
Figure 1: Lightfective ReBorn Non-Invasive Fat Reduction System
|
N=13 |
|
|
Female |
Male |
Gender, n (%) |
10 (76.9%) |
3 (23.07%) |
Age, years (study mean) |
45.08 |
|
Age |
42.6 |
53.3 |
BMI (study mean) |
21.45 |
|
BMI mean |
20.96 |
23.11 |
Skin type (Fitzpatrick scale), n (%) |
|
|
Type 2 |
3 (30%) |
0 (0%) |
Type 3 |
7 (70%) |
(100%) |
Smoker |
2 (20%) |
1 (33%) |
Previous treatments |
|
|
ReBorn |
5 (50%) |
2 (66%) |
Other |
6 (60%) |
2(66%) |
Table 1: Subject demographics
|
Position of applicator |
||||
|
|
AP 1 |
AP 2 |
AP 3 |
AP 4 |
Patient 1 |
Anterior |
8 |
9 |
12 |
13 |
Patient 2 |
Anterior |
6 |
7 |
X |
X |
Patient 3 |
Posterior |
1 |
2 |
3 |
4 |
Patient 4 |
Anterior |
3 |
4 |
X |
X |
Patient 6 |
Anterior |
2 |
3 |
4 |
5 |
Patient 7 |
Anterior |
3 |
4 |
X |
X |
Patient 8 |
Anterior |
3 |
4 |
X |
X |
Patient 9 |
Anterior |
10 |
11 |
X |
X |
Patient 10 |
Anterior |
1 |
3 |
4 |
X |
Patient 11 |
Anterior |
10 |
14 |
11 |
15 |
Patient 12 |
Anterior |
8 |
12 |
13 |
X |
Patient 13 |
Anterior |
2 |
3 |
4 |
5 |
Table 2: Placement of applicators per participant
X indicates no placement of an applicator
|
Initial and final energy levels per each applicator over 10 minutes in Comfort Mode |
|||
|
Applicator 1 |
Applicator 2 |
Applicator 3 |
Applicator 4 |
Patient 1 |
65%-90% |
65%-90% |
65%-90% |
65%-90% |
Patient 2 |
70%-90% |
70%-90% |
X |
X |
Patient 3 |
70%-80% |
70%-80% |
70%-90% |
70%-90% |
Patient 4 |
70%-95% |
70%-95% |
X |
X |
Patient 6 |
70%-90% |
70%-90% |
70%-100% |
70%-100% |
Patient 7 |
65%-90% |
65%-90% |
X |
X |
Patient 8 |
70%-90% |
70%-90% |
X |
X |
Patient 9 |
65%-90% |
65%-90% |
X |
X |
Patient 10 |
65%-80% |
65%-80% |
65%-80% |
X |
Patient 11 |
65%-80% |
65%-80% |
65%-80% |
65%-80% |
Patient 12 |
65%-85% |
65%-85% |
65%-85% |
X |
Patient 13 |
65%-80% |
65%-80% |
65%-80% |
65%-80% |
Table 3: Comfort Mode setting per participant. Comfort mode allows for the desired time-duration for automatic energy raise, as well as initial and final energy levels per each applicator
X indicates no placement of an applicator
|
AP1 + delay |
AP2 + delay |
AP3 + delay |
AP4 + delay |
||||
Patient 1 |
42°C |
100% |
40.9°C |
100% |
42.2°C |
90% |
43.9°C |
90% |
Patient 2 |
48.9°C |
90% |
52°C |
100% |
|
|
|
|
Patient 3 |
43.8°C |
80% |
46°C |
80% |
45.9°C |
90% |
44°C |
90% |
Patient 4 |
42.9°C |
95% |
43.5°C |
100% |
|
|
|
|
Patient 6 |
47.5°C |
90% |
43.1°C |
90% |
46.3°C |
100% |
46.3°C |
100% |
Patient 7 |
42°C |
90% |
41.9°C |
100% |
|
|
|
|
Patient 8 |
44°C |
90% |
48.5°C |
100% |
|
|
|
|
Patient 9 |
42.5°C |
90% |
43.9°C |
100% |
|
|
|
|
Patient 10 |
* |
* |
43.3°C |
90% |
47.6°C |
90% |
|
|
Patient 11 |
45.8°C |
80% |
42.2°C |
80% |
47.7°C |
90% |
43.7°C |
90% |
Patient 12 |
45.7°C |
95% |
40.8°C |
85% |
40.9°C |
85% |
|
|
Patient 13 |
39°C |
80% |
44°C |
80% |
43.3°C |
85% |
40.9°C |
85% |
Table 4: Adjusted adipose tissue temperature (oC) vs percentage LED power output energy after 35-minute treatment period per participant
unsafe area for hypodermic needle microprobe insertion

Figure 1: Lightfective ReBorn Non-Invasive Fat Reduction System
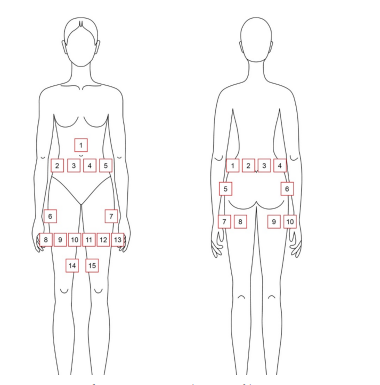
Figure 2: Applicator positioning: a) anterior; b) posterior
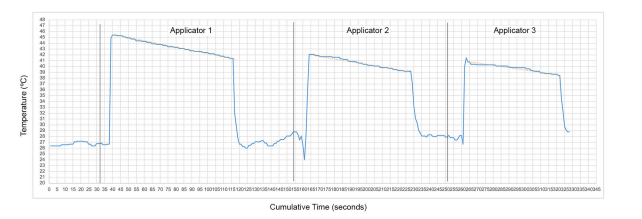
Figure 3: Example of temperature decrease from time of applicator removal to time of final temperature measurement in the treatment areas on a single patient (Patient 12)
Tables at a glance
Figures at a glance