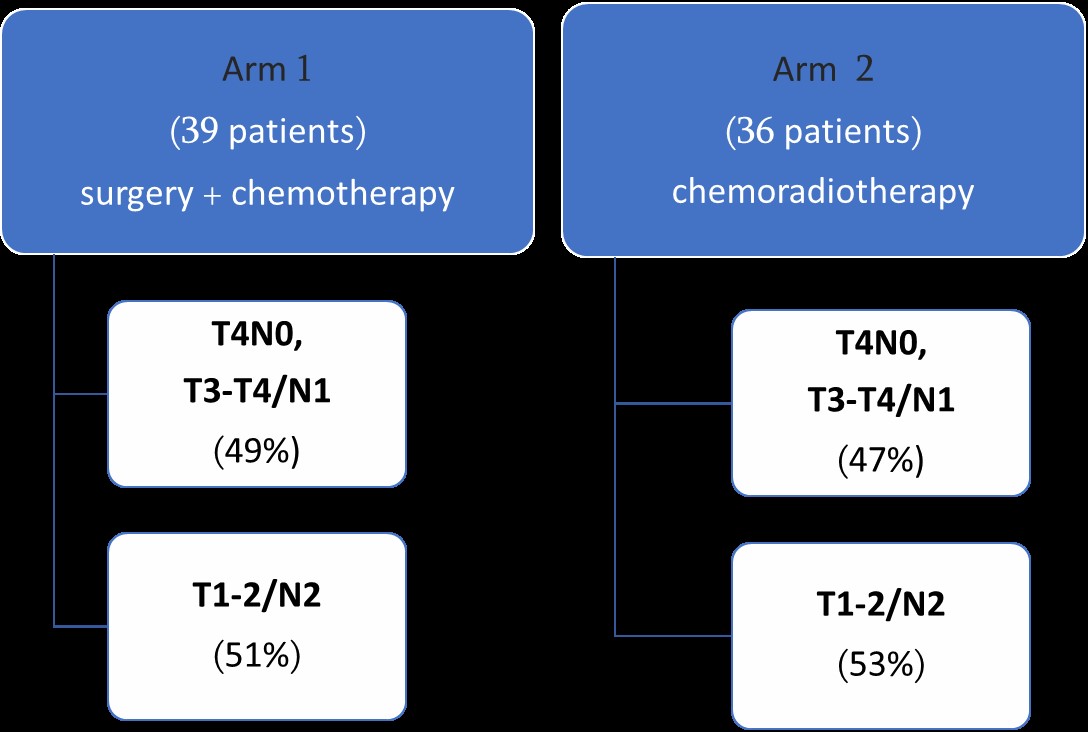
Figure 1: study design
Patients Characteristics |
Surgery+ adjuvant chemotherapy |
Chemoradiation alone (n=36) |
AGE, years |
60 (56.1-62.0) |
63 (58.7-63.9) |
GENDER |
|
|
male |
35 (90%) |
34 (94%) |
female |
4 (10%) |
2 (6%) |
HISTOLOGY |
|
|
Adenocarcinoma |
14 (36%) |
12 (34%) |
Squamous |
18 (46%) |
21(58%) |
other |
7 (18%) |
3 (8%) |
STAGE/TNM |
|
|
T4N0 |
2 (5%) |
2 (6%) |
T3N1 |
13 (34%) |
5 (14%) |
T4N1 |
4 (10%) |
10 (27%) |
T1N2 |
2 (5%) |
2(6%) |
T2N2 |
18 (46%) |
17(47%) |
TYPE OF SURGERY |
|
|
Lob/bilobectomy |
15(39%) |
|
Pneumonectomy |
24 (61%) |
|
CHEMOTHERAPY REGIMEN |
|
|
Cisplatin/Etoposide |
|
22 (61%) |
Carboplatin/Paclitaxel |
|
14 (39%) |
PERFORMANCE STATUS |
|
|
ECOG 0-1 |
36 (92%) |
32(89%) |
ECOG 2 |
3 (8%) |
4(11%) |
Table 1: Characteristics for patients by treatment group
Survival Time |
Surgery+ adjuvant chemotherapy |
Chemoradiation alone (n=36) |
P-value |
1-year |
69% |
64% |
p= 0.623 |
Table 2: 1-year survival rates for patients with stage IIIA lung cancer stratified by treatment modality
|
Surgery+ adjuvant chemotherapy |
Chemoradiation alone (n=36) |
Haematological |
|
|
Anaemia Grade >2 |
7 (17.9%) |
6 (16.6%) |
Thrombocytopenia Grade >2 |
2 (5.1%) |
3 (8.3%) |
Febrile neutropenia |
0 |
3 (8.3%) |
Non-Haematological |
|
|
Fatigue |
N/A |
12 (33.3%) |
Neuropathy |
0 |
7 (19.4%) |
Cardiac |
3 (7.6%) |
6 (16.6%) |
GI complications |
2 (5.1%) |
12 (33.3%) |
Pneumonitis Grade >2 |
0 |
10 (27.7%) |
Thromboembolic event |
4 (10.2%) |
3 (8.3%) |
Esophagitis Grade 2-3 |
0 |
16(44.4%) |
Treatment-related death |
3 (7.6%) |
2 (5.5%) |
Table 3: Grade 2–5 adverse events

Figure 1: study design
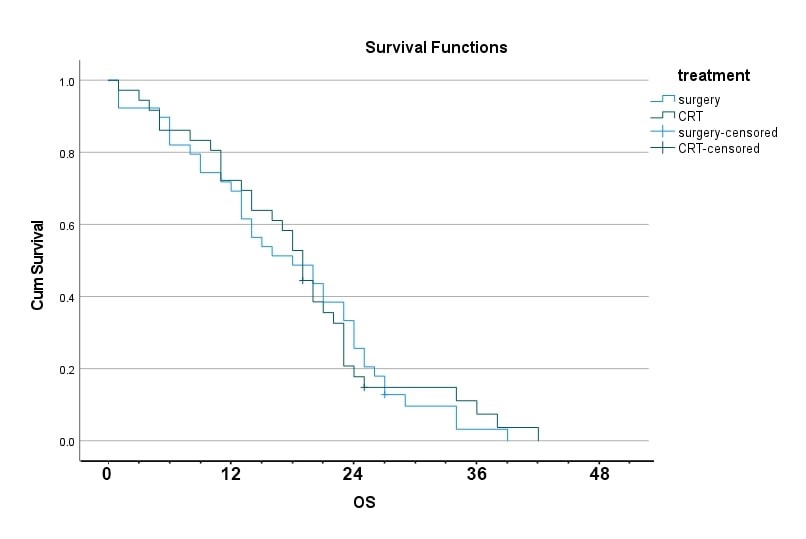
Figure 2: Survival
Tables at a glance
Figures at a glance