A lesions with mild calcification - a finding consistent with Iwamoto et al.'s work [34], though our multivariate analysis uniquely identified inflation pressure as an independent success predictor.
Conclusion
Our study suggests that multiple balloon inflations, particularly using three inflations with in-situ stent balloon at high pressure (~16 atm.) for 10 seconds each, show effectiveness primarily in patients with less severe type A lesions. However, their efficacy diminishes in more complex lesion cases. Similarly, this approach is beneficial for patients with mild angiographically visual calcification but yields unsatisfactory results in cases of moderate to severe calcification, even with three inflations. The success rate decreases with higher lesion grades, especially when this higher grade is due to moderate or severe calcification density, suggesting limited efficacy of multiple balloon inflations in such cases. Nonetheless, the protocol is considered safe regardless of lesion complexity.
Limitations
This study had some limitations. First, it was conducted at a single center with a relatively small sample size, potentially limiting the generalizability of the findings. Second, the follow-up period may be too short to fully assess long-term outcomes such as late stent thrombosis, restenosis, and long-term patient mortality and morbidity. Lastly, the exclusion of certain patient groups, such as female patients, those with acute coronary syndromes, hemodynamically unstable patients, and those with chronic total occlusions, restricts the applicability of the study findings to all patients undergoing PCI.
Recommendation
Further research should prioritize multicenter collaborations with larger sample sizes to substantiate our findings. Emphasizing the broader integration of IVUS in percutaneous coronary interventions, particularly for complex cases with extended lesions, is crucial to refine stent deployment accuracy and safety. Additionally, conducting longitudinal studies with extended follow-up periods is essential to comprehensively evaluate the enduring advantages and risks associated with IVUS-guided stent placement, encompassing outcomes like restenosis, late stent thrombosis, and long-term patient survival.
Funding
None
- Björkegren JL, Lusis AJ (2022) Atherosclerosis: Recent developments. Cell. 18510: 1630–45.
- De Winther MPJ, Bäck M, Evans P, Gomez D, Goncalves I, et al. (2023) Translational opportunities of single-cell biology in atherosclerosis. Eur Heart J. 44: 1216–30.
- Lee CW, Park KH, Kim YH, Hong MK, Kim JJ, et al. (2006) Clinical and angiographic outcomes after placement of multiple overlapping drug-eluting stents in diffuse coronary lesions. J Am Coll Cardiol. 98: 918–22.
- Sharp A, Latib A, Ielasi A, Larosa C, Godino C, et al. (2009) Long-term follow-up on a large cohort of “full-metal jacket” percutaneous coronary intervention procedures. Circ Cardiovasc Interv. 2: 416–22.
- Honda Y, Muramatsu T, Ito Y, Sakai T, Hirano K, et al. (2016) Impact of ultra-long second-generation drug eluting stent implantation. Catheter Cardiovasc Interv. 87: 44–53.
- Lee CW, Ahn JM, Lee JY, Kim WJ, Park DW, et al. (2014) Long-term (8 year) outcomes and predictors of major adverse cardiac events after full metal jacket drug-eluting stent implantation. Catheter Cardiovasc Interv. 84: 361–5.
- Kong MG, Han JK, Kang JH, Zheng C, Yang HM, et al. (2021) Clinical outcomes of long stenting in the drug-eluting stent era: Patient-level pooled analysis from the GRAND-DES registry. EuroIntervention. 16: 1318–25.
- Durante A, Manzillo GF, Burzotta F, Trani C, Aurigemma C, et al. (2016) Long-term follow-up of “full metal jacket” of de novo coronary lesions with new generation zotarolimus-eluting stents. Int J Cardiol. 221: 1008–12.
- Moreno R, Fernández C, Hernández R, Alfonso F, Angiolillo DJ, et al. (2005) Drug-eluting stent thrombosis: Results from a pooled analysis including 10 randomized studies. J Am Coll Cardiol. 45: 954–9.
- Kedhi E, Joesoef KS, McFadden E, Wassing J, van Mieghem C, et al. (2010) Second-generation everolimus-eluting and paclitaxel-eluting stents in real-life practice (COMPARE): A randomised trial. Lancet. 375: 201–9.
- Udipi K, Melder RJ, Chen M, Cheng P, Hezi-Yamit A, et al. (2007) The next generation Endeavor Resolute stent: Role of the BioLinx polymer system. EuroIntervention. 3: 137–9.
- Chevalier B, Silber S, Park SJ, Garcia E, Schuler G, et al. (2009) Randomized comparison of the Nobori Biolimus A9-eluting coronary stent with the Taxus Liberté paclitaxel-eluting coronary stent in patients with stenosis in native coronary arteries: The NOBORI 1 trial—Phase 2. Circ Cardiovasc Interv. 2: 188–95.
- Wiviott SD, Braunwald E, McCabe CH, Ivan Horvath I, Keltai M, et al. (2008) Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: A subanalysis of a randomised trial. Lancet. 371: 1353–63.
- Alfonso F, Sandoval J (2012) New insights on stent thrombosis: In praise of large nationwide registries for rare cardiovascular events. JACC Cardiovasc Interv. 5: 141–4.
- Armstrong EJ, Feldman DN, Wang TY, Kaltenbach LA, Yeo KK, et al. (2012) Clinical presentation, management, and outcomes of angiographically documented early, late, and very late stent thrombosis. JACC Cardiovasc Interv. 5: 131–40.
- de Ribamar Costa Jr J, Mintz GS, Carlier SG, Costa RA, Fujii K, et al. (2005) Intravascular ultrasonic assessment of stent diameters derived from manufacturer’s compliance charts. Am J Cardiol. 96: 74–8.
- Johansson B, Olsson H, Wennerblom B (2002) Angiography guided routine coronary stent implantation results in suboptimal dilatation. Angiology. 53: 69–75.
- Javaid A, Chu WW, Cheneau E, Clavijo LC, Satler LF, et al. (2006) Comparison of paclitaxel-eluting stent and sirolimus-eluting stent expansion at incremental delivery pressures. Cardiovasc Revasc Med. 7: 208–11.
- Ashok Seth A, Gupta S, Singh VP, Kumar VV (2017) Expert opinion: Optimizing stent deployment in contemporary practice: The role of intracoronary imaging and non-compliant balloons. Interv Cardiol. 12: 81–4.
- Thakare VS, Sontakke NG, Wasnik P, Kanyal D (2023) Recent advances in coronary artery bypass grafting techniques and outcomes: A narrative review. Cureus. 15: e45511.
- Abubakar M, Javed I, Rasool HF, Raza S, Basavaraju D, et al. (2023) Advancements in percutaneous coronary intervention techniques: A comprehensive literature review of mixed studies and practice guidelines. Cureus. 15: e41311.
- Shammas NW, Radaideh Q, Shammas WJ, Daher GE, Rachwan RJ, et al. (2019) The role of precise imaging with intravascular ultrasound in coronary and peripheral interventions. Vasc Health Risk Manag. 15: 283–90.
- Malaiapan Y, Leung M, White AJ (2020) The role of intravascular ultrasound in percutaneous coronary intervention of complex coronary lesions. Cardiovasc Diagn Ther. 10: 1371–8.
- Nafee T, Shah A, Forsberg M, Zheng J, Ou J (2023) State-of-art review: Intravascular imaging in percutaneous coronary interventions. Cardiol Plus. 8: 227–46.
- Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, et al. (2021) Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 143: e984–1010.
- Cheng W, Zhuang J, Chen S (2022) Dyslipidemia and the prevalence of hypertension: A cross-sectional study based on Chinese adults without type 2 diabetes mellitus. Front Cardiovasc Med. 9: 938363.
- Dalal J, Chandra P, Chawla R, Kumar V, Abdullakutty J, et al. (2024) Clinical and demographic characteristics of patients with coexistent hypertension, type 2 diabetes mellitus, and dyslipidemia: A retrospective study from India. Drugs Real World Outcomes. 1: 167–76.
- Sharma S, Gaur K, Gupta R (2024) Trends in epidemiology of dyslipidemias in India. Indian Heart J. 76: S20–8.
- Fujii K, Carlier SG, Mintz GS, Yang YM, Moussa I, et al. (2005) Stent under-expansion and residual reference segment stenosis are related to stent thrombosis after sirolimus-eluting stent implantation: An intravascular ultrasound study. J Am Coll Cardiol. 45: 995–8.
- Skowroński J, Wolny R, Jastrzębski J, Tyczyński P, Szlazak K, et al. (2019) Impact of the balloon inflation time and pattern on the coronary stent expansion. J Interv Cardiol. 2019: 6945372.
- Hovasse T, Mylotte D, Garot P, Salvatella N, Morice MC, et al. (2013) Duration of balloon inflation for optimal stent deployment: Five seconds is not enough. Catheter Cardiovasc Interv. 81: 446–53.
- Cook JR, Mhatre A, Wang FW, Uretsky BF (2014) Prolonged high-pressure is required for optimal stent deployment as assessed by optical coherence tomography. Catheter Cardiovasc Interv. 83: 521–7.
- Vallurupalli S, Kasula S, Agarwal SK, Pothineni NVK, Abualsuod A, et al. (2017) A novel stent inflation protocol improves long-term outcomes compared with rapid inflation/deflation deployment method. Catheter Cardiovasc Interv. 90: 233–40
- Iwamoto Y, Okamoto M, Hashimoto M, Fukuda Y, Iwamoto A, et al. (2012) Better stent expansion by two-time inflation of stent balloon and its responsible mechanism. J Cardiol. 59: 160–6.

Tables at a glance
Figures at a glance