Characteristics and Functionality of Tomato Saponin, Esculeoside A
Received Date: August 24, 2024 Accepted Date: September 24, 2024 Published Date: September 27, 2024
doi: 10.17303/jmph.2024.3.202
Citation: Toshihiro Nohara, Yukio Fujiwara, Jian-Rong Zhou (2024) Characteristics and Functionality of Tomato Saponin, Esculeoside A. J Med Plant Herbs 3: 1-13
Abstract
As part of our research on the composition of plants belonging to the genus Solanum, we searched for the components in mature tomatoes and found the overwhelming main ingredient, tomato steroid saponin, named as Esculeoside A. We have published many reports related to this ingredient, but we have now organized them into i) Isolation and structure of tomato saponin, Esculeoside A, ii) Finding of esculeoside B in canned and juice products, iii) Seasonal changes in tomato saponin, iv) Revolutionary chemical transformation of Esculeoside A to pregnane, and v) Internal metabolism of Esculeoside A. Moreover, we provide an overview of the recently reported pharmacological effects relating to atherosclerosis and dermatitis of Esculeoside A and Esculeoside B. Esculeoside A has a hydroxyl group at the C-23 position on spirosolane, so it is easily metabolized to pregnane, a type of steroid hormone, and is thought to have various physiological activities. This paper represents the need for us to reacquaint ourselves with Esculeoside A that may better contribute to health. Esculeoside A is currently under development for application as a health food, so please pay close attention.
Keywords: Tomato; Esculeoside A; Esculeoside B; Pregnane; Atherosclerosis; Dermatitis
Introduction
About 45 years ago, Nohara took part in a study of folk medicine (in the Iya region of Shikoku) conducted at university, and particularly interested in plants of Solanum lyratum and S. nigrum, which were used in folk medicine as anti-tumor and anti-herpes agents. Later, when he visited Kunli Herbal Medicine Store in Shanghai, he was surprised to learn that these plants were the most used as antitumor herbal medicines. He separated the normal glycosides of spirostanol and solanidane from S. nigrum and S. lyratum [1,2]. He wondered why such steroid glycosides were effective against tumors.
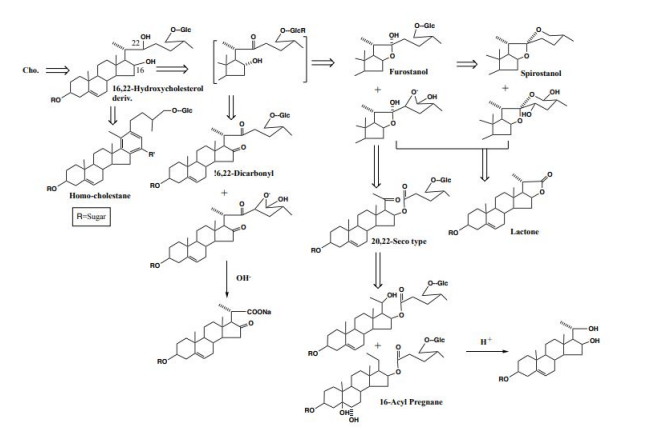
In addition to these medicinal herbs, he and his collaborators immediately began cultivating many other Solanum plants in collaboration with the Ministry of Agriculture, Forestry and Fisheries at the Herb Garden attached to Kumamoto University, they searched for steroid ingredients and obtained many steroid glycosides [3] as shown in Figure 1. Furthermore, they discovered 16,22-hydroxycholesterol derivative [4], which was considered to be a precursor to spirostanol and furostanol, and 16-acyl pregnane compounds [4,5]. In particular, the presence of the latter pregnane derivatives attracted considerable attention, and it was speculated that spirostanol and furostanol might be metabolized to pregnane and exert physiological activity as a steroid hormone. They thought this was a theme that should be considered for further development in the future.
Isolation and structure of Tomato Saponin, Esculeoside A
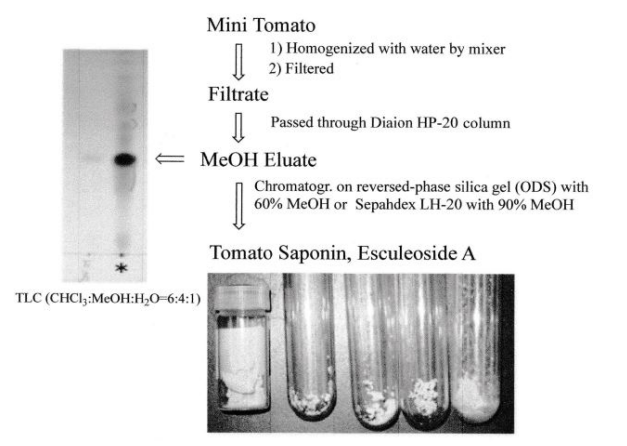
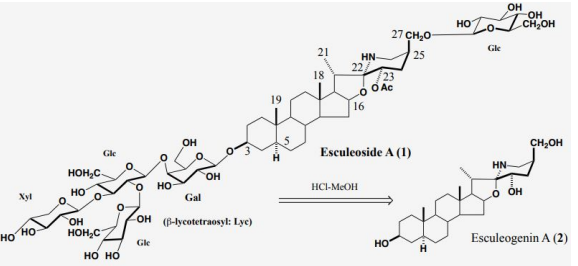
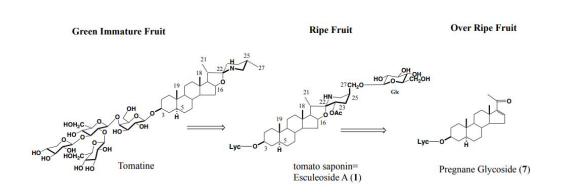
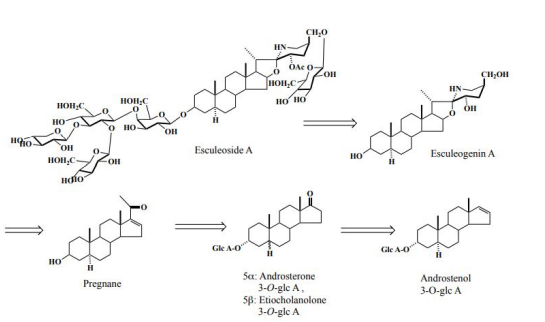
In the future, in order to carry out in vivo tests using laboratory animals and metabolic experiments in humans, it was necessary to obtain large amounts of steroid saponins from everyday foods. We then turned our attention to the tomato (Solanum lycopersicum L.), which had recently been reclassified from the Tomato genus (Lycopersi-con esculentum Mill.) to the Solanum genus. Although it was known that immature fruits contained a steroid saponin called tomatine, it was thought that mature tomatoes no longer contained steroid saponins in both Europe and America. We wondered if tomatine, which was present in large quantities in fruits and leaves, was metabolized by enzymes and converted into other forms when it matured, and could not be extracted using methanol, the normal method. To search for the ingredients of these tomatoes, we usually extracted with methanol, but we extracted with water. A simple procedure, namely, smashing the tomato by hand in water, followed by filtration, gave the filtrate. The filtrate was then subjected to high porous polystyrene gel (Diaion) column chromatography. It was first eluted with water, followed by methanol. The methanolic eluate was subsequently subjected to reversed silica gel column chromatography to yield a major tomato saponin, named as Esculeoside A (1) as colorless needles. Crystals weighing 440 mg were obtained from 1.78 kg of the commercial ripe mini (cherry) tomatoes. From 7.53 kg of Momotaro tomatoes, 331 mg of the same compound was also obtained (Figure 2). Using the above method of extracting raw mini tomatoes, middy tomatoes, and Momotaro tomatoes with water, it was found that mini tomatoes and middy tomatoes contained 3 to 5 times more Escleoside A (1) than Momotaro tomatoes. Also, mini tomatoes contained 5-8 mg/100g of lycopene, Esculeoside A (1) was about 5 times more than lycopene. One mini tomato (av. 13.33 g/one) contained about 3.3 mg of Esculeoside A (1). The structure of esculeoside A (1) was determined as 3-O-β-lycotetraosyl- (5α,22S,23S,25S)-23-acetoxy-3β,27-dihydroxyspirosolane 27-O- β-D-glucopyranoside [6,7] (Figure 3).
Finding of Esculeoside B in Tomatoes Can and Juice
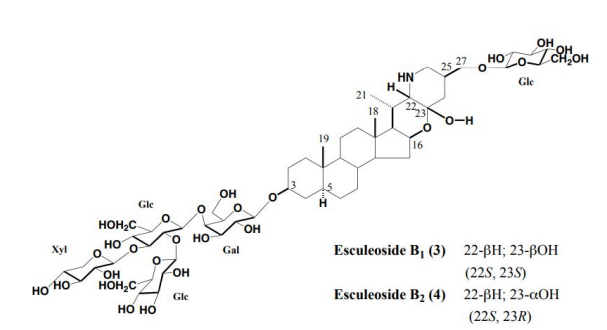
From the canned tomatoes and juice, newly named Esculeoside B1 (3) and Esculeoside B2 (4) were isolated (yields 0.0053, 0.007 %, respectively). Their structures were determined as 3-O-β-lycotetraosyl (5α, 22S, 23S and 5α, 22S, 23R)-22,26-epimino16b,23-epoxy-3β,23,27-trihydroxycholestane 27-O-β-D-glucopyranoside compounds (3, 4) [7-9] (Figure 4). It seems that the canned tomatoes and juice sold in Japan are made in Italy and other countries. When they were canned or bottled, they were heat sterilized, and they appeared that the change from Esculeoside A (1) to Esculeoside B1 (3) and Esculeoside B2 (4) occurred during that process. Actually, Esculeoside A was converted into Esculeosides B1 and B2 by refluxing with water for 6 hours. [10,11] (Figure 5).
Seasonal Changes of Tomato Saponin
We have isolated (5α)-pregna-16-en-3β-ol-20-one 3-O-β-lycotetraoside (7) as a minor component from the over-ripe tomato fruit [12,13]. This indicates that the type of steroidal glycoside varies as the tomato matures, that is, tomatine in the green immature fruit is oxidized at C-23 and at C-27 in the ripe fruit to give Esculeoside A (1). Further, Esculeoside A (1) is converted into the pregnane glycoside in the over-ripe fruit [14] (Figure 6).
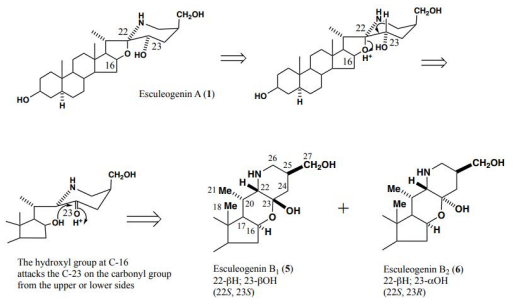
Chemical Conversion of Esculeogenin A into Pregnane Derivative
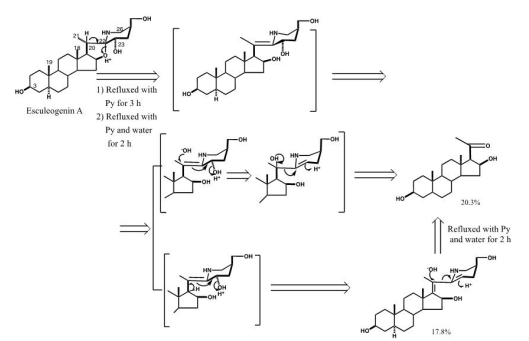
Next, Esculeogenin A (2) was converted into a pregnane derivative by refluxing with aqueous pyridine. This reaction was unexpected as it suggested the presence of a hydroxyl group at C-23 that makes the E, F-ring very fragile, thereby leading to bond fission between C-20 and C-22 to afford a pregnane derivative. The mechanism for this reaction was speculated to be as follows: i) at first, protonation occurred to the 16-C-oxygen, ii) it pulled the electrons between the 16-O and C-22 positions, thus creating a double bond between the C-20 proton and C-22 position, iii) next, H2O was added to the double bond, namely HO- attached to C-20, and H+ to C-22, iv) and then the hydroxyl group at C-23 is dehydrated, along with that the bond between C-20 and C-22 was cleaved, and the proton of the hydroxyl group at C-20 was released, resulting in C20 carbonyl [15 ] (Figure 7).
Metabolic Experiment of Tomato Saponin
Eight males consumed tomatoes-2 kg per adult over a period of 2 days. Their urine was collected for 48 h and passed through a polystyrene gel (Diaion HP-20). The first eluate with water was discarded, and the second eluate with MeOH was collected. The methanolic residue (7.42 g) was subjected to Sephadex LH-20, silica gel. and ODS column chromatographies to afford three androstane derivatives [16]. These androsterone analogues are usually excreted in normal persons; however, since none of these excretions was detected in the control sample, the occurrence of androsterone analogues indicates that they would be excreted via the production of progesterone by those that consumed tomatoes, that is, the tomato steroidal glycoside might stimulate the hormone secretor or would itself be metabolized into the pregnane (Figure 8).
Esculeogenin A, a New Tomato Sapogenol, Ameliorates Hyperlipidemia and Atherosclerosis in ApoE-deficient Mice by Inhibiting ACAT
Fujiwara et al. examined the effects of Esculeoside A (1) and Esculeogenin A (2), a new aglycon of Esculeoside A (1), on foam cell formation in vitro and atherogenesis in apoE-deficient mice. Esculeogenin A (2) significantly inhibited the accumulation of cholesterol ester (CE) induced by acetylated low density lipoprotein (acety1-LDL) in human monocyte-derived macrophages (HMDM) in a dosedependent manner without inhibiting triglyceride accumulation, however, it did not inhibit the association of acetylLDL to the cells. Esculeogenin A (2) also inhibited CE formation in Chinese hamster ovary cel1s overexpressing acyl-- coenzymeA (CoA): cholesterol acyltransferase (ACAT)-1 or ACAT-2, suggesting that Esculeogenin A (2) suppresses the activity of both ACAT-1 and ACAT-2. Furthermore, Esculeogenin A (2) prevented the expression of ACAT-1 protein, whereas that of SR-A and SR-BI was not suppressed. Oral administration of Esculeoside A (1) to apoE-deficient mice significantly reduced the levels of serum cholesterol, triglycerides, LDL-cholesterol, and the areas of atherosclerotic lesions without any detectable side effects. Their study provides the first evidence that purified Esculeogenin A (2) significantly suppresses the activity of ACAT protein and leads to reduction of atherogenesis [17].
Since Esculegenin A (2) suppressed the foaming of macrophages, which is an early lesion of arteriosclerosis, in order to examine whether this compound actually suppresses arteriosclerosis in vivo, we conducted a study in hyperlipidemia model mice. They conducted animal experiments using apoE-deficient mice. It is known that after ingestion of glycosides contained in natural products, the sugars and sugar chains are cleaved by the action of intestinal bacteria, and the aglycones are absorbed into the body. Therefore, it was considered that Esculeoside A (1) was also converted to Esculeogenin A (2) and absorbed (Figure 9).
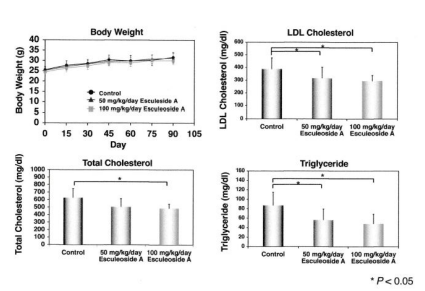
Therefore, in this experiment, Esculeoside A (1), which has the structure of a glycoside actually contained in tomatoes, was orally administered. First, apoE-deficient mice were divided into two groups: a control diet administration group and a 100 µg/kg/day Esculeoside A (1) administration group, and oral administration was performed for 90 days. Blood was collected after oral administration, and blood biochemical tests were performed every 15 days. No change in body weight was observed in all groups. On the other hand, total cholesterol levels were reduced by approximately 25% in the Esculeoside A-treated group compared to the control. Furthermore, LDL cholesterol levels and triglyceride levels were also significantly reduced in the Esculeoside A-treated group, by approximately 25% and 45%, respectively. In other words, these results suggested that administration of Esculeoside A (1) improves lipid metabolism in apoE-deficient mice (Figure 10).
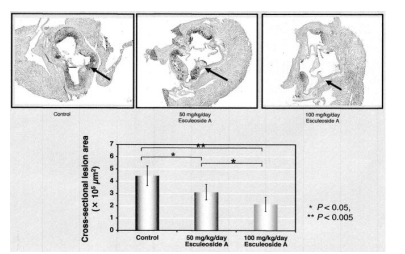
Furthermore, after the completion of oral administration, we pathologically analyzed the arteriosclerotic lesions at the root of the aorta in mice, and as shown in Figure 11, we found that the cross-section of the aorta in the Esculeoside A-treated group was significantly lower than that in the control group. The area of arteriosclerosis has decreased. These results revealed that Esculeoside A (1) inhibits arteriosclerosis even in animal experiments.
Anti-hyaluronidase Activity in vitro and Amelioration of Mice Experimental Dermatitis by Tomato Saponin, Esculeoside A and Esculeoside B
Allergic diseases, such as atopic dermatitis have an increasing incidence during recent decades, it is important to develop safe and effective agents for prevention of atopic diseases. Meanwhile, it is well known that hyaluronidase, an enzyme for hyaluronic acid degradation, is related to inflammation and allergic responses. The hyaluronidase inhibitory activity test is one of the screening methods for developing anti-allergy drugs. Zhou et al. firstly determined the effects of Esculeoside A (1) / Esculeoside B (3+4) and its aglycon Esculeogenin A (2) / Esculeogenin B (5+6) on mammalian hyaluronidase activity in vitro, and then investigated anti-allergy of Eesculeoside A (1) / Esculeoside B (3+4) in experimental dermatitis models.
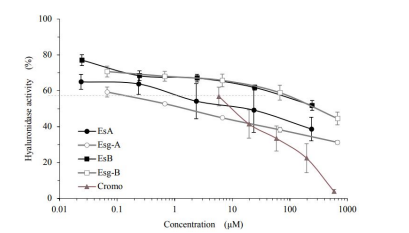
As shown in Figure 12, Esculeoside A (1) / Esculeogenin A (2) / Esculeoside B (3+4) / Esculeogenin B (5+6) inhibited hyaluronidase activity with a concentration-dependent manner, and also indicating that the inhibitory effect of Esculeogenin A (2) on hyaluronidase activity is stronger than that of cromoglycate, a known anti-allergy agent. Moreover, we also have reported that the effect on hyaluronidase by Esculeoside A (1) is a mode of competitive inhibition [18-20].
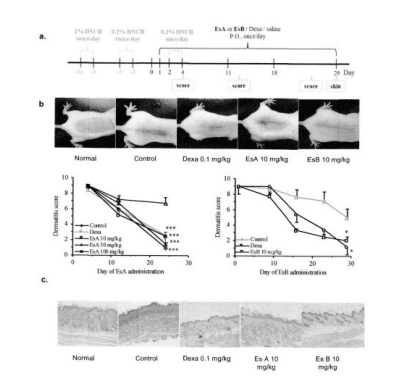
To evaluate on the possible effects of Esculeoside A (1) / Esculeoside B (3+4) on ameliorating atopic dermatitis, experimental dermatitis was induced by 2, 4- dinitrochlorobenzene (DNCB) for sensitize and challenge on mice dorsal skin, and then Esculeoside A(1) / Esculeoside B (3+4) was administrated orally every day for 4 weeks. As shown in Figure 13, Esculeoside A (1) / Esculeoside B (3+4) treatment significantly decreased the skin clinical score without any detectable side effects. In the end the dorsal skin were collected for histopathological analysis. Histological evaluation showed hypertrophy, hyperkeratosis of the epidermis and inflammatory cells infiltration in the control group, and the treatment with Esculeoside A (1) or Esculeoside B (3+4) decreased significantly epidermal and dermal thickening and inflammatory cell infiltration [18,21].
Meanwhile, it has been reported that treatment of human keratinocyte cell with DNCB induced a significant degradation of hyaluronic acid at the pericellular matrix of keratinocyte cell, and it would be correlated to an upregulation of hyaluronidase [8]. Accordingly, it is speculated that the inhibition of hyaluronidase activity by tomato saponin and its aglycon may partly contribute to tomato saponin-- mediated alleviation of experimental dermatitis. In future study, it will be important to evaluate the effect of tomato saponin on hyaluronidase in vivo.
Conclusion
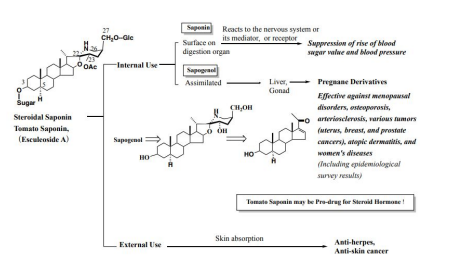
In summation, the use of steroidal glycosides is classified into internal and external uses. Regarding the former, there are two cases: one is action on the surface of the digestive tract, and the other is action after assimilation and metabolism. Unassimilated steroidal glycoside acts on the nervous system or its mediator or receptors to suppress the rise in blood sugar levels. On the other hand, the assimilated glycoside is first metabolized into C-23-hydroxylated spirostane or furostane, and further into pregnane derivatives, which demonstrate various bio-activities. It is possibly effective against menopausal disorders, osteoporosis, arteriosclerosis, various tumors (uterine cancer, breast cancer, prostate cancer), atopic dermatitis, and women's diseases (Including epidemiological survey results). In external use. steroidal glycoside is absorbed via the skin and demonstrates anti-herpes and anti-skin-cancer activities as mentioned before (Figure 14). Steroidal glycosides are regarded as natural pro-drugs of steroidal hormone.
Conflict of Interest
The authors declare no conflict of interest.
- Saijo R. Murakami K, Nohara T, Tomirnatsu T. Sato A. Matsuoka K (1982) Studies on the constituents of Solanum plants. II. On the constituents of the immature berries of Solanum nigrum L. Yakugaku Zasshi. 102: 300-5.
- Murakami K, Ejima H, Takaishi Y, Takeda Y, Fujita T, Sato A, Nagayama Y, Nohara T (1985) Studies on the constituents of Solanum plants. V. The constituents of S. lyratum Thunb. II. Chem Pharm Bull, 33: 67-73.
- Nohara T, Ikeda T, Fujiwara Y, Matsushita S, Noguchi E, Yoshimitsu H, Ono M (2007) Physiological functions of solanaceous and tomato steroidal glycosides. J Nat Med. 61: 1-13.
- Zhu X, Tsumagari H, Honbu T, Ikeda T, Ono H, Nohara T (2001) Peculiar steroidal saponins with opened E-ring from Solanum genera plants. Tetrahedron Lett. 42: 8043-6.
- Ikeda T, Tsumagari H, Okawa M, Nohara T (2004) Pregnane- and furostane-type oiigoglycosides from the seeds of Allium tuberosum. Chem Pharm Bull, 52: 142-5.
- Fujiwara Y, Yahara S, Ikeda T, Ono M, Nohara T (2003) Cytotoxic major saponin from tomato fruits. Chem Pharm Bull, 51: 234-5.
- Fujiwara Y, Takaki A, Uehara Y, Ikeda T, Okawa M, Yamauchi K, Ono M, Yoshimitsu H, Nohara T (2004) Tomato steroidal alkaloid glycosides, esculeosides A and B, from ripe fruits. Tetrahedron, 60: 4915-1820.
- Manabe H, Fujiwara Y, Ikeda T, Ono M, Murakami K, Zhou JR, Yokomizo K, Nohara T (2013) Saponins esculeosides B-1 and B-2 in Italian canned tomatoes. Chem Pharm Bull 61: 764-7.
- Nohara T, Fujiwara Y, Zhou JR, Urata J, Ikeda T, Murakami K, El-Aasr M, Ono M (2015) Saponins, esculeosides B-1 and B-2, in tomato juice and sapogenol, esculeogenin B1. Chem Pharm Bull, 63: 848-50.
- Yoshizaki M, Matsushita S, Fujiwara H, Ono M, Nohara T (2005) Tomato new sapogenols, isoesculeogenin A and esculeogenin B. Chem Pharm Bull, 53: 839-40.
- EL-Aasr M, Oshiro Y, Fujiwara Y, Miyashita H, Ikeda T, Ono M, Yoshimitsu H, Nohara T (2008) Conversion of esculeoside A into esculeogenin B. Chem Pharm Bull, 56: 926-9.
- Fujiwara Y, Yoshizaki M, Matsushita M, Yahara S, Yae E, Ikeda T, Ono M, Nohara T (2005) A new tomato pregnane glycoside from overripe fruits. Chem Pharm Bull, 53:580-5.
- Nohara T, Iwakawa E, Matsushita S, Fujiwara Y, Ikeda T, Miyashita H, Ono M, Yoshimitsu H (2008) A pregnane glycoside from over ripe tomato. Chem Pharm Bull, 56: 1013-4.
- Manabe H, Murakami Y, EL-Aasr M, Ikeda T, Fujiwara Y, Ono M, Nohara T, (2011) Content variations of tomato-saponin, esculeoside A, in various processed tomatoes. J Nat Med. 65:176-9.
- Matsushita S, Yoshizaki M, Fujiwara Y, Ikeda T, Ono M, Okawara T, Nohara T (2005) Facile conversion of 23-hydroxyspirosolane into pregnane. Tetrahedron Lett. 46: 3549-51.
- Noguchi E, Fujiwara Y, Matsushita S, Ikeda T, Ono M, Nohara T (2006) Metabolism of tomato steroidal glycosides in human. Chem Pharm Bull, 54: 1312-4.
- Fujiwara Y, Kiyota N, Hori M, Matsushita S, Iijima Y, Aoki K, Shibata D, Takeya M, Ikeda T, Nohara T, Nagai R (2007) Esculeogenin A, a new tomato sapogenol, ameliorates hyperlipidemia and atherosclerosis in apoE-deficient mice by inhibiting ACAT. Arterioscler Thromb Vasc Biol: 27: 2400-6.
- Zhou JR, Kanda Y, Tanaka A, Manabe H, Nohara T, Yokomizo K (2016) Anti hyaluronidase activity in vitro and Amelioration of mouse experimental dermatitis by tomato saponin, esculeoside A. J Agric Food Chem. 64: 403-8.
- Zhou JR, Kitahara N, Nakamura H, Ono T, Karashima R, Fang J, Nohara T, Yokomizo K (2022) Decrease of hyaluronidase activity and suppression of mouse CD4+ T lymphocyte activation by tomato juice saponin esculeoside B, and its sapogenol esculeogenin B. J Pers Med: 12: 579.
- Zhou JR, Kimura S, Nohara T, Yokomizo K (2018) Competitive Inhibition of Mammalian Hyaluronidase by Tomato Saponin, Esculeoside A. Nat Prod Communic: 13: 1461-3.
- Zhou JR, Urata J, Shiraishi T, Tanaka C, Nohara T, Yokomizo K (2018) Tomato juice saponin, esculeoside B ameliorates mice experimental dermatitis. Funct Foods in Health & Dis: 8: 228-41.


Figures at a glance