Aflatoxin B1 and Ochratoxin A- Induced Some Airway Epithelial Cell Innate Immune Responses Inhibited by a Extract from Punica Granatum
Received Date: January 26, 2022 Accepted Date: February 26, 2022 Published Date: February 28, 2022
doi: 10.17303/jmph.2022.1.101
Citation: Minyue Li, Xiaojing Shen, Ruiqi Zhang, Xuexiang Nong, Zichao Mao et al. (2023) Bibliometric Analysis of Stephania Research: Trends and Hotspots. J Med Plant Herbs 2: 1-11
Abstract
Ethno-pharmacological relevance: The Punica granatum L. var. granatum (pomegranate) has been demonstrated to exert some antitumor effects on various types of cancer cells. In this study, we aimed to investigate the effect of Punica granatum seed extract on the apoptosis of A549 human lung adenocarcinoma cell line and to assess the expressions of Toll-like (TLR)2 and TLR4 genes as well as the secretion of Th2-promoting cytokines interleukin (IL)-25/IL-33/ thymic stromal lymphopoietin (TSLP) induced by ochratoxin A (Ochra).
Materials and methods: The quantitative analysis of P. granatum extract was performed by HPLC/MS. In vitro early and late apoptotic effects of P. granatum, AFB1, Ochra, P. granatum and AFB1, and P. granatum and Ochra were assessed in A549 human lung adenocarcinoma cell line using flow cytometry. After the treatments, ELISA was also performed to measure the concentrations of IL-25, IL-33, and TSLP in the supernatants. Quantitative real-time PCR (qPCR) was performed to assess the expression levels of TLR2 and TLR4 genes.
Results: Four main compounds including ellagic acid, anthocyanins, gallic acid, and tannins were found in ethanolic extract from P. granatum. Also, P. granatum extract could induce lung cancer cells apoptosis, which was accompanied by the decreased expressions of TLR2 and TLR4 genes as well as the decreased secretion of Th2-promoting cytokines IL-25/IL-33/TSLP induced by mycotoxins AFB1 and Ochra.
Conclusion: Downregulation of TLR2 and TLR4 expressions as well as IL-25/IL-33/TSLP secretion showed association with apoptosis of lung cancer cells. Thus, our study suggests that, P. granatum extract could be considered as a promising drug candidate to treat lung cancer, which can show us a better way to develop novel drugs from natural compounds.
Keywords: Punica granatum; aflatoxin B1; ochratoxin A; apoptosis; TLRs expression; Th2-promoting cytokines
Listof abbreviations: Toll-like (TLR), interleukin (IL), thymic stromal lymphopoietin (TSLP), aflatoxin B1 (AFB1), ochratoxin A (Ochra), Quantitative real-time PCR (qPCR), International Agency for Research on Cancer (IARC), Punica granatum (P. granatum), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT), dimethyl sulfoxide (DMSO), Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS), ellagic acid (EA), punicalagin (PC), delta delta CT (ddCT),
Introduction
Mycotoxins aflatoxin B1 (AFB1) and ochratoxin A (Ochra) have been found as contaminants of various commodities worldwide (Pinotti et al., 2016). Also, toxicity and carcinogenicity of AFB1 and Ochra to experimental animals are well documented (IPCS, 2001). Accordingly, they have teratogenic, mutagenic, and immunotoxic effects (Bhatti et al. 2016). Due to the proven carcinogenicity to humans, International Agency for Research on Cancer (IARC) classified AFB1 as a Group 1 carcinogen (IARC, 2002), while Ochra was classified into group 2B (possible human carcinogen) (IARC, 1993). To protect the human and animal health, AFB1 maximum level in all products derived from cereals, was set to 2 μg/kg, while for Ochra maximum level in all products derived from unprocessed cereals including processed cereal products and cereals intended for direct human consumption, was set to 3 μg/kg (EU, 2006).
Both AFB1 and Ochra elicit different cellular disorders and adverse effects such as oxidative stress, inhibition of translation, DNA damage, and apoptosis in host cells, thus causing various kinds of cytotoxicities (Wen et al., 2016). Evasion of apoptosis can be considered as a part of a cellular stress and a hallmark of various types of cancer (Hanahan et al., 2011). It was shown that, the signaling through Toll-like receptors (TLRs) may activate an apoptotic response in eukaryotic cells. Activation of transcription factor NF-kB by TLR2 or TLR4 signaling, can promote apoptosis as well as pro-inflammatory responses (Ozkan et al., 2018). Dysregulation of apoptosis results in the uncontrolled growth of cancer and development of resistance to the treatment. Therefore, drugs that could restore cancer cell apoptosis may be effective on many types of cancer, which can make this as a promising strategy to treat cancer (Nicholson, 2000). Recently, there is more interest to natural active biological compounds that are derived from plants (El-Khoury et al. 2017). Punica granatum, commonly known as pomegranate, is a member of the monogeneric family, Punicaceae, and is mainly found in Iran. P. granatum and its chemical components possess variList of abbreviations: Toll-like (TLR), interleukin (IL), thymic stromal lymphopoietin (TSLP), aflatoxin B1 (AFB1), ochratoxin A (Ochra), Quantitative real-time PCR (qPCR), International Agency for Research on Cancer (IARC), Punica granatum (P. granatum), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT), dimethyl sulfoxide (DMSO), Dulbecco’s modified eagle medium (DMEM), fetal bovine serum (FBS), ellagic acid (EA), punicalagin (PC), delta delta CT (ddCT),ous pharmacological and toxicological properties including anti-oxidant, anti-inflammatory, anti-cancer, and anti-angiogenesis activities (Rahimi et al., 2012; Deng et al., 2017). Previous studies demonstrated that, P. granatum possesses some inhibitory effects on different type of cancers such as prostate (Ma et al., 2015), breast (Sturgeon and Ronnenberg, 2010), and colon (Kasimsetty et al., 2010). The role of P. granatum in AFB1 and Ochra-induced apoptosis in lung cancer cells remained still unclear. In the current study, we investigated the effect of P. granatum extract on A549 human lung adenocarcinoma cell line and then suggested some specific objectives as follows:
- determining the early and late apoptotic effects of P. granatum extract, AFB1, and Ochra A549 human lung adenocarcinoma cell line;
- determining the expressions of TLR2 and TLR4 genes induced by P. granatum extract, AFB1, and Ochra in A549 human lung adenocarcinoma cell line;
- determining the secretion of Th2-promoting cytokines IL-25/IL-33/TSLP induced by P. granatum extract, AFB1, and Ochra in A549 human lung adenocarcinoma cell line;
- determining the inhibitory effect of P. granatum extract on TLR2 and TLR4 expressions as well as IL-25, IL-33 and TSLP secretions induced by AFB1 and Ochra (combination effects).
Materials and Methods
Preparation of P.granatum seed extract
Fresh pomegranate fruits were purchased from a local market. The sample was authenticated by Department of Pharmacognosy, University of Tehran, Iran. The seeds of pomegranate were manually removed, and were then air-dried at room temperature, and ground into powder by a hammer mill with 80sizes. To prepare the extract, 200 g of each powder was soaked in 70% ethanol solution (1:10 ratio) in a closed container, and after that was shaken for 24 hours at darkness. The extract was filtered through Whatman No. 41 filter paper, and concentrated under vacuum at 40°C using a rotary machine, and the obtained powder was stored at −80°C and later used (Nozohour et al., 2018).
Reagents
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT), dimethyl sulfoxide (DMSO), aflatoxin B1 (AFB1), ochratoxin A (Ochra), and Dulbecco’s modified eagle medium (DMEM) were purchased from Sigma Chemical Co. (St Louis, MO, USA). Also, fetal bovine serum (FBS) was purchased from Merck Co. (Darmstadt, Germany). Annexin V-FITC apoptosis detection kit was purchased from BD biosciences (San Jose, CA, USA). RNeasy mini-kit was purchased from Qiagen GmbH (Hilden, Germany). iScript cDNA synthesis kit was purchased from Bio-Rad (Hercules, CA, USA). IL-25, IL-33, and TSLP sandwich-type ELISA kits were purchased from R&D Co. (Minneapolis, MN 55413, USA).
Quantitative analysis of P. granatum extract by HPLC and MS
A Waters 2695 HPLC system (Waters Corp., Milford, Massachu-setts, USA) was used in this experiment. The method and chromatographic conditions were followed by earlier described methods (Deng et al., 2017). Also, the obtained data were processed using Waters Empower 3 software. The chromatographic separation was conducted on a Waters Symmetry C18 column (5 μm, 4.6 × 250 mm). The mobile phase was composed of deionized water with glacial acetic acid (A, 99:1, v/v, pH 3.0) and methanol (B) with a flow rate of 1 ml/ min. The gradient program was set as follows: 0-70 minutes, 10-45% B; 70-80 minutes, 45% B. The chromatogram was detected at a wavelength of 256 nm using the assay. An Absciex Qtrap 5500 was used in the MS. Moreover, we detected the samples by HPLC and MS after establishing the standard working curves of ellagic acid (EA) and punicalagin (PC).
Cell culture
A549 human lung adenocarcinoma cell line was obtained from Pasteur Institute of Iran (Tehran, Iran). Cells were cultured in DMEM media containing 10% heat-inactivated FBS and 1% antibiotics (penicillin and streptomycin) in 5% CO2, at the temperature of 37oC and a relative humidity of 90%.
Trypan blue assay
The Trypan blue assay was measured by above-mentioned method (Chung et al., 2015). By passing one to three days from culture, cells were detached by trypsinization and the number of viable cells was counted using a Trypan blue stain reagent. The viability of the control (untreated cells) was regarded as 100%.
Apoptosis analysis by flow cytometry
An Annexin V-FITC apoptosis detection kit was used to further confirm P. granatum induced apoptosis as described earlier (Shi et al., 2014). In brief, A549 human lung adenocarcinoma cell lines (2.5 × 105 cell/ml) were seeded in 12-well plates. 24 hours later, the cells were treated with different substances including P. granatum extract (8 μg/ml), AFB1 (0.05 μg/ml), Ochra (3.5 μg/ml), P. granatum extract and AFB1, and P. granatum extract and Ochra. In each experiment, a negative control (PBS-buffer treated) was included. Then, the cells were harvested, and washed twice with cold PBS followed by staining with 1 μl V-FITC and 1 μl PI for 10 minutes. The apoptosis state in each treatment was detected using flow cytometry, and the data were analyzed by Flow Jo software. This experiment was repeated for three times.
Quantitative real-time PCR (qPCR)
For TLR2 and TLR4 genes expressions, A549 human lung adenocarcinoma cell lines (2.5 × 105 cell/ml) were treated by different substances including P. granatum extract (8 μg/ml), AFB1 (0.05 μg/ml), Ochra (3.5 μg/ml), P. granatum extract and AFB1, and P. granatum extract and Ochra. Total RNA was extracted from cell cultures using the RNeasy mini-kit in terms of the manufacturer’s instructions. RNA was eluted from column into 50 μl nuclease-free water, and was then stored at -80°C until the time of use. RNA concentration and quality were measured using a Nanodrop system (NanoDrop Technology, San Diego, CA, USA). Reverse transcription of total RNA was also performed using iScript cDNA synthesis kit in terms of the manufacturer’s protocol. Real time PCR was performed using the SYBR Green method by the following primers: TLR2, Forward: TGCTGCCATTCTCATTCTTCTG, Reverse: AGGTCTTGGTGTTCATTATCTTCC, TLR4, Forward: CAACCAAGAACCTGGACCTG, Reverse: GAGAGGTGGCTTAGGC, GAPDH,Forward: CCACTCCTCCACCTTTGACG, Reverse: CCACCACCCTGTTGCTGTAG.
qPCR data were analyzed by the delta delta CT (ddCT) method, and then normalized to GAPDH (Livak and Schmittgen, 2001). Also, qPCR was performed in terms of the manufacturer’s protocol using an ABI 7900HT system (Applied Biosystems, Foster City, CA, USA). Raw threshold cycle values were normalized using RNU-6B (Cat#4427975, Thermo Fisher Scientific, San Jose, CA, USA) as the internal control.
Measurement of cytokines
For cytokines assessment, A549 human lung adenocarcinoma cell lines (2.5 × 105 cell/ml) were treated with different substances including P. granatum extract (8 μg/ml), AFB1 (0.05 μg/ml), Ochra (3.5 μg/ml), P. granatum extract and AFB1, and P. granatum extract and Ochra. Levels of IL-25, IL-33, and TSLP in cell culture supernatants were assessed using commercially available sandwich-type ELISA kits. Also, the assays were performed in terms of the manufacturer’s instructions. Briefly, a 96-well flat bottom plate was coated with capture polyclonal antibody specific to each cytokine. Serially diluted specific standards were added to the respective wells. Following a series of washing, the captured cytokine was detected using the specific conjugated detection antibody. The chromogen/substrate reagent was added into each well, and after color development, the plate was read at 450 nm using an ELISA plate reader.
Statistics
Cell culture-based experiments were performed in biological triplicates. Data were expressed as means±SD, and the analysis was compared by one-way analysis of variance (ANOVA) using SPSS 16.0 software. A statistically significant p value was considered at p<0.05.
Results and discussion
Numerous studies reported that, natural substances, especially from plants, can trigger apoptosis in a lot of cancer cells with different signaling pathways (Kasimsetty et al., 2010). Therefore, this inspired us to investigate the anti-cancer effect of extract from pomegranate seeds on A549 human lung adenocarcinoma cell lines.
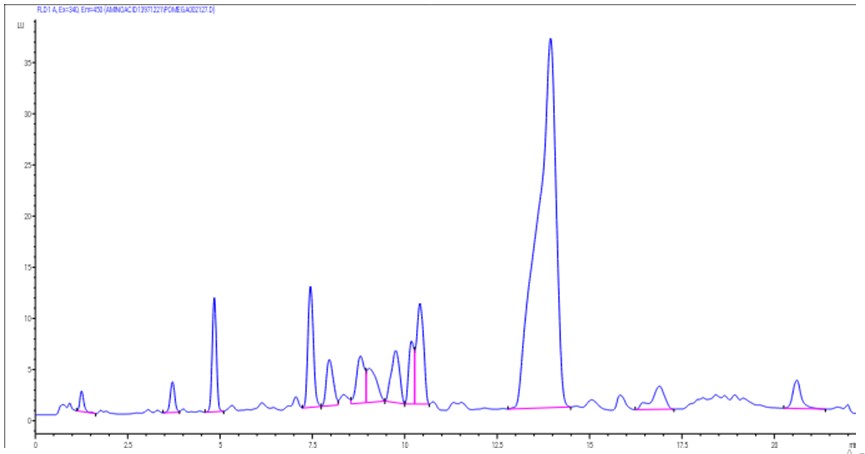
In the current study, the compositions from P. granatum extract are illustrated in Table 1 and Figure 1. Four main compounds were identified in ethanolic extraction method, namely ellagic acid, anthocyanins, gallic acid, and tannins. A previous study reported by Rahimi et al. (2012) found that, P. granatum extract contains ellagic acid, cyaniding (anthocyanins), and punicalagin (gallotannins) as the major compounds. Also, Deng et al. (2017) showed that, the main chemical compositions of P. granatum extract were ellagic acid and punicalagin.
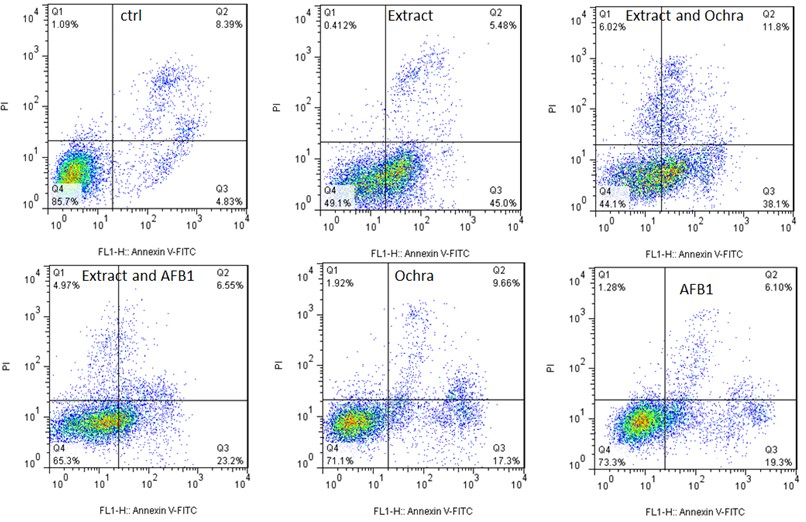
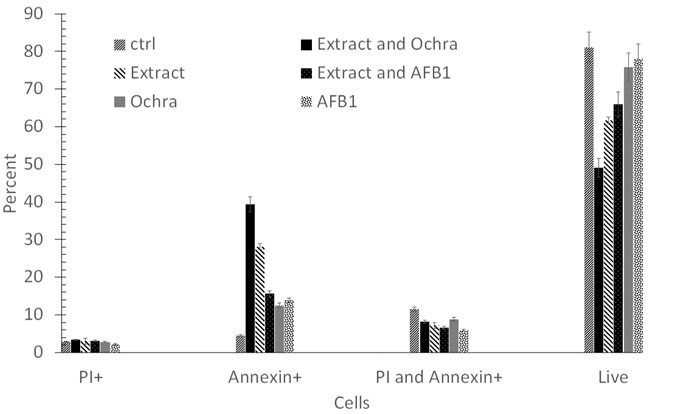
Dysregulation of apoptosis is a hallmark of cancer, and in this regard, many anti-cancer drugs exhibit the activities by inducing cancer cells apoptosis, which make it a promising anti-cancer target (Hanahan et al., 2011). In this study, to quantify the necrosis, early and late apoptosis induced by P. granatum extract, AFB1, Ochra, extract and AFB1, and extract and Ochra in A549 human lung adenocarcinoma cell lines, we analyzed the cells using flow cytometry after Annexin V-FITC/PI dual-labeling. As revealed in Figure 2A and B and Table 2, the live rates of the cells significantly decreased in various treatments from 85.7% in control to 49.1% in P. granatum extract-treatment, 73.3% in AFB1-treatment, 71.1% in Ochra-treatment, 65.3% in extract and AFB1-treatment, and 44.1% in extract and Ochra-treatment after 24 hours (P<0.05). Indeed, P. granatum, as a natural substance, was potently toxic for cancer cells.
The proportion of necrotic A549 cells sharply increased upon 24 hours incubation with P. granatum extract up to 4.12%, AFB1 up to 1.28%, Ochra up to 1.92%, extract and AFB1 up to 4.97%, and extract and Ochra up to 6.02% when compared to control (1.09%). Indeed, P. granatum extract significantly increased AFB1 and Ochra-induced necrosis in cancer cells (P<0.05) (Figure 2A and B). Annexin-V analysis revealed that, there were 45%, 19.3%, and 17.3% increases in early apoptotic cell population of A549 exposed to P. granatum extract, AFB1, and Ochra when compared to control, respectively, whereas the combination of extract with AFB1 (23.2%)/or Ochra (38.1%) significantly increased the early apoptotic cell population more than AFB1 and Ochra alone (P<0.05). In addition, the late apoptotic rates decreased in P. granatum extract-treatment (5.48%), extract and AFB1-treatment (6.55%), and AFB1-treatment (6.10%) when compared to control (8.39%); however, the rates increased in extract and Ochra-treatment (11.8%) and Ochra-treatment (9.66%) compared to control (Table 2). Overall, our results show that, 24 hours treatment with P. granatum extract can increase the necrotic and apoptotic effects of AFB1 and Ochra in A549 human lung adenocarcinoma cell lines. In agreement with our results, Deng et al. (2017) found that, P. granatum inhibited DU145, PC3, and TRAMP-C1 prostate cancer cells viabilities, and also induced apoptosis via mitochondrial intrinsic pathway. In a study conducted by Vidal et al. (2003), human PCa PC-3 cancer cells treated with P. granatum extract (10-100 μg/ml) for 48 hours resulted in a dose-dependent inhibition of cell growth and induction of apoptosis. Notably, these observations were in agreement with apoptosis of other cancer cells induced by P. granatum extract that were reported earlier (Li et al., 2014; Bingbing et al., 2016; Li et al., 2016). In addition, Khan et al. (2007) revealed that oral consumption of P. granatum can effectively inhibit lung tumor multiplicity and down-regulate the activation of MAPK and NF-kB signaling. Various studies indicated that, proteins Bax, Bcl2 and caspase-3 are crucial signaling regulators of intrinsic apoptosis (Rahimi et al., 2012; Zuo et al., 2014). It has been demonstrated that, protein Bcl2 inhibits apoptosis at the level of mitochondrial function by preventing cytochrome c efflux to the cytosol, thus inhibiting Bax and caspase-3 activations (Redza-Dutordoir and Averill-Bates, 2016). Previous studies indicated that, AFB1 and Ochra trigger apoptosis by increasing the expressions of Bax and Caspase-3, while Bcl-2 level decreases (Domijan et al., 2019). Deng et al. (2017) proved that, P. granatum -induced apoptosis was accompanied by the decreased expression of anti-apoptotic Bcl2 and the increased expressions of caspase-3 and pro-apoptotic Bax. In a study by Edderkaoui et al. (2008), ellagic acid from P. granatum stimulated the mitochondrial pathway of apoptosis associated with mitochondrial depolarization, cytochrome C release, the downstream caspase activation, and the decreased NF-kB binding activity.
TLRs promote the growth of lung cancer cells through anti-apoptotic signals and associated with the invasion of lung cancer cells (Gu et al., 2018). The expression of TLRs in lung cancer tissues and the interaction among them have been the focus of research in recent years (Yang et al., 2014; Lagiedo et al., 2015).
In this study, qRT-PCR confirmed the decreased expressions of TLR2 and TLR4 genes in the presence of P. granatum extract in A549 human lung adenocarcinoma cell lines. As revealed in Table 3, AFB1 and Ochra up-regulated the expressions of both TLR2 (65.18% and 65.18%) and TLR4 (127.05% and 65.88%), respectively, in cancer cells, whereas P. granatum extract reduced the expressions of TLR2 and TLR4 genes induced by AFB1 and Ochra. Also, In agreement with our results, Du et al. (2018) indicated that, P. granatum extract and their main components could significantly inhibit the expression of TLR genes. Moreover, in another study conducted by Zhong et al. (2014), the extract from P. granatum reversed the over-expressions of genes of the TLR2 and TLR4, and down-regulated the activation of NF-κB signal transduction pathway. TLR2 and TLR4 signaling is responsible for MAPKs activation and NF-κB translocation (Du et al., 2018). NF-κB induces genes whose products prevent apoptosis such as Bcl-2 family members, and thus exerts pro-survival activity (Khan et al. (2007). These observations provide conclusive evidence for a prominent role of NF-κB signaling pathway in tumor cells survival via TLR2 and TLR4. Indeed, TLR signaling pathway could also promote cancer initiation and progression.
Compelling evidence suggest that, Th2-promoting cytokines, apoptosis, and cancer are linked with a central role played by NF-κB. In fact, TLRs can trigger inflammatory responses through activation of NF-κB, a master switch for inflammation (Khosravi and Erle, 2016). In this regard, Khosravi et al. (2018) showed that, IL-25, IL-33, and TSLP released by lung epithelial cells promote Th2-type airway inflammation, suggesting pivotal roles in the pathophysiology of lung diseases. In this study, the amounts of cytokines are summarized in Table 4. The fold changes in IL-25, IL-33, and TSLP secretions were 11.17-, 7.77-, and 4.38-fold by passing 12 hours from the treatment with AFB1, whereas the fold changes in the above-mentioned cytokines were 37.05-, 13.33-, and 13.84-fold after the treatment with Ochra, respectively. In contrast, P. granatum extract significantly reduced the secretion of IL-25, IL-33, and TSLP induced by AFB1 and Ochra (P<0.05). Up to the best of our knowledge,there are no reports conducted on the effect of P. granatum on IL-25, IL-33, and TSLP secretions in cancer cells. Cytokines play central roles in cellular interaction in the inflammatory tumor microenvironment (Candido and Hagemann, 2013). It is widely accepted that, immune deviation toward Th1 response including induction of CD8+ cytotoxic T cells, results in tumor rejection; however, deviation toward Th2 response prevents tumor rejection(Terabe et al., 2004). Several lines of evidences have demonstrated an important role of Th2-promoting cytokines IL-25/IL-33/TSLP in controlling the progress and metastasis of tumors(Coussens et al., 2013). Also, Jiang et al. (2017) found that, IL-25 promoted tumor invasion to the lung, possibly via regulating type 2 immune responses in the tumor micro-environments. In recent reports, IL-33 is also highly involved in several cancers where this cytokine exerts protumorigenic and anti-tumorigenic functions up to the environment (Lu et al., 2016). In addition,Burkard-Mandel et al. (2018) showed that, TSLP plays a key role in lung tumor outcome via its action on alveolar macrophages in the lung microenvironment. This is consistent with a study in human gastric carcinoma (Barooei et al., 2015), which showed a significant relationship between the TSLP overexpression and tumor growth. Thus, while more works are required to determine if these are feasible approaches in cancer models; our data suggest that, this may be considered as an appropriate line of investigation in cancer types that produce TSLP.
In conclusion, we have demonstrated that, P. granatum extract presented obvious inhibition effect on the growth and viability of A549 human lung adenocarcinoma cell lines. P. granatum extract could also induce lung cancer cells apoptosis, which was accompanied with the decreased expressions of TLR2 and TLR4 genes as well as the decreased secretion of Th2-promoting cytokines IL-25/IL-33/TSLP induced by mycotoxins AFB1 and Ochra. In fact, downregulation of TLR2 and TLR4 expressions as well as IL-25/IL-33/TSLP secretions showed an accordance with apoptosis of lung cancer cells. These findings provide the theoretical basis for further investigations of P. granatum and their natural compounds, which give us a better way to find the drugs for treating lung cancer.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgment
This work was supported by the Research Council of the University of Tehran, Tehran province, Iran.

Tables at a glance
Figures at a glance