Glycemic Control with 5% Weight Loss using the Zélé Method (Very Low-Calorie Low-Fat Ketogenic Diet)
Received Date: May 01, 2024 Accepted Date: June 01, 2024 Published Date: June 04, 2024
doi: 10.17303/jfn.2024.10.104
Citation: Gabriela E. Saldaña-Davila,Francisco J. Nachón-García (2024) Glycemic Control with 5% Weight Loss using the Zélé Method (Very Low-Calorie Low-Fat Ketogenic Diet). J Food Nutr 10: 1-11
Abstract
Background: There is ongoing debate regarding the optimal nutritional intervention for glycemic regulation in patients with obesity and prediabetes. Classic ketogenic diets have been proposed for weight control and stabilizing plasma glucose levels. This study introduces a novel approach: the Very Low-Calorie Very Low Fat Ketogenic Diet (VLCLFKD).
Material and Methods: In a controlled clinical trial, we compared the effects of VLCLFKD and a hypocaloric diet on weight reduction and fasting plasma glucose levels.
Results: VLCLFKD achieved a 5% weight loss in half the time compared to the hypocaloric diet (p < 0.001) and resulted in a total weight reduction of 12.39 ± 2.8 kg versus 6.95 ± 1.9 kg (p < 0.001), respectively. Additionally, there was a significant modification in fasting plasma glucose levels from 94.3 ± 22.9 mg/dl to 82.3 ± 10.1 mg/dl (p = 0.009).
Conclusion: VLCLFKD leads to greater weight loss and significant improvement in fasting plasma glucose levels compared to the hypocaloric diet.
Keywords: Ketogenic Diets; Obesity; Glucose Metabolism Disorders
Introduction
Obesity represents a major risk factor for glucose metabolism disorders [1], with 80-90% of type 2 diabetes patients being overweight or obese [1]. Globally, type 2 diabetes affects approximately 537 million adults, accounting for 10.5% of the population aged 20 to 79 years [2]. In Mexico, the prevalence of diabetes is alarmingly high, with a reported rate of 18.3%(3). Prediabetes, characterized by glycemic dysfunction preceding type 2 diabetes onset, affects 38% of adults in the United States [4] and 22.1% in Mexico [3].
Obesity stands out as the primary modifiable risk factor for prediabetes and type 2 diabetes. A weight loss of 5 to 10% through reduced energy intake is essential for managing obesity and preventing prediabetes and type 2 diabetes [4,5]. Therefore, addressing obesity and glycemic disorders requires a multifaceted approach, encompassing lifestyle modifications, dietary changes, increased physical activity, pharmacotherapy, and surgical interventions [4].
The optimal nutritional intervention for glycemic regulation in obese, prediabetic, or type 2 diabetic patients remains a topic of debate [4]. Both hypocaloric diets and classic ketogenic diets have demonstrated efficacy in glycemic control [5]. Ketogenic diets have been shown to help maintain normal glucose levels and reduce blood glucose peaks in individuals with type 2 diabetes, potentially decreasing the need for insulin [6]. Notably, a 5% reduction in total body weight has been associated with improved glycemic control and reduced reliance on diabetes medications, enhancing overall quality of life [7]. Systematic reviews have further highlighted the significant weight loss, glucose level reduction, and decreased cardiovascular risk factors associated with Very Low-Calorie Ketogenic Diets (VLKDs) [8].
Numerous descriptions of ketogenic diets and low-fat diets exist. The Zélé method integrates nutritional intervention, featuring 1.2 grams of protein per kilogram of ideal weight, very low-calorie content (800 kcal), and 20 grams of fat per day, constituting a ketogenic, normoproteic, low-fat diet (VLCLFKD). However, evidence comparing VLCLFKD to hypocaloric diets in glycemic control is lacking. Thus, we conducted a double-blind, randomized prospective study to compare the effects of a hypocaloric diet (LCD) with a controlled low-fat ketogenic diet. Our study aimed to evaluate the impact of VLCLFKD compared to a hypocaloric diet on fasting plasma glucose levels at 5% and 10% weight loss thresholds.
Materials and Methods
This segment of the Zélé 2021 study, registered as a prospective, longitudinal, randomized, double-blind clinical trial under the code NCT06275347, focused on the comparative analysis of glycemic behavior in Mexican patients with grade I obesity. Two metabolically distinct nutritional interventions were compared: a very-low-calories low-fat ketogenic diet (VLCLFKD) and a low-calorie diet (LCD).
All patients underwent multifrequency bioelectrical impedance analysis (In-Body 570 body composition analyzer) and weekly fasting plasma glucose (FPG) measurements throughout the study. The time points at which an average body weight reduction of 5% and 10% was achieved were also identified for each group.
Initially, baseline glycemic conditions were established in the study group and following randomization. Subsequently, FPG was evaluated when a 5% weight loss was achieved, and finally, when a 10% body weight loss was achieved
Participants
Participants of any gender, aged between 18 and 60 years, with a body mass index (BMI) between 30 and 35 kg/m2, were eligible for inclusion. Exclusion criteria comprised individuals who had undergone weight loss treatments or used hypoglycemic agents within the last 6 months. Recruitment was conducted within the metropolitan area of Mexico City via open invitations through Zélé®'s social media platforms. Excluded were pregnant or lactating individuals, those with severe eating disorders, alcoholism, or substance abuse, as well as patients with severe psychiatric disorders (e.g., schizophrenia, bipolar disorder). Additionally, patients with liver dysfunction, defined as ALT, AST, GGT elevation more than 4 times the reference value, and those diagnosed with endocrine, metabolic, cardiovas cular, or renal diseases under treatment prior to the study, or using medications for glycemic control or weight loss, were excluded.
Sample Size
Sample size determination was primarily based on resource availability and secondarily on population representativeness. With a power of 95% and a 95% confidence interval, maintaining a 3:1 ratio of cases to controls, and accounting for a dropout rate of up to 35%, the Fleiss equation was used for calculation (computed with EPIinfo STATCALC for sample size). Consequently, the study included 56 cases and 32 controls.
Randomization
The initial 100 eligible individuals were invited for an interview with the researchers. During this session, project details and conditions were explained, and informed consent was obtained, approved by the research ethics committee of the CAE of the Secretary of Health of the State of Veracruz. Participants were assigned random numbers via computerized allocation, which were then blindly assigned at the food production and distribution facility, ensuring both researchers and participants remained unaware of the group assignments. Nutritional treatment commenced only after baseline laboratory analyses had been reviewed and interpreted.
Nutritional Intervention
Participants underwent a 12-week nutritional intervention program, complemented by a standardized physical exercise regimen and emotional support. Throughout the study period, participants received vitamin and trace element supplements (sodium chloride, magnesium oxide, calcium carbonate) to ensure compliance with recommended daily requirements.
Participants allocated to the VLCLFKD group progressed through four stages:
- Ketosis Phase: Participants consumed between 650 and 730 kcal/day in 5 meals consisting of Zélé® commercial products and low glycemic index vegetables. This phase provided an average of 1.2 g of protein/kg of ideal body weight/day, 20 g/day of lipids derived from essential fatty acids, and less than 60 g/day of absorbable carbohydrates. This phase lasted for 4 weeks.
- Mixed Ketosis Phase: Over 4 weeks, two meals of commercial products were gradually replaced with animal protein sources (meat, fish, eggs, etc.), increasing caloric intake by 100 to 150 kcal/day while maintaining ketosis.
- Transition Phase: Simple carbohydrates from fruits and some complex carbohydrates from cereals were introduced, facilitating the transition from ketosis to a controlled hypocaloric diet of approximately 1300 to 1500 kcal/- day. This phase typically lasted for one or two days.
- Integral and Maintenance Phase: Participants received a tailored hypocaloric diet aligned with their energy expenditure, measured by IBMF and ranging from 1300 to 2250 kcal/day. The macronutrient distribution followed the DiOGenes study recommendations, comprising 50% carbohydrates, 25% protein, and 25% fat [9].
Participants assigned to the LCD group received a balanced hypocaloric diet, providing 20% fewer calories than their basal metabolic rate, either measured by multifrequency bioelectrical impedance or calculated using the FAO/WHO/UNU formula. The typical caloric intake ranged from 1200 to 1400 kcal/day, with a macronutrient distribution consistent with the DiOGenes study recommendations (50% carbohydrates, 25% protein, and 25% fat) [9].
Food Delivery During the Study
All participants received, free of charge, the necessary products for meal and snack preparation throughout each phase of the study. Fresh vegetables were the only items that needed to be added to complete their diet. Meals were delivered weekly, providing menu items for 7 days per delivery at the time of their clinical review. This ensured that participants received a weekly set of items to customize their menu according to their assigned nutritional intervention, in a blinded process for each patient. Nutritional counseling was provided to all participants weekly throughout the study, along with menu item suggestions.
Anthropometric and Metabolic Data
Anthropometric measurements were preferably taken at the same time and under consistent conditions each week. Height was measured at the beginning of the study using a Seca brand wall-mounted stadiometer with an accuracy of 0.1 cm. Weight and body composition were recorded without shoes and in light clothing using an In-- Body 570 body composition analyzer with an accuracy of 0.1 kg.
Fasting blood extractions were conducted in the medical laboratory through venipuncture by trained personnel.
Statistical Analysis
Descriptive statistical tests were performed establishing general and group measures, demographic data and initial clinical characteristics of the participants are summarized presenting the n and mean (SD) and the Confidence Interval at 95% (CI 95%) for continuous variables categorical and percentage (%) for categorical variables. To assess the difference between variables in each group, t-tests and general lineal model repeated measures ANOVA were performed for continuous variables, while the X2 test was contrasted with Mann-Whitney U and one-way ANOVA for categorical variables, assuming differences with P values ≤ 0.05. The primary outcome was the percentage and/or absolute change in fasting glucose values. Statistical analysis was conducted using Microsoft Excel 365 and SPSS V 21.
Results
A total of 88 participants were enrolled in the study, comprising 75 females and 13 males. Following randomization, 32 participants were assigned to the LCD group and 56 to the VLCLKFD group. The overall dropout rate at the end of the study was 12.5%, with 7 participants from the LCD group and 4 from the VLCLFKD group. Data analysis was conducted using information from participants who completed the study, accounting for 81.2% in the LCD group and 91.1% in the VLCLFKD group.
The mean BMI of the participants at baseline was 32.6 ± 1.86 (95% CI 32.2 – 33.08) kg/m2. After group allocation, there were no significant differences observed, with BMIs of 32.4 ± 1.5 kg/m2 and 32.7 ± 2.02 kg/m2 for the LCD and VLCLFKD groups, respectively (p = 0.47). Regarding baseline weight determination, the mean weight for all participants was 85.3 ± 8.81 kg (95% CI 83.176 – 87.411). After randomization, the mean weight was 81.9 ± 8.03 kg (95% CI 78.128 – 85.651) for the LCD group and 86.7 ± 8.08 kg (95% CI 84.152 – 89.215) for the VLCLFKD group (f = 4.412, df 1, p = 0.039).
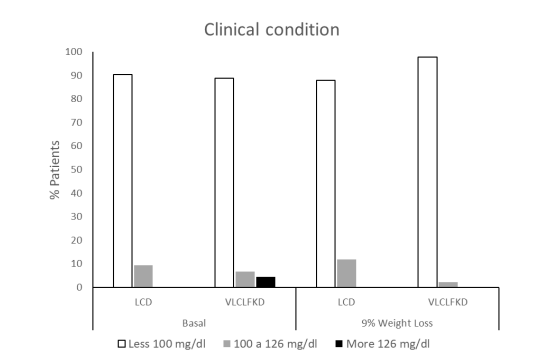
The fasting glucose determination upon admission was 93.7 ± 21.1 mg/dl (95% CI 88.25 – 99.14). Patients were categorized according to the diagnostic criteria of the American Diabetes Association (10), considering fasting plasma glucose (FPG) levels. Among them, 89.4% had levels below 100 mg/dl (Normal), 7.6% between 100 and 126 mg/dl (Prediabetic), and 3% above 126 mg/dl (Diabetic). Following group integration, the mean FPG level was 91.14 ± 11.98 (95% CI 85.68 – 96.59) for the LCD group and 95.07 ± 24.66 (95% CI 87.08 – 103.87) mg/dl for the VLCLFKD group (p = 0.49). The distribution after randomization according to the ADA was as follows: LCD 90.5% Normal, 9.5% Prediabetic, and for VLCLFKD 88.9% Normal, 6.7% Prediabetic, and 4.4% Diabetic (U = 436, p = 0.8).
The initial analysis of glycemic behavior was conducted when patients achieved a 5% weight loss, occurring after 9.5 ± 3.1 (95% CI 8.05 – 10.9) weeks in the LCD group and 5.3 ± 1.5 (95% CI 4.8 – 5.7) weeks for VLCLFKD (f = 56.15 df 1, p < 0.0001).
The FPG levels were 87.39 ± 7.68 mg/dl (95% CI 84.06 – 90.71) for the LCD group and 84.62 ± 13.53 (95% CI 80.77 – 88.46) mg/dl for the VLCLFKD group. According to ADA criteria, 86.4% of LCD group patients fell within normal ranges, while 13.6% were classified as prediabetic, consistent with the baseline distribution (X2 = 0.173 df 1, p = 0.678). In contrast, 98% of VLCLFKD group patients reported levels below 100 mg/dl, with only 2% falling between 100 and 126 mg (X2 = 0.182 df 1, p = 0.117).
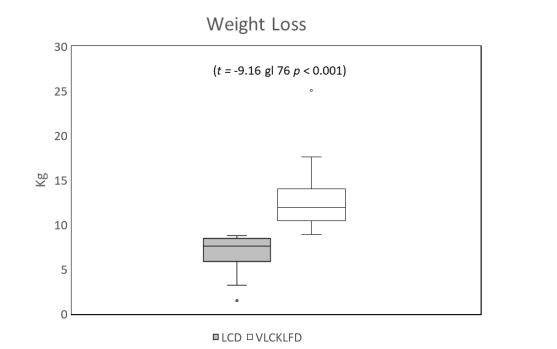
The maximum weight loss achieved by week 12 was 6.95 ± 1.9 kg (95% CI 6.03 to 7.83 kg) in the LCD group, approximately 9% of total body weight, compared to 12.39 ± 2.8 kg (95% CI 11.7 to 13.3 kg) in the VLCLFKD group, approximately 12% of total body weight (t -9.16 df 76 p < 0.001). Consequently, the final analysis was performed when both groups reached a 9% weight loss, occurring on average during week 8 for the VLCLFKD group. At 9% weight loss, LCD group patients maintained a mean blood glucose level of 91.2 ± 11.5 mg/dl (95% CI 86.5 to 96.0 mg/dl). After ADA classification, 88% exhibited normal glucose levels, while 12% had levels between 100 and 126 mg/dl. In the VLCLFKD group, the mean glucose level was 82.3 ± 10.1 mg/dl (95% CI 79.2 to 85.4 mg/dl), with 97.7% of patients reporting normal levels and only 2.3% with prediabetic levels.
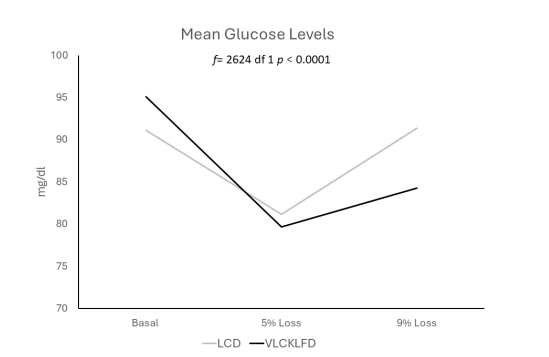
The analysis of mean glucose levels in both groups revealed an intriguing trend. In the LCD group, levels started at 92.8 ± 12 mg/dl (95% CI 86.5 to 98.9 mg/dl), decreased to 87 ± 25.5 mg (95% CI 73.9 to 100.1 mg/dl) with a 5% weight loss, and then returned to 91.5 ± 13.5 mg/dl (95% CI 84.5 to 98.3 mg/dl) after achieving maximum weight loss (f = .561 df 2 p = 0.576). Conversely, the VLCLFKD group exhibited an initial glucose determination of 94.3 ± 22.9 mg/dl (95% CI 86.6 to 101.9 mg/dl), which decreased to 81 ± 21.9 mg/dl (95% CI 73.7 to 88.4 mg/dl) with a 5% weight loss. By week 8, with a 9% weight loss, glucose levels stabilized at 84.9 ± 8.8 mg/dl (95% CI 82.1 to 87.9 mg/dl). Notably, the generalized linear analysis of repeated measures (f = 5.26 df 2 p = 0.009) and the inter-subject contrast test between both groups demonstrated a highly significant difference (f= 2624.1 df 1 p < 0.001).
During the study, two cases of rash occurred, which were resolved by changing the vitamin supplementation, and one case of diarrhea that was self-limiting and could not be attributed to the consumption of the foods provided.
Discussion
Obesity's role as a risk factor for type 2 diabetes (T2DM) and its complications, including chronic cardiovascular diseases, is well established in literature [11]. Numerous studies have underscored the effectiveness of ketogenic diets in weight management [12,13].This study, supported by robust evidence, reaffirms that with a structured weight control program such as the Very Low-Calorie Low-Fat Ketogenic Diet, FPG control can be enhanced in patients with grade I obesity, starting from a 5% weight loss. Participants in the intervention group achieved a remarkable weight loss of 12.39 ± 2.8 kg over the 12-week study period, while those in the LCD group experienced a weight loss of 6.95 ± 1.9 kg (p < 0.001). These findings indicate that weight loss milestones were reached at different time points during the study, with a 5% loss occurring at 9.5 weeks for the LCD group and at 5.3 weeks for the VLCLFKD group (p < 0.0001). This data suggests that weight loss is expedited in patients managed with VLCLFKD, thereby reducing obesity and diabetes-related risk factors at an accelerated pace.
Various approaches have been explored to minimize fluctuations in glucose levels. While medications such as metformin, gliquidone, and acarbose have demonstrated efficacy in this regard, nutritional interventions, particularly ketogenic diets, have emerged as promising strategies for stabilizing glucose levels [5,14]. Recent studies have highlighted the potential of such interventions as effective weight control measures for obese individuals. Among the four main types of ketogenic diets studied—standard ketogenic diet, cyclical ketogenic diet, targeted ketogenic diet, and high-protein ketogenic diet [15], the primary novelty of this article lies in the utilization of a very low-calorie ketogenic diet, which is low in fats and normal in protein content (Zélé method). This diet comprises macronutrients with 50 g of carbohydrates, 20 g of medium-chain fatty acids, and 1.2 g of protein per kg of ideal weight. Through this approach, ketone bodies are generated via hepatic oxidation of fatty acids released from adipocytes [16-18].
The mechanisms underlying the relationship between ketogenic diets and T2DM remain a subject of debate [19] and the efficacy of ketogenic diets in managing body weight and glycemic and lipid profiles in T2DM patients is not yet definitively established [5]. Nevertheless, interventions such as the one proposed herein, which involve stringent restriction of carbohydrate intake, suggest that glucose level fluctuations may be mitigated by reducing monosaccharide absorption, thereby lowering blood glucose levels and positively regulating glucose metabolism, as previously hypothesized [20,21].
Furthermore, obesity is associated with chronic low-grade inflammation that, via activation mediated by proinflammatory cytokines, blocks tyrosine phosphorylation of the insulin receptor, resulting in insulin resistance, type 2 diabetes, and many other diseases. and endocrine abnormalities [22] it is know that weight loss, especially that associated with a decrease in the percentage of body fat, reduces the chronic inflammatory process and contributes to the reduction of insulin resistance [23,24].
Similarly, systematic reviews and meta-analyses, such as the study conducted by Yuan et al., have demonstrated significant improvements in glycemic profiles associated with ketogenic diets [25]. By inducing a slight increase in peripheral blood ketosis, ketogenic diets may enhance insulin sensitivity, alleviate peripheral insulin-related stress, reduce external insulin requirements, and inhibit insulin secretion, thereby improving glycemic profiles and mitigating insulin resistance [26].
In relation to complications, the possibility of "Keto Flu" [27] Headache, dehydration, hypoglycemia and vomiting is described [28], none of them occurred in this study group, the adverse effects that were detected were not attributable to the foods provided.
Despite the promising outcomes observed in this controlled clinical trial, it is acknowledged that both the sample size and trial duration serve as limiting factors for reaching definitive conclusions. However, it is evident that VLCLFKD leads to a substantial decrease in FPG levels, coupled with greater weight loss achieved in a shorter duration. And it can be included in the treatment protocols of patients with obesity in whom insulin resistance and type 2 diabetes is an associated factor, reducing fasting plasma glucose levels in the short term and with them the associate risk factors.
Conclusions
Achieving a 5% weight loss in half the time underscores the significantly faster reduction in glucose levels with VLCLFKD. We recognize that these findings represent preliminary steps in the investigation of this controlled form of ketogenic diet. Further studies, particularly longitudinal follow-up studies, are warranted to delineate the long-term benefits of VLCLFKD in individuals with obesity and prediabetes. Nonetheless, this research contributes valuable insights into dietary interventions for glycemic control and offers avenues for enhancing metabolic health within this demographic.
Author Contributions
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used “Conceptualization, Francisco J. Nachón García and Gabriela E. Saldaña-Dávila.; methodology, Francisco J. Nachón García; software, Gabriela E. Saldaña-Dávila; validation, Francisco J. Nachón García and Gabriela E. Saldaña-Dávila; formal analysis, Francisco J. Nachón-García.; investigation, Gabriela E. Saldaña-Dávila; resources, Gabriela E. Saldaña-Dávila; data curation, Francisco J. Nachón García; writing—original draft preparation, Francisco J. Nachón García; writing—review and editing,Gabriela E. Saldaña Dávila; visualization, Francisco J. Nachón García; supervision, Franciso J. Nachón García; project administration, Gabriela E. Saldaña-Dávila; funding acquisition, Francisco J. Nachón García. All authors have read and agreed to the published version of the manuscript.” Please turn to the Credit taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work re-ported.
Funding
This research received no external funding
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics committee of the CAE of the Secretary of Health of the State of Veracruz (protocol code CONBIOETICA-30-CEI-001 -20170221, ID 1/114/2021 CI-ICS approved at September 21st, 2021. And Protocol Registration and Result System (Clinical Trials.gov PRS), with the protocol ID 19CI 30 087 041 and the ClinicalTrials.gov ID NCT06275347.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
- Nianogo RA, Arah OA (2022) Forecasting Obesity and Type 2 Diabetes Incidence and Burden: The ViLA-Obesity Simulation Model. Front Public Health, 10:1-13.
- Sun H (2022) IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 183: 109-19.
- Basto-Abreu A, López-Olmedo N, Rojas-Martínez R, Aguilar-Salinas CA, Moreno-Banda GL, Carnalla M, et al. (2023) Prevalence of prediabetes and diabetes in Mexico: Ensanut 2022. Salud Publica Mex. 65.
- Kalayjian T, Westman EC (2022) Re: Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: the interventional Keto-Med randomized crossover trial. American Journal of Clinical Nutrition. 116: 1184.
- Zhou C, Wang M, Liang J, He G, Chen N (2022) Ketogenic Diet Benefits to Weight Loss, Glycemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. 19
- Sellahewa L, Khan C, Lakkunarajah S (2017) IIA. Systematic Review of Evidence on the Use of Very Low Calorie Diets in People with Diabetes. Curr Diabetes Rev. 13: 35-46.
- Aras M, Tchang BG PJ (2021) Obesity and Diabetes. Nurs Clin North Am. 56: 527-41.
- Hallberg SJ, Gershuni VM, Athinarayanan SJ (2019) Reversing type 2 diabetes: A narrative review of the evidence. Nutrients. 11: 1-16.
- Larsen TM, Dalskov SM, Van Baak M, Jebb SA, Papadaki A, Pfeiffer AFH, et al. (2010) Diets with High or Low Protein Content and Glycemic Index for Weight-Loss Maintenance. 363.
- ADA. https://diabetes.org/about-diabetes/diagnopsis. 2022. Diabetes Diagnosis & test.
- Kachur S, Lavie CJ, de Schutter A, Milani RV, Ventura HO (2017) Obesity and cardiovascular diseases. Minerva Med. 108: 212-28.
- Castellana M, Conte E, Cignarelli A, Perrini S, Giustina A, Giovanella L, et al. (2020) Efficacy and safety of very low calorie ketogenic diet (VLCKD) in patients with overweight and obesity: A systematic review and meta-analysis. 21: 5-16.
- Yu Z, Nan F, Wang LY, Jiang H, Chen W, Jiang Y (2020) Effects of high-protein diet on glycemic control, insulin resistance and blood pressure in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Clinical Nutrition. 39: 1724-34.
- Lambrinou E, Hansen TB, Beulens JWJ (2019) Lifestyle factors, self-management and patient empowerment in diabetes care. Eur J Prev Cardiol. 26: 55-63.
- Shilpa J, Mohan V (2018) Ketogenic diets: Boon or bane? 148: 251-3.
- Joshi S, Ostfeld RJ, McMacken M (2019) The Ketogenic Diet for Obesity and Diabetes - Enthusiasm Outpaces Evidence. 179: 1163-4.
- Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LES, et al. (2016) Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. American Journal of Clinical Nutrition. 104: 324-33.
- Hamdy O, Tasabehji MW, Elseaidy T, Tomah S, Ashrafzadeh S, Mottalib A (2018) Fat Versus Carbohydrate-Based Energy-Restricted Diets for Weight Loss in Patients With Type 2 Diabetes. Current Diabetes Reports. Current Medicine Group LLC. 18: 1.
- Alsharairi NA (2021) The role of short-chain fatty acids in mediating very low-calorie ketogenic diet-infant gut microbiota relationships and its therapeutic potential in obesity. 13.
- Bolla AM, Caretto A, Laurenzi A, Scavini M, Piemonti L (2019) Low-Carbs and Ketogenic Diets in Type1 and Type 2 Diabetes. 1-14.
- Yancy WS, Foy M, Chalecki AM, Vernon MC, Westman EC (2005) A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr Metab (Lond). 2.
- Margioris AN, Dermitzaki E, Venihaki M, Tsatsanis C (2013) Chronic low-grade inflammation. In: Diet, Immunity and Inflammation. Elsevier Ltd. 105-20.
- Nicklas BJ, You T, Pahor M (2005) Behavioural treatments for chronic systemic inflammation: Effects of dietary weight loss and exercise training. CMAJ. Canadian Medical Association Journal. Canadian Medical Association. 172: 1199-209.
- Paoli A (2014) Ketogenic diet for obesity: Friend or foe? International Journal of Environmental Research and Public Health. MDPI; 11: 2092-107.
- Yuan X, Wang J, Yang S, Gao M, Cao L, Li X, et al (2020) Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutrition and Diabetes. Springer Nature 10.
- Gupta L, Khandelwal D, Kalra S, Gupta P, Dutta D, Aggarwal S (2017) Ketogenic diet in endocrine disorders. J Postgrad Med. 63: 242-51.
- Bostock ECS, Kirkby KC, Taylor BV, Hawrelak JA (2020) Consumer Reports of “Keto Flu” Associated With the Ketogenic Diet. Front Nutr. 7.
- Wheless James W (2001) The Ketogenic Diet An Effective Medical Therapy with side effects. J Child Neurol. 16: 633-5.

Figures at a glance