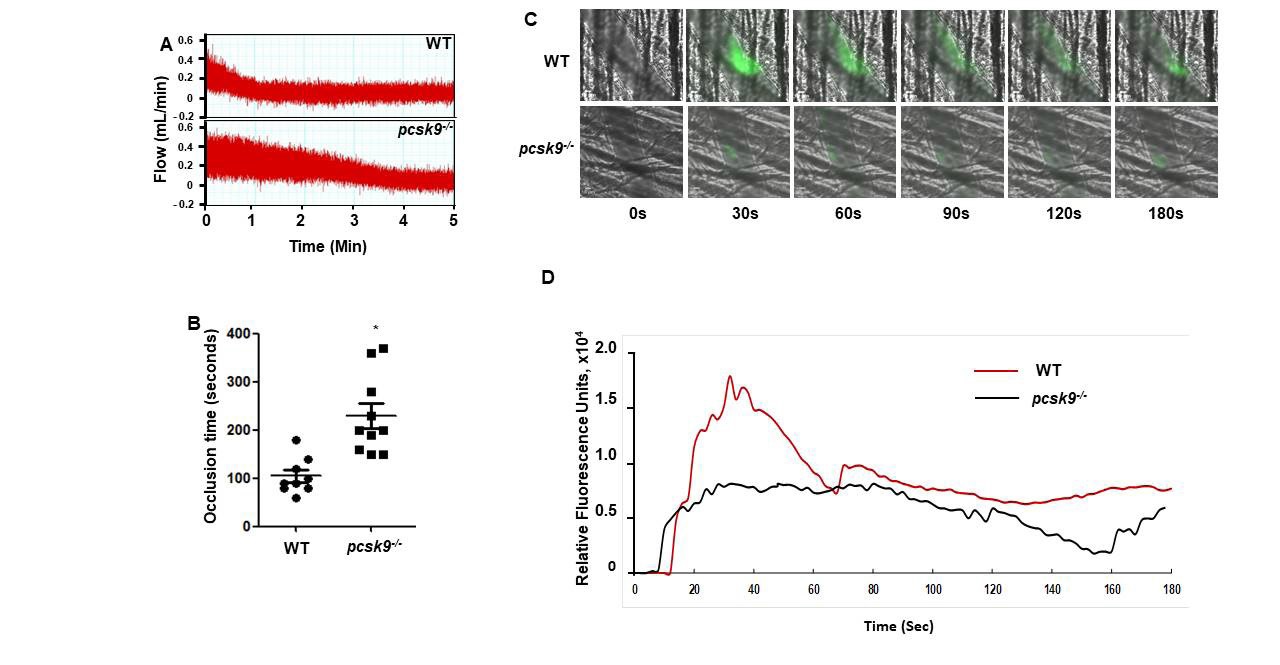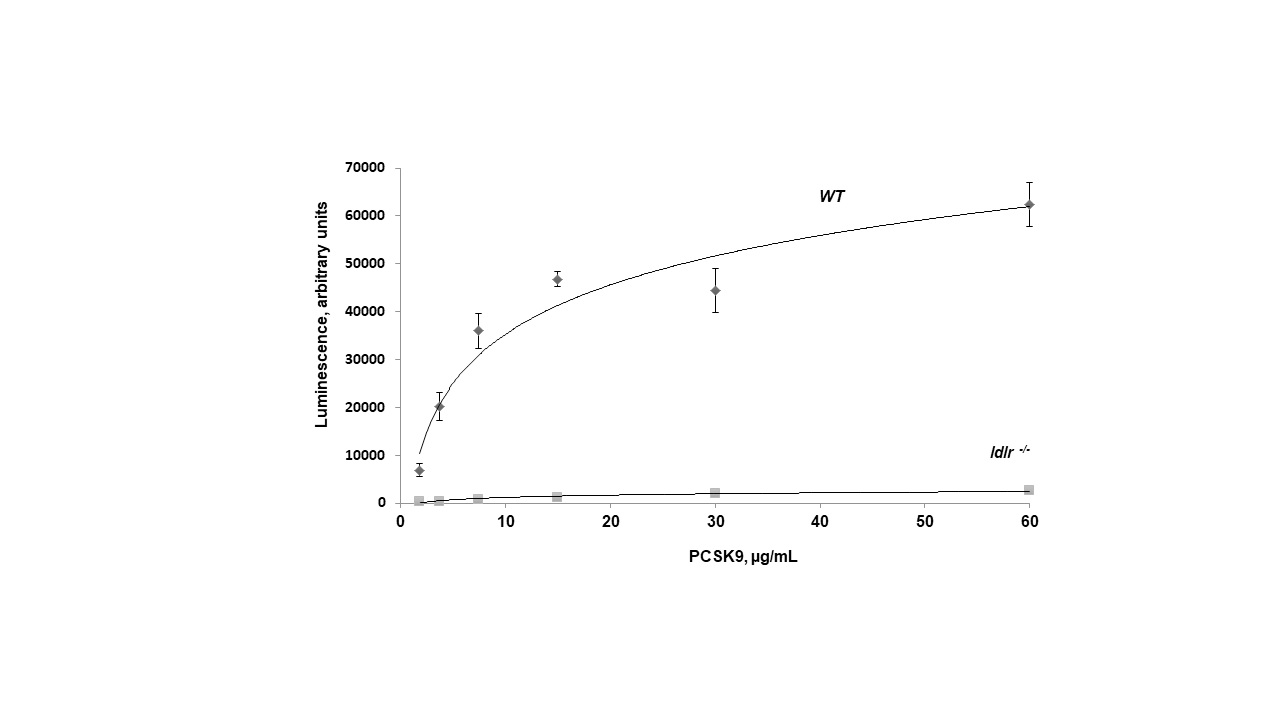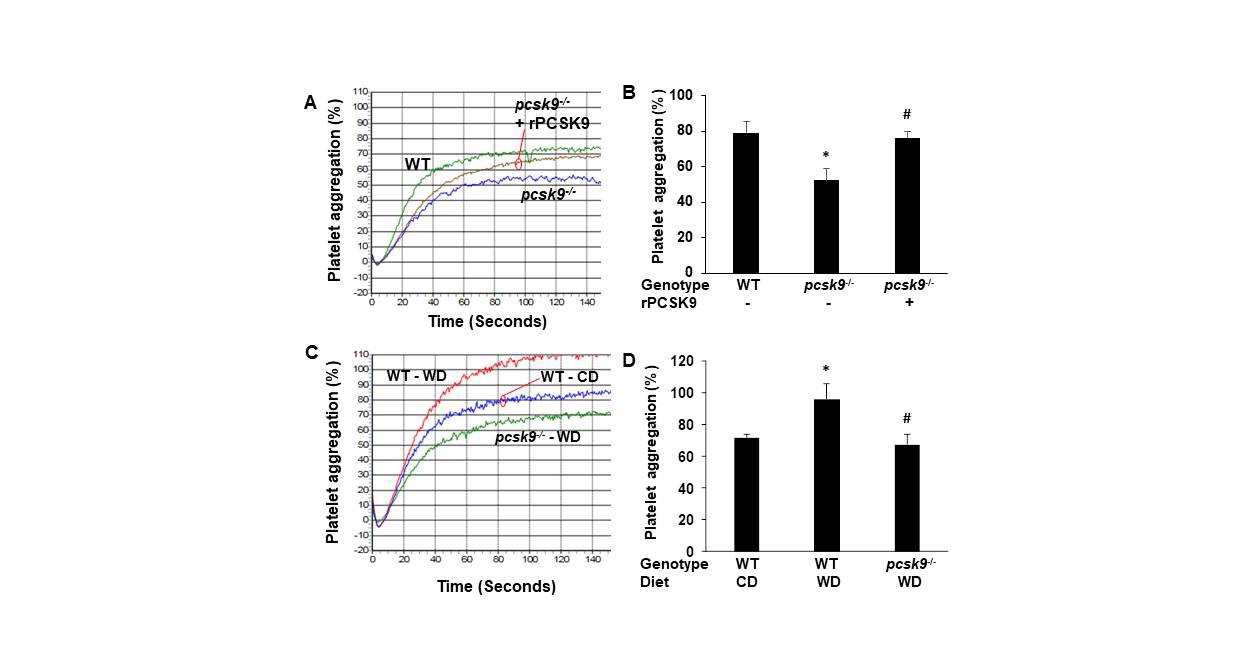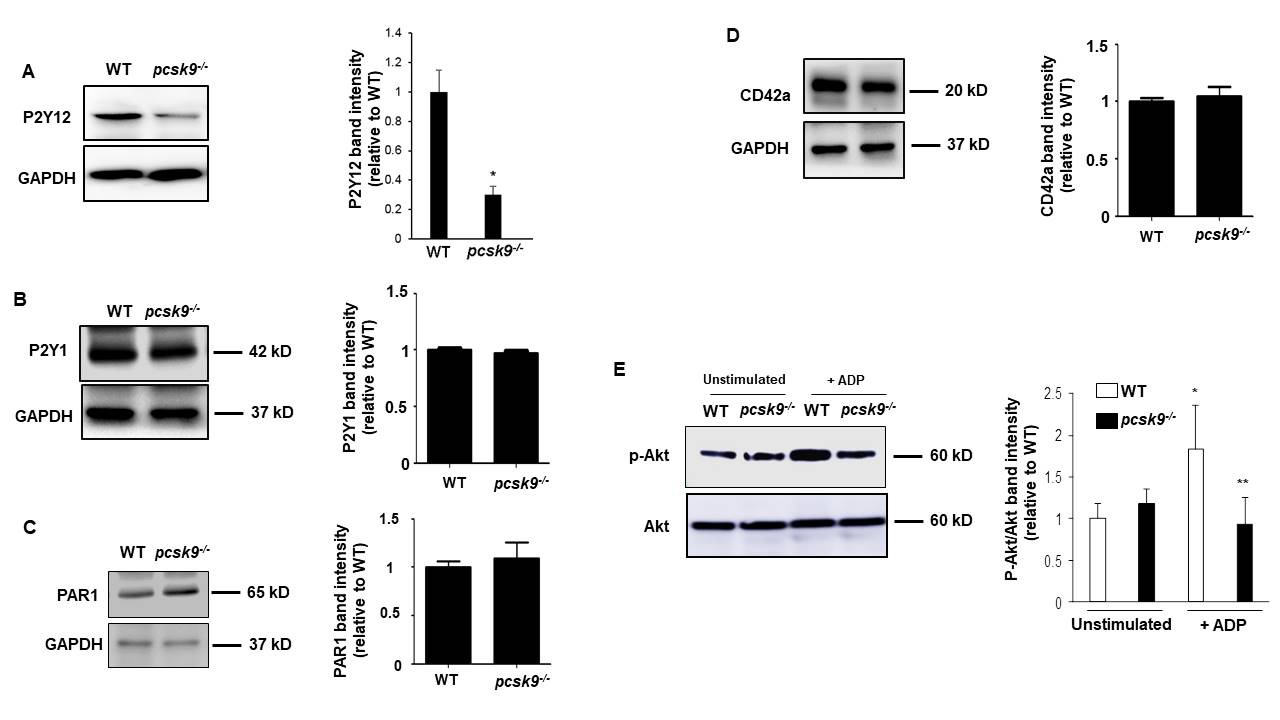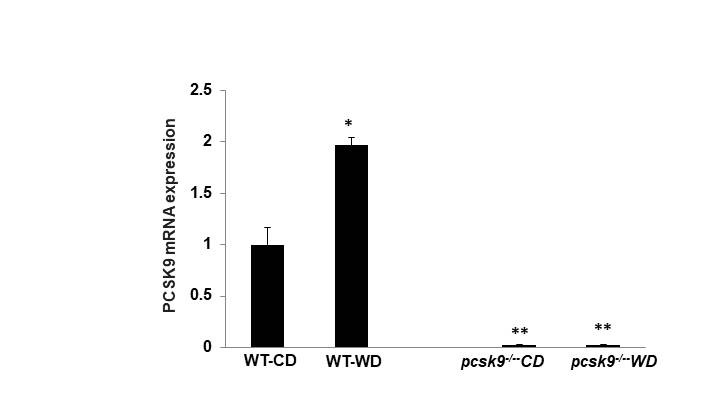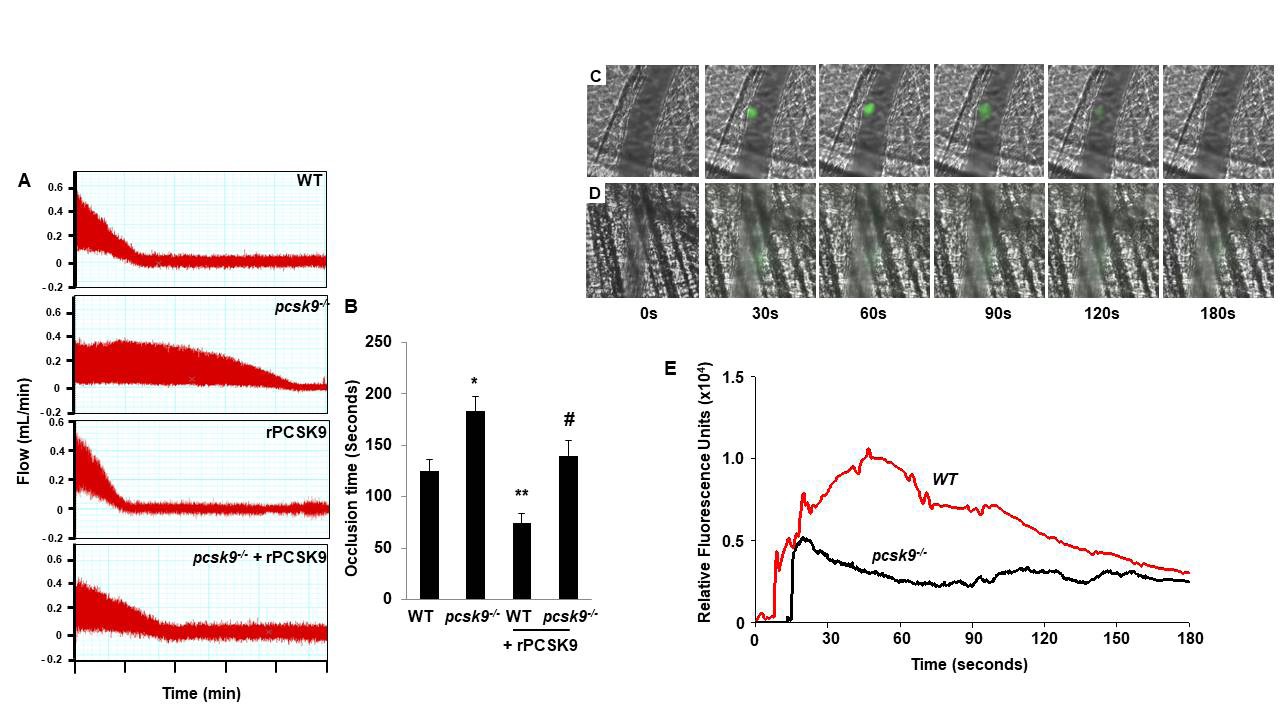
PCSK9 regulates thrombus formation in mice fed normal chow. (A) Recombinant PCSK9 (30 μg) or vehicle control was administered to WT mice via the tail vein 5 min before injuring carotid arteries with topical FeCl3. Carotid blood flow tracings are shown. (B) Mean carotid artery occlusion times after FeCl3 injury (n=11/group); *P<0.05 vs vehicle control group; **P>0.05 vs vehicle control group; #P<0.05 vs vehicle control group. WT, n=5; pcsk9-/-, n=13; pcsk9-/- + rPCSK9, n=7. The data were shown as mean ± SEM. *P<0.05 vs WT; #P<0.05 vs pcsk9-/- mice that did not receive rPCSK9. WT, wild-type. (C) Time-lapse images showing formation of platelet thrombus (green) in a cremasteric arteriole of a WT mouse after laser injury. Alexa Fluor 488-conjugated anti-CD41 antibody was used to label platelets. Scale bar (shown in 0s images) = 10 μm. (D) Corresponding images of a laser-injured cremasteric arteriole of a pcsk9-/- mouse. (E) Median integrated platelet fluorescence intensity (thrombus formation) in cremasteric arterioles after laser injury (n=3 mice/group). s, seconds; WT, wild-type.