Vitamin D Supplementation in Type I Obese Patients in Mexico
Received Date: April 19, 2024 Accepted Date: May 19, 2024 Published Date: May 22, 2024
doi: 10.17303/jfn.2024.10.102
Citation: Francisco J. Nachon Garcia, Gabriela E. Saldaña-Davila (2024) Vitamin D Supplementation in Type I Obese Patients in Mexico. J Food Nutr 10: 1-10
Abstract
Introduction: Vitamin D, primarily recognized for its skeletal functions, extends its influence beyond bone health, impacting various physiological systems. Serum vitamin D levels below optimal thresholds have been associated with an array of disorders, including cardiovascular diseases, diabetes, and mental health conditions. Moreover, obesity exacerbates vitamin D deficiency, with proposed mechanisms including volumetric dilution, sequestration in adipose tissue, and compromised hepatic metabolism. In Mexico, studies demonstrate high prevalence rates of vitamin D deficiency among obese individuals, further compounded by insulin resistance. Supplementation guidelines vary, with recommendations ranging from 4000 IU/- day to 10,000 IU/day.
Materials and Methods: To address this complex interplay, a longitudinal study was conducted in Mexico City, involving type I obese patients.
Results: Indicated a negative correlation between body mass index (BMI) and vitamin D levels. Supplementation with 4000 IU of vitamin D3 for 90 days significantly increased serum levels, particularly among initially deficient and insufficient participants. A substantial proportion of patients achieved normalization, emphasizing the efficacy of this dosis and this presentation.
Conclusion: These findings underscore the intricate relationship between obesity, vitamin D status, and metabolic health, advocating for early interventions to mitigate deficiencies and associated comorbidities in patients with obesity.
Keywords: Vitamin D Deficiency; Obesity; 25-Hydroxycholecalciferol; Vitamin D Supplementation; Body Mass Index (BMI)
Introduction
The 1,25-dihydroxyvitamin D (VitD), resulting from endogenous synthesis of vitamin D, is widely regarded more as a hormone than a vitamin, especially considering its skeletal functions such as calcium stimulation and absorption, osteoclastic bone resorption, osteoblastic function, and reduction in parathyroid hormone (PTH) secretion. However, its functions beyond the skeletal structure, such as decreasing type 1 collagen production, increasing muscle function, stimulating cell differentiation, participating in insulin secretion, and involvement in the immune system, confirm its hormone status [1].
Numerous disorders unrelated to calcium metabolism, such as cardiovascular diseases, hypertension, dyslipidemia, type 2 diabetes, cancer, multiple sclerosis, depression, dementia, psychiatric diseases, among others, have been associated with serum levels of VitD below 50 nmol/L or 20 ng/mL [2].
As is known, obesity, defined as excess fat, is associated with lower vitamin D levels. Data from large observational studies (NHANES III and Framingham) suggest that obesity is associated with a higher risk of hypovitaminosis D [3,4].
There are basically three theories that could explain the inverse relationship between increased body fat and low plasma concentrations of VitD. Volumetric dilution of VitD is the most likely mechanism of the inverse relationship between serum vitamin D levels and BMI [5-7]. On the other hand, the hypothesis of VitD sequestration in adipose tissue suggests lower increases in plasma levels in obese individuals than in normal-weight individuals after sun exposure [8]. Another possible mechanism for decreased plasma VitD is compromised hepatic 25-hydroxylation associated with non-alcoholic fatty liver disease, a very common condition in obese patients [9].
In Mexico, an interesting study demonstrates the association of vitamin D deficiency, obesity, and insulin resistance. Official figures from the National Institute of Public Health report 70% of patients with normal levels, 20% with deficiency, and only 9.8% with insufficiency [11]. These data do not coincide with more recent studies where 61% of pregnant women and 98% of newborns have levels below 29.9 ng/dL, as reported by the National Institute of Perinatology [12]. This study also found pregestational obesity in 34.8% of the patients studied, with 51.4% presenting insufficiency and 33.7% deficiency of vitamin D [13]. Similarly, another study in Mexico with individuals over 50 years old found 31.54% insufficient and 47.47% deficient [14]. It has been difficult to establish optimal levels of vitamin D in the blood. Finally, several authors agree to define vitamin D deficiency as 25(OH)D < 20 ng/mL, insufficiency at concentrations between 21 and 29 ng/mL, and sufficiency at > 30 ng/mL, as it is from levels of 30-40 ng/mL of 25(OH)D when parathyroid hormone (PTH) begins to decrease [15-18].
In response to the need for supplementation, the European Food Safety Authority recommends administering 4000 IU/day or 100 μg [19,20], while the Endocrine Society establishes a maximum daily limit of 10,000 IU [21]. More recently, after a systematic review involving 16,515 patients, it is proposed that in obese patients, supplementation with 2000 IU daily for 2 years achieves an improvement in plasma levels of 25(OH)D and reduces diseases associated with its deficiency or insufficiency [22]. This establishes that there is no optimal dose for supplementation or a set time to do so.
Considering the aforementioned points, our decision was to initially ascertain the prevalence of deficiency, insufficiency, and sufficiency in a cohort of type I obese patients residing in Mexico City. Subsequently, we aimed to investigate the existence of a negative correlation between BMI and vitamin D levels, along with exploring the correlation between body fat percentage and vitamin D status. Finally, we sought to determine whether the resolution of deficiency and insufficiency could be attained within a short timeframe through the administration of 4000 IU daily for 3 months. This supplementation method was chosen to enhance absorption, delivered in the form of drops within an oily vehicle containing Oleic, Linoleic, and Palmitic acids (Olive Oil) [1].
Materials and Methods
We conducted a prospective, longitudinal study involving Mexican patients diagnosed with grade I obesity. Initially, we determined baseline levels of 25(OH)D, classifying patients as insufficient, deficient, or normal based on these results. Anthropometric measurements including weight, height, and BMI calculation were performed for all participants, along with multifrequency bioelectrical impedance analysis using the In-Body 570 body composition analyzer.
Participants
Our study included both male and female patients aged between 18 and 60 years, with a body mass index (BMI) falling within the range of 30 to 35 kg/m^2. Recruitment of participants took place within the metropolitan area of Mexico City, facilitated through open invitations posted on Zélé® social media platforms. Exclusion criteria encompassed individuals who had undergone previous weight loss treatments, consumed vitamin D or calcium supplements, used glucocorticoids, testosterone, hormonal replacement therapy, or anabolic steroids within the preceding 6 months. Additionally, pregnant or lactating individuals, those with severe eating disorders, alcoholism, or drug addiction, as well as those with severe psychiatric disorders (e.g., schizophrenia, bipolar disorder) were excluded. Liver insufficiency, defined by ALT, AST, GGT levels exceeding four times the reference value, along with the presence of endocrine, metabolic, cardiovascular, or parathyroid diseases, absorption disorders, kidney diseases, or any other pre-existing conditions known to affect vitamin D metabolism, also served as exclusion criteria.
Sample Size
The sample size was determined arbitrarily based on resource availability. The first 100 patients who met the inclusion criteria were invited to an informational meeting, where the study purpose was explained, and their acceptance was requested through signing an informed consent form. (This study was approved by the Research Committee of the Health Sciences Institute at Universidad Veracruzana and audited by the Health Sciences Research Center at Universidad Anáhuac Norte. Ethical aspects were evaluated by the Research Ethics Committee of the High Specialty Center of the State of Veracruz CONBIOÉTICA-30-- CEI-001-20170221).
Intervention
Each patient was instructed to take a daily dose of 4,000 IU of vitamin D3 in an oily vehicle (D3 400 Zélé®). This equated to 10 drops in the morning after the first meal of the day, which could be taken sublingually or dissolved in their preferred liquid.
Anthropometric and Metabolic Data
Anthropometric measurements were preferably taken at the same time and under the same conditions at each follow-up visit. Height was measured at the beginning of the study using a Seica wall-mounted stadiometer with an accuracy of 0.1 cm. Weight and body composition were recorded without shoes and with lightweight clothing using an In-Body 570 body composition analyzer with an accuracy of 0.1 kg.
Fasting blood samples were collected in the medical laboratory through venipuncture by trained phlebotomists.
Follow-Up
Interviews, clinical reviews, anthropometric measurements, and multifrequency bioelectrical impedance analysis (MFBIA) were conducted every 30 days, and patients were questioned for adverse reactions. Finally, at the 90-day mark of treatment, in addition to clinical review, interview, and anthropometric measurements, final 25(OH)D levels were determined.
Statistical Analysis
Data were recorded in an Excel database. Descriptive statistics tables were generated for baseline demographic data, presenting means with measures of dispersion for continuous variables and percentages for categorical variables. Pearson's correlation coefficient was calculated between 25(OH)D levels and BMI, as well as against body fat percentage using Pearson's linear regression test. Comparative tests of repeated measures were conducted on initial and final VitD determinations using non-parametric tests (Kruskal-Wallis) and chi-square tests. SPSS V21.0 software was used for analysis.
Results
Between January and March 2022, 70 patients were enrolled, with only 56 (80%)—comprising 45 women and 11 men—completing the protocol. Women had an average age of 39.56 ± 10.7 years, while men had an average age of 35.73 ± 7.5 years. Their respective weights were 83.3 ± 7.8 kg and 97.59 ± 5.7 kg, resulting in BMIs of 32.7 ± 2.06 and 32.4 ± 1.47 kg/m^2 (Table 1).
Notably, the body fat percentage was significantly higher in women compared to men: 44.8 ± 4% in women versus 34.4 ± 4% in men (t = 7.8, df 53, p < 0.0001).
Based on the blood measurements of 25(OH)D, patients were categorized as follows: 28 patients (24.6%) had insufficient levels, with a mean level of 15.8 ± 2.6 ng/ml (95% CI 14.75 to 16.79 ng/ml); 22 patients (19.3%) were deficient, with a mean value of 23.7 ± 1.98 ng/ml (95% CI 22.8 to 23.6 ng/ml); and finally, 6 patients (5.3%) reported normal or sufficient levels, with a mean value of 39.3 ± 5.5 ng/ml (95% CI 33.4 to 45.1 ng/ml).
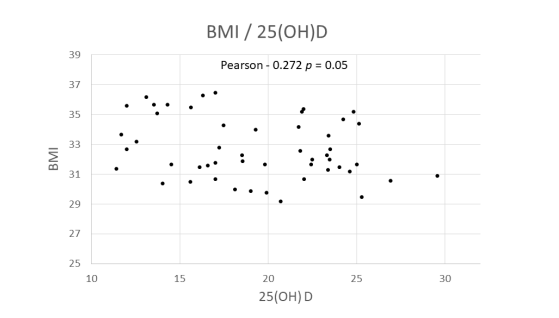
Correlation coefficients were calculated using Pearson's linear regression with the obtained data, revealing a negative correlation between BMI and 25(OH)D levels (Correlation -0.272, p = 0.05). (see correlation graph) Similar tests conducted with body fat percentage divided by sex and fat mass in kilograms did not yield significant results.
No adverse effects or intolerance to the administration of VitD in this oily vehicle were reported. Patients were specifically questioned about weakness, dry mouth, nausea, and vomiting.
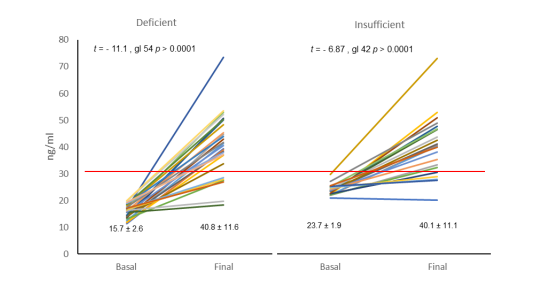
Following the administration of 4000 IU of Vitamin D3 at the end of winter and during spring in Mexico City, 24.6% of the initially insufficient sample experienced an increase in the mean value of 25(OH)D levels from 15.8 ± 2.6 to 40.8 ± 11.5 ng/ml (95% CI from 36.4 to 45.3 ng/ml). After conducting the generalized linear model of repeated measures, highly significant differences were observed both within subjects (f = 136.1, df 1, p < 0.0001) and between subjects (f = 587.2, df 1, p < 0.0001). Additionally, 19.3% of the deficient patient group saw an increase from 23.7 ± 1.98 to 40.1 ± 11.2 (95% CI from 35.1 to 45.0 ng/ml), with significant differences within subjects (f = 56.0, df 1, p < 0.0001) and between subjects (f = 590.4, df 1, p < 0.0001). (see Mixed linear graph panels A and B) respectively.
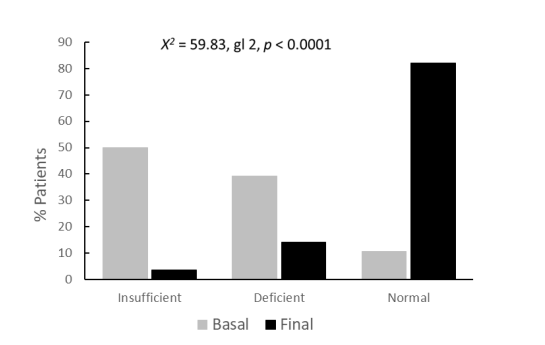
Lastly, upon analyzing the final condition in relation to the initial status, only 2 out of the 28 patients initially classified as insufficient remained in this category, while 4 remained deficient, and 22 normalized. Similarly, among the 22 patients initially classified as deficient, 4 remained deficient, and 18 migrated to the normal condition. This analysis reveals that out of the 56 patients studied, where 28 had insufficient levels of 25(OH)D, 22 had deficient levels, and only 6 had normal levels, after receiving 4,000 IU of VitD3 in an oily medium for 90 days, 2 remained insufficient, 8 remained deficient, and 46 reached normal levels. (χ2 = 59.8, df 2, p < 0.0001) Percentage normalization graph.
Discussion
This study delves into various aspects related to Vitamin D3. Initially, a random sample of the Mexican population with grade I obesity was examined, and their baseline levels of 25(OH)D were assessed. The prevalence rates of insufficiency, deficiency, and normalcy align closely with findings from previous studies conducted in Mexico [12-14,18], except for the study by Flores et al. [11]. Moreover, the presence of obesity in the study group accentuates the deficiency of 25(OH)D, consistent with the observations of other studies [3,4].
Another aspect explored in this study was elucidating the vitamin deficiency observed in the context of obesity. In line with previous research by Lorenzo et al. [23], we confirm the inverse correlation between BMI and 25(OH)D deficiency. Attempts were made to investigate correlations between fat percentage and fat quantity in kilograms; however, no significant correlations were found. Given the variations in body composition and fat distribution between genders, separate analyses were conducted for males and females, yielding nonsignificant results.
Another consideration was the use of olive oil as the administration vehicle. Although there is no definitive conclusion regarding the optimal vehicle for enhancing the absorption of vitamin D3, studies have shown that oil-soluble vehicles result in the most significant changes in serum 25(OH)D3 levels, particularly following the administration of 10,000 IU. Given the liposolubility of 25-Hydroxycholecalciferol, its intestinal absorption is facilitated by micelle formation and is absorbed alongside other lipids through passive diffusion in enterocytes. The efficiency of absorption is contingent upon the presence of fats in the intestinal lumen, which triggers the release of bile acids and pancreatic lipase.
Regarding the daily supplemented dose, we opted for the therapeutic replacement dose of 4,000 IU per day, as recommended by the European Food Safety Authority. Although the proposed consensus dose for daily supplementation is 50 μg, equivalent to 2000 IU, the Endocrine Society sets a maximum daily limit of 10,000 IU in cases of supplementation need. Long-term oral supplementation studies in obese patients with 2,000 IU daily for 2 years of Vit D3 have shown restoration of normal 25(OH)D levels. This study demonstrates that a daily dose of 4,000 IU for 3 months is effective in restoring sufficient levels of VitD. The transition from 50% of patients with insufficiency, 39.3% with deficiency, and 10.7% with normal levels to 3.6% with insufficiency, 14.3% with deficiency, and 82.1% with normal values was highly significant (χ2= 59.8, df 2, p < 0.0001).
In this study, as in many others, no adverse effects have been reported for vitamin D administered in doses up to 4,000 IU [28-30]
It is acknowledged that this study involved a limited number of patients, and to strengthen the findings, a larger and more inclusive sample encompassing patients with obesity across all grades is warranted. Additionally, further studies are needed to establish the optimal therapeutic dose, including testing doses from 800 IU to 4,000 IU in patients with insufficiency and deficiency, as well as determining the optimal supplementation dose for normal patients. Trials should also modify the duration of vitamin D3 administration dissolved in this vehicle. Finally, it is necessary to compare the administration of this oily vehicle against other presentations of 25(OH)D supplements.
Conclusion
89.3% of the studied population exhibited subnormal levels of 25(OH)D. Supplementation with 4,000 IU of Vitamin D3 daily in an oily vehicle for 90 days proved effective in resolving insufficiency and deficiency in 78.6% of patients with insufficiency and 81.8% of patients with deficient levels.
Disclosure
The authors declared no conflict of interest
- Vranić L, Mikolašević I, Milić S (2019) Vitamin D deficiency: Consequence or cause of obesity? Vol. 55, Medicina (Lithuania). MDPI AG.
- Milic S, Mikolasevic I, Krznaric-Zrnic I, Stanic M, Poropat G, Stimac D, et al. (2015) Nonalcoholic steatohepatitis: Emerging targeted therapies to optimize treatment options. Drug Design, Development and Therapy. Dove Medical Press Ltd. 9: 4835-45.
- Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. (2010) Adiposity, cardiometabolic risk, and vitamin D status: The framingham heart study. Diabetes. 59: 242-8.
- Yetley EA (2008) Assessing the vitamin D status of the US population. Am J Clin Nutr. 88: 558S-64S.
- Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, et al. (2007) The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency 13.
- Gallagher JC, Yalamanchili V, Smith LM (2013) The effect of vitamin D supplementation on serum 25OHD in thin and obese women, Journal of Steroid Biochemistry and Molecular Biology. 136: 195-200.
- Drincic A, Fuller E, Heaney RP, Armas LAG (2013) 25-Hydroxyvitamin D response to graded vitamin D3 supplementation among obese adults. Journal of Clinical Endocrinology and Metabolism. 98: 4845-51.
- Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 72: 690-3.
- Targher G, Bertolini L, Scala L, Cigolini M, Zenari L, Falezza G, et al. (2007) Associations between serum 25-hydroxyvitamin D3 concentrations and liver histology in patients with non-alcoholic fatty liver disease. Nutrition, Metabolism and Cardiovascular Diseases. 17: 517-24.
- Loya-López GM, Godínez-Gutiérrez SA, Chiquete E, Valerdi-Contreras L, Taylor-Sánchez V (2011) Vitamin D levels in overweight and obese patients and their association with insulin resistance. Journal of Endocrinology and Nutrition. 19: 140-5.
- Flores M, María L, Romero S, Macías N, Lozada A, Díaz E, et al. (2006) Serum vitamin D concentrations in Mexican children, adolescents and adults. ENSANUT results, 2006.
- Ochoa-Correa E del C, García-Hernández PA, Villarreal-Pérez JZ, Treviño-Garza C, Rodríguez-Balderrama I, Martínez-de Villarreal LE, et al. (2017) Vitamin D deficiency in Mexican mothers and neonates. Gac Med Mex. 153: 559-65.
- Perera OP, González-Leyva CP, González-Ludlow I, Tolentino-Dolores M, Solis-Paredes M, Reyes-Muñoz E, et al. (2020) Vitamin D deficiency in mexican pregnant women: Is supplementation with ≤400 iu/day enough? Nutrients. 12: 1-11.
- Díaz de León González E, Gutiérrez Hermosillo H, Morales Torres J (2023) Serum vitamin D levels and mortality in Mexicans: results from the Mexican Health and Aging Study. Nutr Hosp. 40: 732-8.
- Bloomgarden ZT (2011) The American Diabetes Association’s 57th Annual Advanced Postgraduate Course: Diabetes risk, vitamin D, polycystic ovary syndrome, and obstructive sleep apnea. In: Diabetes Care.
- Calatayud M, Jódar E, Sánchez R, Guadalix S, Hawkins F (2009) Prevalence of deficient and insufficient vitamin d levels in a young healthy population. Endocrinologia y Nutricion. 56: 164-9.
- Torres del Pliego E. Nogues Solan X (2014) How to use vitamin D, and what supplementary dose would be the optimum to achieve the best balance between efficacy and security?.
- Loya-López GM, Godínez-Gutiérrez SA, Chiquitete E, Valerdi-Contreras L, Taylor-Sánches V (2011) Vitamin D levels in overweight and obese patients and their association with insulin resistance. Journal of Endocrinology and Nutrition, 19: 140-5.
- Ross AC, Manson JAE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. Vol. 96, Journal of Clinical Endocrinology and Metabolism. Endocrine Society; 53-8
- EFSA Panel on Dietetic ProductsEuropean Food Safety Authority (2011) Scientific Opinion on the Tolerable Upper Intake Level of vitamin D. EFSA Journal. 10.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. (2011) Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. Vol. 96, Journal of Clinical Endocrinology and Metabolism. 1911-30.
- Tobias DK, Luttmann-Gibson H, Mora S, Danik J, Bubes V, Copeland T, et al. (2023) Association of Body Weight With Response to Vitamin D Supplementation and Metabolism. JAMA Netw Open. 6: e2250681.
- Lorenzo J, Boente R, Sas Fojón M (2012) Vitamin D deficiency and obesity. Vol. 59, Endocrinology and Nutrition. 401-2.
- Grossmann RE, Tangpricha V (2010) Evaluation of vehicle substances on vitamin D bioavailability: A systematic review. Vol. 54, Molecular Nutrition and Food Research. 1055-61.
- Bolland MJ, Grey A, Gamble GD, Reid IR (2014) The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: A trial sequential meta-analysis. Lancet Diabetes Endocrinol. 2: 307-20.
- Valero-Zanuy MA, Hawkins Carranza F (2007) Review Metabolism, Endogenous and Exogenous Sources of Vitamin D Introduction 16.
- Vieth R (2006) What is the optimal vitamin D status for health? Vol. 92, Progress in Biophysics and Molecular Biology. 26-32.
- Pérez-Castrillón JL, Dueñas-Laita A, Gómez-Alonso C, Jódar E, del Pino-Montes J, Brandi ML, et al. (2023) LongTerm Treatment and Effect of Discontinuation of Calcifediol in Postmenopausal Women with Vitamin D Deficiency: A Randomized Trial. Journal of Bone and Mineral Research. 38: 471-9.
- Chevalley T, Brandi ML, Cashman KD, Cavalier E, Harvey NC, Maggi S, et al. (2022) Role of vitamin D supplementation in the management of musculoskeletal diseases: update from an European Society of Clinical and Economical Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) working group, Aging Clinical and Experimental Research. Springer Science and Business Media Deutschland GmbH, 34: 2603-23.
- Rizzoli R (2021) Vitamin D supplementation: upper limit for safety revisited? Aging Clinical and Experimental Research. Springer Science and Business Media Deutschland GmbH; 33: 19-24.

Tables at a glance
Figures at a glance