Spectral Search of Azulene-Containing Plants of Temperate Climate as Possible New Biological Resources for Pharmacy
Received Date: November 14, 2024 Accepted Date: December 14, 2024 Published Date: December 17, 2024
Citation: Victoria V. Roshchina, Mikhail M. Shovkun, Vasilii E. Demidov (2024) Spectral Search of Azulene-Containing Plants of Temperate Climate as Possible New Biological Resources for Pharmacy. J Med Plant Herbs 3: 1-14
doi: 10.17303/jmph.2024.3.304
Abstract
Spectral methods of cell analysis, including microspectrophotometry and spectrophotometry, were used to study the phenomenon of “leaf blue color” for the search of new herbal resources for pharmacy. The seasonal appearance and accumulation of azulenes, starting with the early development of intact leaves of eighteen plant species Adoxa moschtellina L., Allium oleraceum L., Anthriscus sylvestris L., Artemisia campestris L., Centaurea phrygia L., Centaurea scabiosa L., Euphorbia virgata Waldst. et Kit., Festuca pratensis L., Filipendula vulgaris Hill., Galium verum L., Hylotelephium maximum L. (Holub.), Phlomoides (Phlomis) tuberosa L., Saponaria officinalis L., Scabiosa ochroleuca L., Sedum acre L., Tanacetum vulgare L., Thalictrum minor (minus) L. have been estimated. The presence of azulenes was based on the appearance of blue color and characteristic maxima of 580-640 nm in the absorption spectra and 405-430 nm in the fluorescence spectra. This was confirmed in experiments with extracts of these hydrophobic pigments with acetone or ethanol solvents, respectively, from the surface of the leaves and from the inside. The results obtained may be of interest as an spectral approach to search new plant resources of azulenes for pharmacy.
Keywords: Absorption; Azulenes; Blue Coloration; Fluorescence; Spectra; Extracts from Leaves
Introduction
When observing the appearance of blue or silver coloration of leaves of some plants, it was supposed that this may be due to the presence of blue azulene pigments that it has the interest for pharmacology [1,2]. This phenomenon has not been studied before, as well as possible function of these pigments for the plants themselves. Today, azulenes, known with the application in cosmetics and partly in medicinal care [3,4] or industrial use [4] as antioxidants, found in some medicinal plants and isolated by distillation of essential oils. It should be noted that their chemical characterization based on the known structure of synthetic azulene has also been carried out for a very small number of plants [5,6]. It has recently been suggested that azulenes, as powerful antioxidants, can participate in protecting the plant surface from damaging effects [7,8]. Plants secrete these natural compounds in liquid form or are deposited on the surface, and the surface of plant cells is sensitive to changes in ultraviolet radiation, tropospheric ozone concentration, salinity, traumatic factors, etc. [7].
Observations in natural conditions have shown that the blue or silver color of the leaves can be either constant or noticeable only in spring. This problem is important both for the ecological monitoring of these compounds and for the search of the resources of natural azulenes with medicinal properties. It should mark that earlier nobody linked blue leaf color with concrete pigments. However, assortment of the azulene-containing plants is narrow, and analysis of the occurrence of azulenes is rare and very expensive. The task of present work to test plant species with blue or silver color leaves for the presence of azulenes with spectral methods that fast and does not needed big finances. We suppose that it may help to find new azulene-containing species as possible biological resources for pharmacy.
Materials and Methods
Objects
The leaves of plant species, common for temperate climates and having blue or silver leaf color in spring (Table 1), taken in April-August 2024 year in the Prioksko-Terrasny Nature Reserve and small reservations as the Lugovoi and Karst in the vicinity of the city of Pushchino, Moscow region, were collected as objects of research.
Spectral Measurements of Intact Leaves
Absorption (absorbance) of the intact leaves was measured directly on slides using the microspectrophotometer/microspectrofluorimeter MSF-15 (LOMO, St. Petersburg, Russia) [8-10].The position of the maxima in the absorption spectra of intact cell surfaces was determined according to the Zolotarev method by the option of the reflection spectra differentiation [11]. Limitation in the sensitivity of spectrophotometry in the analysis depends on the apparatus and the blue pigment concentration of the leaf. Microspectrophotometer with Zolotarev method [11] of the small maxima spectra permits to see the absorbance maxima with optical density about 0.05 units. However, if blue leaf color intensive, one could see characteristic maxima with higher optical density by usual spectrophotometry of whole intact leaf.
Spectral Measurements of Leaf Extracts
The absorption and fluorescence spectra of extracts with 100% acetone or 95% ethanol from cells (1:10 w/v for 10 min to 1 hour or more) in 1-0.5 cm cuvettes were recorded using the Unicam Helios-epsilon spectrophotometer (USA), spectrophotometer Specord M-40 (Germany) and Perkin Elmer 350 MPF-44 B spectrofluorometer (Great Britain). Although azulenes were received earlier by distillation of essential oils, mainly from Artemisia, Achillea, Matricaria species, and in physics used very hydrophobic medium like hexane or cyclohexane, etc., our choose of solvents for the extraction for our aim was explained by natural metabolites of plants as solvents. Azulenes also may be dissolved in ethanol because the reagents are used for the medicinal infusions. The concentration of azulenes (A) was estimated in the ethanol or acetone extracts according to the [7,8]. The results were expressed as an average value of ±SEM. For each variant, the average error of the experiment was calculated with three to four repetitions. The relative standard deviation was 5-6% (n=3-4 samples per species), P=0.95.
Results and Discussion
Common View of Plants Studied
According to earlier data with woody plants [7], the choose of species for the testing of azulenes should look for among herbs with blue or silver color. Figure 1 shows clear blue color in similar plants. At the beginning of experiments with young seedlings, they demonstrated it. We continued the observation and collected probes for the azulenes’ testing from April up to July 2024, period when the plants started to die off. Most intensive blue color in April was for Allium oleraceum, Artemisia campesrtris, Hylotelephium maximum and Saponaria officinalis. Brightly silver tone was characteristic for leaves of Phlomoides (Phlomis) tuberosa.
After photography, we studied our leaf probes in the following order : 1. receiving of the absorbance spectra of intact leaves for the presence of the characteristic maxima of natural azulenes in the region 540-640 nm, according protocol published earlier [7]; 2. The short 10 min- or 24 h max- extraction of azulenes by organic solvents (w/v) such as acetone or ethanol; 3. Receiving the absorbance and fluorescence maxima of the extracts to observe possible maxima of azulenes.
Spectral Measurements on Intact Leaf Surfaces
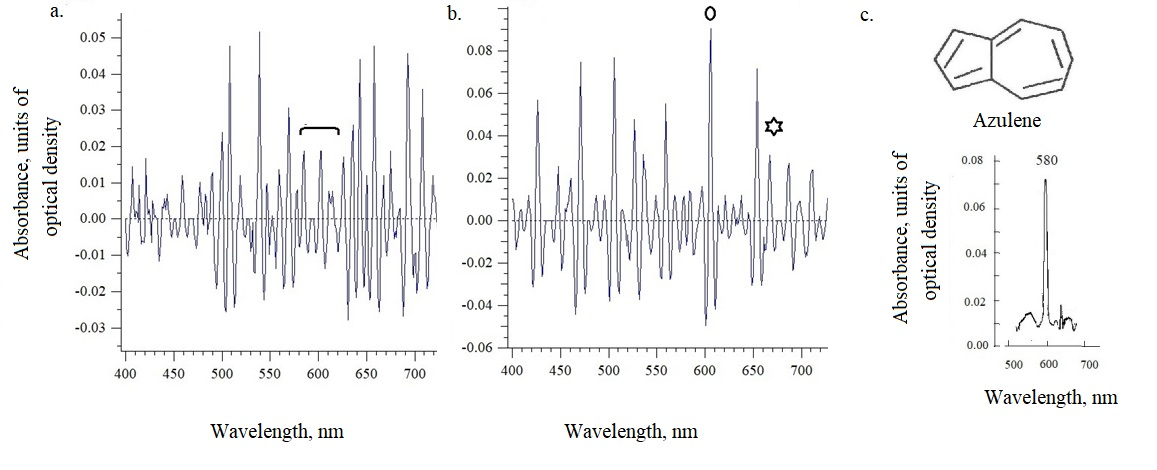
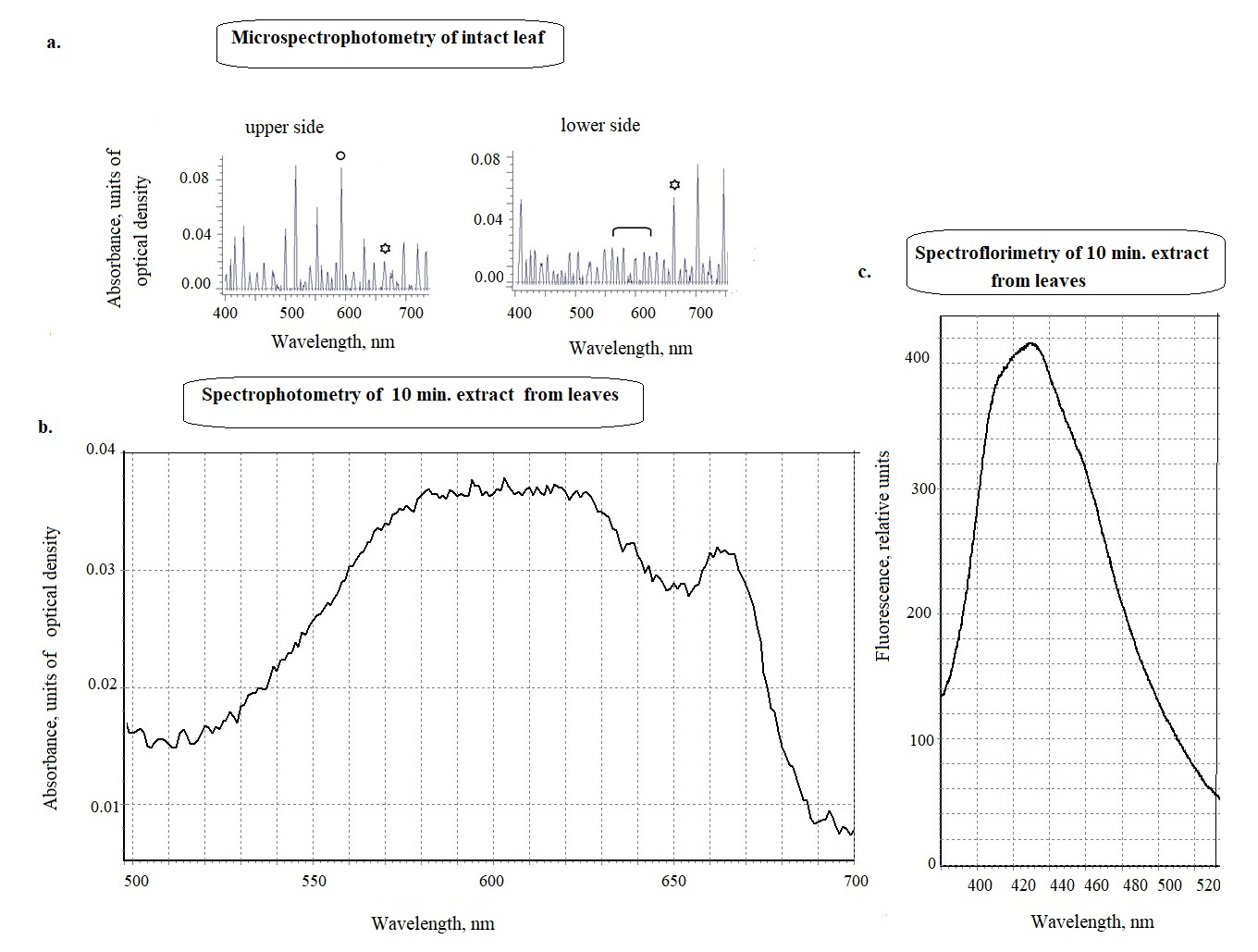
The absorption (absorbance) of the intact leaves was measured directly on slides using the microspectrophotometer/microspectrofluorimeter MSF-15 (LOMO, St. Petersburg, Russia) [10]. The position of the maxima in the absorption spectra of intact cell surfaces was determined according to the Zolotarev method by the option of the reflection spectra differentiation [11]. The first derivative of the external reflection spectrum for low-intensity bands made it possible to directly determine the presence in the spectra after differentiation the peaks characteristic for azulenes and chlorophyll of 580-620 nm and 660-666 nm, respectively (Figure 2 and 3). As seen on the example Figure 2, to identify the maxima in the 550-670 nm region, it was enough to provide information with positive values (at the top of the graph), and then we used only similar images for other samples (see following example on Figure 3a for Centaurea phrygia). Which is done further for all other data, according to the work [2]. Classical synthetic azulene crystal has maximum 580 nm (с), while, according natural azulenes have more wider range of the absorbtion maxima - from 550 up to 650 nm in a dependence on the solvent (Figure 2 shows the differences between upper and lower of the Adoxa leaf). There are no maxima characteristic for azulenes on upper side, while the lower side has such a maximum of 600 nm. The same microspectrometry method shows another, opposite example for Centaurea phrygia (Figure 3a) - where there is a maximum of 590 nm at the upper side of the leaf. The lower part of the leaf of this plant is quite green, since the large maximum absorption of chlorophyll, unlike the upper part, where the green pigment is masked by a blue pigment. The absorption maxima found in the spectra of all leaves studied in our work were observed in the region of 580-640 nm, they are presented further in Table 2.
As can be seen from Table 2, the maximum optical density value of 0.07- 0.09, measured using microspectrophotometry, was generally noted for Adoxa moschtellina, Centaurea phrygia, Euphorbia virgata, Saponaria officinalis, and the lowest value of about 0.05 was for Allium oleracium and Fescue pratensis. The largest amount of absorption was recorded in April for Adoxa, Fescue, Saponaria– the beginning of intense ultraviolet insolation. In May-June, when maximum similar illumination is observed, almost all other species have an increase in optical density in the azulene region. Shifts of maxima in the azulene region are also noted. For example, in musk weed Adoxa position of maxima varied from 606 nm in April to 580-595 nm in June. It is possible that these are azulenes with different chemical structures. Most often, the classic maximum of azulenes of 580-585 nm is noted for Adoxa, Anthriscus sylvestris, Centaurea scabiosa and Filipendula vulgaris. For other studied species, peaks are observed in the longer wavelength range 590-610 nm. This region is peculiar for phenylazulenes [1].
Spectral Analysis of azulenes in the Leaf Extracts
To confirm the data of microspectrophotometry, blue pigment extraction with organic solvents was done. The absorption and fluorescence spectra of extracts with 100% acetone or 95% ethanol from cells (1:10 by fresh weight for 10 min or 24 h) .To make sure that the absorption maxima of intact leaves really reflect the presence of azulenes in the studied plants, ethanol and acetone extracts from intact leaf leaves were obtained. As it was shown earlier on the leaves of subtropical plants [1,2], azulenes in cells can be localized on the surface mainly in the cuticle, and leachates of intact leaves for 5-10 minutes are extracts including blue pigments of the surface. Longer extraction from intact samples led to the appearance of chlorophyll in the extracts. This indicated that the solvent had penetrated into the cell to the chloroplasts. Figure 3b shows that Centaurea phrygia has a clear maximum 600 nm in 10-minute ethanol extracts, characteristic for azulenes, as microspectrophotometry showed earlier for woody plants containing similar blue pigments. Small peak of 666 nm relates to chlorophyll. The fluorescence spectrum shows a maximum of 420-430 nm, as in pure azulene [1,2]. The blue pigments of intact extracts do not change color in an acidic environment, like anthocyanins, and therefore may belong to azulenes. Thus, microphotometric data for whole leaves have been confirmed for extracts as well. Then azulene concentrations were subsequently determined based on the maxima of the absorbance spectra of the extracts.
Azulene Content in Extracts in the Dependence on the Season
Two fractions of extracts were analyzed – 1. Extracts (leachates) from intact leaves for 10 minutes, where the outer layer of azulenes is washed off from the surface [1,2] and prolonged infusions for 24 or 48 hours, when intracellular azulenes can be extracted [2].
Min Extracts
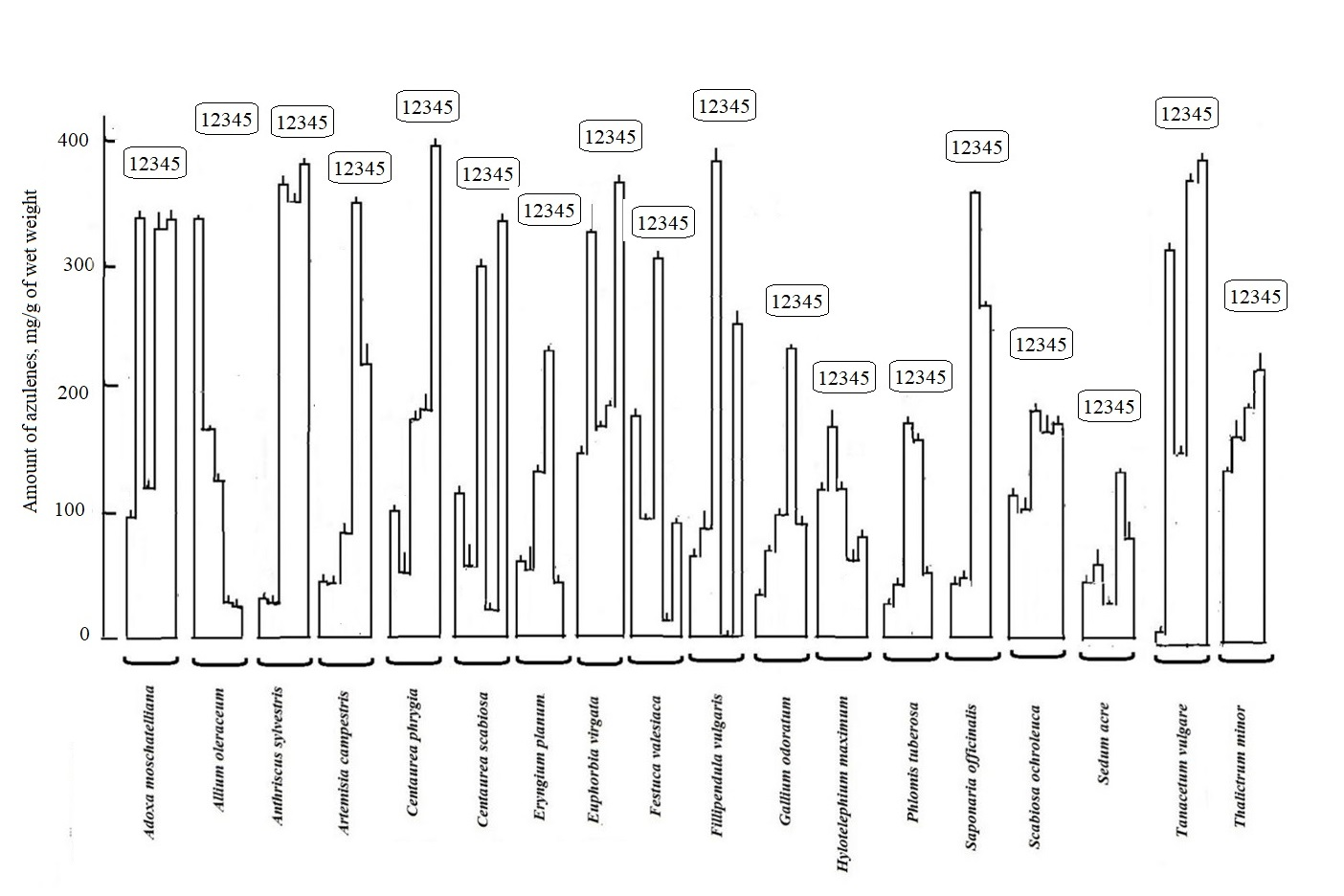
Figure 4 shows that the content of azulenes in the 10-minute fraction can be divided into three types: 1. with maximal amount of pigments (mg/g of fresh mass), 2- with an average amount and 3- with a small amount. In April, the first group of plants contains azulenes in the range of 120-300 mg/g of fresh mass. It looks like this Allium oleraceum > Festuca pratensis > Euphorbia virgate > Centaurea phrygia > Hylotelephilium > Scabiosa. Allium and Festuca have the most pigments within this group of plants. In the second group, the concentration range of blue pigments is 50-100 mg/g of fresh mass in this order: Adoxa moschtellina > Eryngium > Filipendula vulgaris > Artemisia campestris. In the third group with a minimal initial content of 10-50 mg/g of fresh mass, such a ratio was observed among Sedum acre= Artemisia absinthium = Gallium = Saponaria > Anthriscus sylvestris = Phlomoides > Tanacetum vulgare species. The content of azulenes in the 10 min extracts shows that blue pigments are contained precisely in the surface layer of cells – in the cell wall and, possibly, in secretory cells (for example, in milkweed Euphorbia virgata).
If we compare the number of azulenes at the beginning of the appearance of plant leaves in the Prioksko-Terrasny Reserve (April 13) and the end of April (April 29). It is noticeable that among a number of samples by the end of April there was the increase in blue pigments (Adoxa, Euphorbia, Hylotelephium, Filipendula, Gallium, Phlomoides) to varying degrees, and in species Allium, Centaurea, Festuca, Scabiosa, on the contrary, a decrease is visible. In the species Anthriscus, Artemisia, Sedum, the initial number (from April 13, experience) was practically preserved on April 29 at the same level. In a number of studied species (Adoxa, Allium, Centaurea, Artemisia, Hylotelephium), the initial content in such extracts (from April 13) decreased to April 29.
The phenomenon of the appearance of blue coloration may be associated with the time of the most intense ultraviolet radiation and the appearance of high values of ozone as sources of reactive oxygen species, since azulenes have antioxidant properties [3,4]. They protect cell integrity from the damaging oxidative effects of reactive oxygen species.
The further development of the leaves in May and June led to the fact that the initial (April) amount of blue pigments in the surface layer (extracts for 10 minutes) consistently decreased in Allium by almost 300 times compared to the initial level in April. In other species, for the most part, an increase in the concentration of blue pigments was noted until about the end of May, and then either the number of blue pigments stabilized (with fluctuations in the samples) at about the same level by the beginning of June, the maximum number of blue pigments was reached, which then begins to decrease. The presence of azulenes as antioxidants on the surface of leaves is important not only as protection against reactive oxygen species formed under the influence of ultraviolet radiation and ozone [1,2], but also in chemical relationships in the biocenosis [12]. If the plant is under stress, then stressful biogenic amines – dopamine and histamine - appear on the surface, and in this case the role of azulenes as a factor reducing the negative effects of these compounds is also noted [2]. The reduction by artificial synthesized azulene also was salt stress [12].
24-hour Extracts
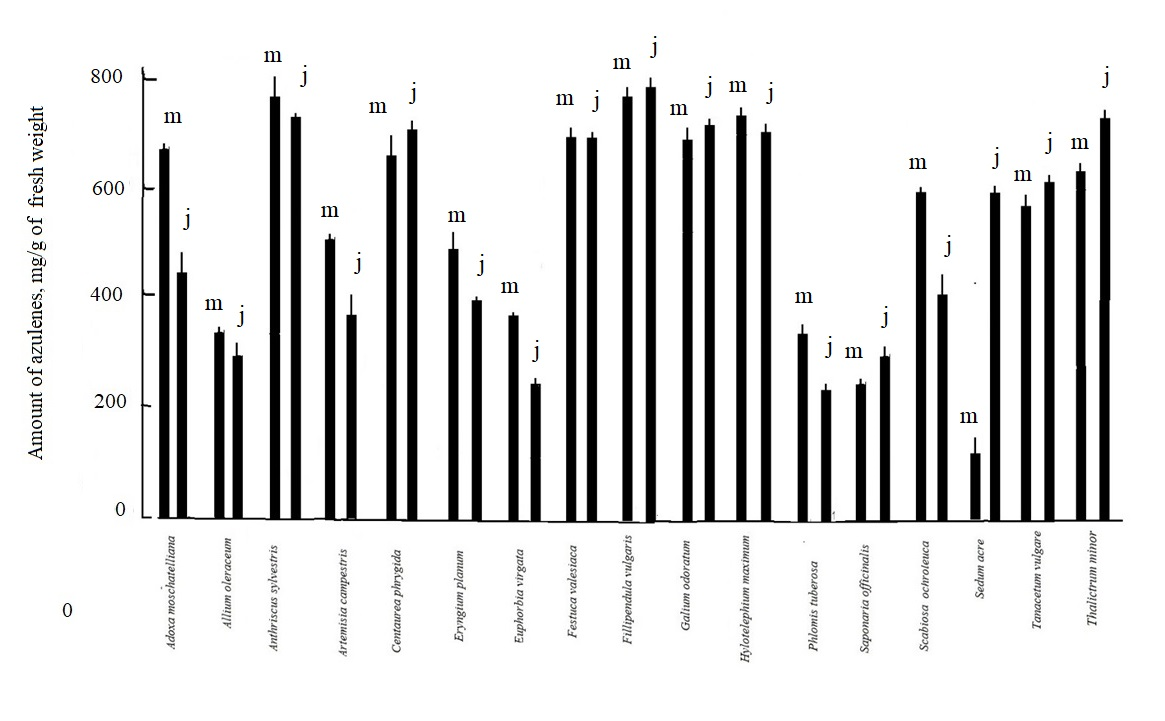
Let's turn now to the samples with a longer extraction time - of azulenes in 24 hours. According to these data, it is possible to estimate the intracellular concentration of azulenes. As seen on Figure 5, especially rich in azulenes inside cells (24-hour extracts) from the studied species are Adoxa moschtellina, Centaurea phrygia, Anthriscus sylvestris, Tanacetum vulgare, Scabiosa ochroleuca, Filipendula vulgare. It is significant that in all studied plants in June, compared with May, the concentration of these pigments either decreases or remains approximately at the same level. Only two species, Saponaria officinalis and Sedum acre, have an increased amount of pigments in June. Further experiments with extracts in July and August did not reveal any increase in azulene concentrations compared to June or noted a drop associated with plant wilt. However, as we can see, there is more significant amount of blue pigments inside the cells in spring. Figure 5 shows the results for the samples in May-June, when the insolation is especially high. By June, the number of azulenes decreases to varying degrees depending on the species in Adoxa moschtellina, Anthriscus sylvestris, Eryngium planum, Euphorbia virgata, Saponaria officinalis, Phlomis tuberosa. However, in the case of Sedum acre, on the contrary, this value increases markedly, as well as in 10 minute extracts. Other species have small fluctuations compared to May.
However, as we can see, there are more significant amount of blue pigments inside the cells in spring. Figure 5 shows the results for the samples in May-June, when the insolation is especially high. By June, the number of azulenes decreases to varying degrees depending on the species in Adoxa moschtellina, Anthriscus sylvestris, Eryngium planum, Euphorbia virgata, Saponaria officinalis, Phlomis tuberosa. In the case of Sedum acre, on the contrary, this value increases markedly, as well as in 10 minute extracts. Other species have small fluctuations compared to May.
Thus, our plants with blue colored leaves have azulenes not only on the outside of the cells, but inside too.
The first steps towards localization of azulenes within cells were taken for isolated chloroplasts of three clover species, where they were found in significant quantities [14]. It has also been shown that azulenes can be electron donors in the electron transport chain of photosynthesis [8,12,15]. Perhaps this is the protective role of azulenes, especially in case of damage to the electron transport chain. The presence of azulenes in other parts and organelles of the cell cannot be excluded, which requires special study.
Common Look on the Problem
Ecological significance of azulenes in biocenosis and in Nature as a whole never considered before. Main attention paid to earlier folk application of few azulene-containing plant species [1], use in cosmetics and partly in medicine [3] and to the significance as antioxidants for artificial synthesis of new azulenes for various industrial aims [4]. Unlike above-mentioned things, we based on the observation and fixation of “blue leaf phenomenon” in Nature. First phenomenon was marked visually in Russia on woody plants of Caucasian flora [7,12], herbs and woody plants of Crimea and central steppe regions [9,10]. Then the attention paid to the registration of the maxima in the intact leaf absorption spectra and the short and long extraction of blue pigments from the leaves [7,15]. The estimation of significance of azulenes was spread to known antioxidant features of azulenes. Parallelly, there were experiments with the influence of azulene on the plant cells surface under oxidants like ozone [7] and dopamine [8]. Ozone-tolerant species have blue color of leaves, having azulene maxima in their extracts [7]. The blue color decreased or missed under ozone exposure or direct interaction of synthetic azulene with dopamine. At the beginning of the studies of protectory antioxidant effects of azulenes on plant [10,13] and animal [16] model cells have been they undergone the stress. As a whole, we saw positive action. For pharmacy, it is significant to the know about new perspective azulene-containing species and the best time for their collection in a dependence on seasonal accumulation of azulenes. Here the visual estimation of the species with blue leaf color and spectral technique for the confirmation of the azulene presence both in intact surface (fast first selection of rue material) and in the extracts (secondary selection of rue material). Future studies of the azulene-containing plant species may be useful for Pharmacy.
Conclusion
Spectral methods of cell analysis made it possible to use blue color leaf phenomenon of herbs for search new perspective azulene resources for pharmacy. In temporary climate they permitted to observe seasonal appearance and accumulation of azulenes, starting from the early development of intact leaves, based on the appearance of characteristic coloration and characteristic peaks in the absorbance spectra. This has been confirmed in experiments with extractions of these hydrophobic pigments. The results obtained may be of interest not only for cellular monitoring of azulene-containing herbaceous plants, but also for the search for species useful for pharmacology in the future. Data on spectral analysis of plants with blue or silver coloration show that the accumulation of azulenes on the cell surface, possibly in the cuticle, occurs in April-May, when the highest ultraviolet insolation and the maximum formation of ground-level ozone. Then the appearance and accumulation of azulenes can be considered as a protection against the formation of reactive oxygen species. Spectral methods serves as fast and non-expensive tools for testing of presence of natural azulenes in intact leaves and their extractions by organic solvents.
Author Contributions
V.V.Roshchina, biochemist and biophysist, is the author of main conception, receiver of all experimental data, and she has written the paper. M.M.Shovkun, botanist of native reserve, photo, identifier and collector of plants and V.E.Demidov, vice-head of Prioksko-Terrasnyi Reserve, specialist inplant geography and collector of the plantprobes. All authors have read and agreed to the published version of the manuscript.
Funding
The author declare that no funds, grants, or other support were received during the preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors thank leading constructor/engineer–Alexander R. Kunyev for the help with the computer program, and engineers of Optical Department of Federal Scientific Center Lubov' M. Khaibulaeva and Nadezhda K. Prizova.
Conflicts of Interest
The authors declare no conflict of interest.
- Heilbronner E (1959) Azulenes. In: Non-Benzenoid Aromatic Compounds (Ed., D. Ginsburg). Interscience. Publ., London, p.171-276
- Konovalov DA (1995) Natural azulenes. Biological Resources (Biologicheskie Resursy, Russian) 31: 101-30
- Bakun P, Czarczynska-Goslinska B, Goslinski T, Lijewski S (2021) In vitro and in vivo biological activities of azulene derivatives with potential applications in medicine. Med Chem Res. 30: 834-46.
- Murfin LC, Lewis SE (2021) Azulene - a Bright core fore sensing and imaging. Molecules, 26: 353-62.
- Nakagawa S, Katoh K, Kusumi T, Komura H, Nomoto K, Konno H, Huneck S, Takeda R (1992) Two azulenes produced by liverwort, Calypogeia azurea, during in-vitro culture. Phytochemistry, 31: 1667-70
- Siegel U, Mues R, Dönig D, Eicher Th, Blechschmidt M, Becker H (1992) Ten azulenes from Plagiochila longispina and Calypogeia azurea. Phytochemistry, 31: 1671-8
- Roshchina VV, Kuchin AV, Kunyev AR, Soltani GA, Khaibulaeva LM, Prizova NK (2022) The presence of azulene on the surface of plant cells as a test for ozone sensitivity. Biochemistry (Moscow), Supplement ser A: Membrane and Cell (Biological Membranes). 16: 167-74.
- Roshchina VV, Yashin VA, Kynyev AR (2023) Study of the spectral characteristics of the plant cell surface: Occurrence of azulenes and biogenic amines. Biochemistry (Moscow), Supplement Series A: Membrane and Cell Biology, 17: 276-85.
- Roshchina VV, Kunyev AR, Fateryga VV, Shovkun MM (2023) Application of microspectrofluorimeter/ microspectrophotometer for the study of the surface of plant cells. Russian Journal of Biological Physics and Chemistry, 8: 137-42.
- Roshchina VV, Prizova NK, Khaibulaeva LM (2022) Azulenes of the leaf surface as a protective optical filter. RussianJournal of Biological Physics and Chemistry, 7: 36-9.
- Zolotarev VM (2012) Application of differentiation in reflection spectroscopy. Optics and Spectroscopy, 112: 150-4.
- Roshchina VV (2023) Plant leaf surface as a sensory system in allelopathic relations: 1. Role of azulenes. Allelopathy Journal., 59: 109-22.
- Roshchina VV, Konovalov DA (2022) Single cell plant model of Equisetum arvense for the study antihistamine effects of azulene and sesquiterpene lactones. Future Pharmacol, 2: 126-34.
- Roshchina VV (2024) Azulenes in Plant Cell: Clover as their useful resource . Annual Agricultural and Crop Sciences, 9: 02-7.
- Roshchina VV (2022) Possible role of azulenes in plant life: Experiments with Models. SMP Environmental Science and Technology, 1: 1-10
- Roshchina VV, Sergievich LA (2024) The study of the azulenes’ effects as protectory factors on the mouse vitality. Russian Journal of Biological Physics and Chemistry, 9: 168-73.


Tables at a glance
Figures at a glance