Some Vegetable Peels Have the Potential to Ameliorate Alloxan Induced Hyperglycemia in Male Mice
Received Date: February 02, 2025 Accepted Date: March 02, 2025 Published Date: March 05, 2025
doi:10.17303/jmph.2025.4.101
Citation: Yamini Dixit, Anand Kar (2025) Some Vegetable Peels Have the Potential to Ameliorate Alloxan Induced Hyperglycemia in Male Mice. J Med Plant Herbs 4: 1-11
Abstract
To examine the possible therapeutic value of some common vegetable peels, ethanolic extracts of Beta vulgaris, Daucus carota, Ipoema batatas, Pisum sativum and Raphanus sativus were evaluated for their potential in ameliorating hyperglycemia in mice. In a preliminary study, five separate experiments were conducted to examine their serum glucose and hepatic lipid peroxidation (LPO) lowering efficacy in normoglycemic mice. In the pilot experiment, the effective and safe dose of each peel extract was administered for 15 days in alloxan induced diabetic mice and the alterations in serum glucose, insulin, hepatic LPO, superoxide dismutase (SOD) and catalase (CAT) were evaluated. Peel extracts of I. Batatas and D. carota proved to be antihyperglycemic in nature as both could markedly reduce the serum glucose and hepatic LPO with an increase in SOD, CAT and serum insulin levels. These effects were well correlated with polyphenolic and flavonoids contents of the peel extracts.
Keywords: Alloxan; Insulin; Lipid Peroxidation; Mice; Vegetable Peels
Introduction
Diabetes mellitus is prevalent in all parts of the world and the number of patients suffering from this disorder is rapidly increasing. The global burden of diabetes is rising, with developing countries like India being significantly affected. This increase is primarily driven by higher rates of overweight/obesity and unhealthy lifestyles. In 2019, there were an estimated 77 million people with diabetes in India, a number expected to surpass 134 million by 2045. Alarmingly, about 57% of these individuals are undiagnosed [1]. It is estimated that approximately 3% of the population worldwide are affected by this disease [2]. For a long time, diabetics have been treated with the extracts of several medicinal plants based on the folklore medicine [3]. As synthetic antidiabetic drugs are often believed to produce side effects [4], the search for more effective and safer hypoglycemic agents has continued to be an important area of active research.
Fruits and vegetables are considered as rich source of natural antioxidants like polyphenols and flavonoids as well as dietary fibers. Therefore, consumption of fruits and vegetables are thought to be beneficial from health point of view [5]. Their peels are also gradually emerging as potential source of antioxidants with possession of rich amount of flavonoids, polyphenolics, ascorbic acid and dietary fibers [6-10]. In fact, many of the plant extracts are reported to be antiperoxidative in nature [11-13]. While lots of studies have been made on the medicinal values of herbs/plants in general, only limited investigations are there on the peels ameliorating diabetes mellitus/hyperglycemia. Although on fruit peels some recent reports indicate their possible hypoglycemic and anti-oxidative properties [14-17], on vegetable peels, scientific studies are mostly restricted to their anti-oxidative values [18-23]. On the anti-hyperglycemic potential of vegetable peels only two reports are available so far [24,25]. Therefore, the objective of the present study was to investigate the hitherto unknown hypoglycemic nature, if any, of the peels of some commonly used vegetables, which are otherwise thrown away as kitchen wastes, in the prevention of chemically induced diabetes mellitus and associated changes in hepatic lipid peroxidation and antioxidant system. The study is based on the previous experiments where initially 14 vegetable peels were chosen and dose was standardized thereafter further studies conducted. The Research work started taking 14 vegetable peels having some literature over antidiabetic potential. Also, the vegetables which can be peeled off were required so these were selected, which were further screened and finally these 5 vegetable peels were taken to study further.
Materials and Methods
Animals and Diets
Healthy colony bred Swiss albino male mice (2 months old, weighing 28 ± 2 gm) were housed in polypropylene cages under constant temperature (27 ± 1 ºC) and photo schedule (14 h light & 10 h dark). They were provided with mice feed (Golden feeds, New Delhi, India) ad libitum and had free access to drinking water. Standard ethical guidelines for maintenance and handling of animals of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India were followed.
Chemicals
Alloxan (2, 4, 5, 6-tetraoxypyrimidine), tricarboxylic acid (TCA) and thiobarbituric acid (TBA) were obtained from E. Merck, Mumbai, India, radioimmunoassay (RIA) kit for serum insulin was supplied by Bhabha Atomic Research Centre (BARC), Mumbai, India. Pyrogallol, sodium dodecyl sulphate (SDS) and all other chemicals were purchased from Loba Chemicals, Mumbai, India.
Peel Extracts Preparation
Fresh vegetables such as Beta vulgaris (BV), Daucus carota (DC), Ipoema batatas (IB), Pisum sativum (PS) and Raphanus sativus (RS) were collected from the local market, Indore.
Vegetables were cleaned properly and were peeled off. Peels were shade-dried, pulverized and their 50 % ethanolic extracts were prepared as done earlier. In brief, peels were removed from the vegetables, shade-dried for one week and pulverized with the help of an electric grinder to get a free flowing powder. The dry powder was subjected to extraction with 50% ethanol (v/v) at room temperature (RT) for 24 h [23]. The extract was filtered through Whatman filter No. 1 and was dried at 37°C. The powder so obtained was dissolved in double-distilled water (DDW) to prepare different concentrations of the test material.
Experimental Design
Effects of peel extracts in normoglycemic male mice
Five separate experiments, each involving one test peel, were conducted to evaluate the effect of the five test materials in the alterations in serum glucose and hepatic LPO in normoglycemic mice. In experiment 1, while group 1 animals receiving the vehicle (0.1 ml DDW) served as control; animals of group 2 and 3 were administered with 1000 & 500 mg/kg/day of BV extract. After 15 days of treatments overnight fasted animals were sacrificed with cervical dislocation, blood was collected from each animal and serum was separated for the estimation of glucose concentration. Liver was quickly removed, cleaned and then homogenized in phosphate buffer (0.1 M, pH 7.4) with the help of a motor-driven teflon homogenizer. The homogenate was centrifuged at 15,000 × g at 4°C for 30 min to obtain a clear supernatant, which was used for the estimation of LPO. Experiment 2 to5 were performed in the similar manner as experiment 1, considering DC (400 and 200 mg / kg/day), IB (480 and 240 mg /kg/day), PS (100 and 50 mg/kg/day) and RS (480 and 240 mg/kg/day) extracts respectively. The administered doses of peel extracts were taken from the earlier studies, based on the use on whole vegetables [39-43]. Serum glucose and hepatic LPO were considered as the end parameters to find out the best effective and safe dose for each peel extract.
Effects of peel extracts in diabetic mice
From the results of the previous experiment, 500, 200, 240, 50 and 240 mg/kg/day of B. vulgaris, D. carota, I. batatas, P. sativum and R. sativus peel extracts respectively were found to exhibit a better glucose and LPO lowering activities. Therefore, these doses were considered in the pilot experiment.
Mice were divided into 7 groups of 7 each. After acclimatization for a week in laboratory conditions, animals of groups 3 to 7 received 500, 200, 240, 50 and 240 mg/kg/day of B. vulgaris, D. carota, I. batatas, P. sativum and R. sativus peel extracts respectively in 0.1 ml of DDW by gastric incubation (p.o) for 10 consecutive days. On 11th and 12th day, along with the peel extracts 100 mg /kg /day alloxan monohydrate was administered (i.p.) to induce diabetic condition as done earlier [26]. On the 16th day of the experiment, all overnight fasted mice were weighed and sacrificed by cervical dislocation and then the end parameters were studied as done earlier in our laboratory [27,28].
Biochemical Estimations
Estimation of LPO
LPO was determined using the protocol of [29] as modified by [30]. The method is based on the principle that the peroxidation products of membrane lipids {majority being malondialdehyde (MDA)}, when heated with TBA in acidic medium, form pink colored complex which has an absorption maxima at 532 nm. Liver homogenate (0.2 ml) was allowed to react with 1.5 ml of TBA (1% solution) in the presence of 0.2ml SDS and 1.5 ml of acetic acid (20%, pH 3.5) and the final volume was adjusted to 4.0 ml with DDW. A tissue blank was prepared by using distilled water in place of TBA. The reaction mixture was then heated at 95°C for 1 hour. Tubes were then cooled and samples were transferred to centrifuge tubes containing equal volume of 10% TCA. The tubes were centrifuged at 3000 rpm for 5 minutes and the absorbance of the supernatant was measured at 532 nm .Finally LPO was expressed as nM of MDA formed/ h/ mg protein.
Estimation of superoxide dismutase (SOD)
The activity of hepatic SOD was estimated by measuring the percent inhibition of the pyrogallol auto-oxidation by the enzyme. Diethylene-triaminepenta acetic acid (DTPA) was used as a cheater to prevent the interferences of Fe++, Ca++ and Mn++. Two ml of Tris-HCl buffer (50 mM Tris containing 1mM DTPA; pH 8.2), 0.02 ml of liver homogenate and 1 ml of pyrogallol (0.2mM) were added in a 3 ml cuvette and read at 420 nm against a homogenate blank. The rate of autoxidation of pyrogallol in absence of SOD was calculated by recording the increase in the absorption at 420 nm at an interval of every 30 seconds for 3 minutes. The inhibition in the pyrogallol autoxidation in the presence of SOD was recorded similarly. SOD activity was expressed as units/ mg protein, where one unit of SOD was defined as the amount of enzyme that inhibits the autoxidation of pyrogallol by 50% and was finally expressed as units/mg protein [31].
Estimation of catalase (CAT)
Catalase activity was estimated by the method of [32] and was expressed as μM of H2O2 decomposed / min /mg protein. Two ml of phosphate buffer (50 mM; pH 7.0) was taken in a cuvette and 0.02 ml of liver homogenate was added to it. Reaction was initiated by the addition of 1 ml of H2O2 (30 mM) in the cuvette and the decomposition of H2O2 was followed for 90 seconds with an interval of 15 seconds. A substrate blank was prepared by replacing the homogenate with buffer. The catalase activity was expressed as µM of H2O2 decomposed/ min/ mg protein.
Estimation of protein
Assay of protein was carried out by following the routine protocol of [33]. In brief, 1 ml of the test solution was added to 5 ml of the alkaline copper reagent, in 1% sodium potassium tartarate. The solution was vortexed and allowed to stand at RT for 10 min. After the incubation at RT, 0.5 ml of the Folin-Ciocalteau reagent (1:1 diluted form of commercially available 2N) was added, mixed immediately and again incubated at RT for 30 minutes. The absorbance was taken at 660 nm against a substrate blank (containing D.W. in place of sample) and the amount of protein (mg/ ml) was calculated from the standard curve prepared using BSA.
Estimation of glucose
Serum glucose concentration was measured by glucose oxidase (GOD) method based on the protocol of [34]. Glucose oxidase oxidizes glucose to D-gluconic acid and hydrogen peroxide, latter the presence of enzyme peroxidase (POD) oxidizes phenol, which combines with 4-Aminoantipyrine to produce a pink colored quinoneimine dye. The intensity of the color developed is proportional to glucose concentration in the sample.
Insulin radioimmunoassay
Assay of serum insulin was performed following the protocol provided in RIA kit as routinely done in our laboratory [35,36]. In brief, the tubes containing 200 μl of assay buffer with 100 μl of serum sample or standard was mixed and then 100 μl of primary antibodies (anti-porcine guinea pig IgG) were added to the mixture and incubated at 4 °C for overnight. After incubation, 100 μl of 125I-labeled insulin hormone was added in this mixture. After three hours of further incubation at room temperature, 100 μl second antibodies (anti-guinea pig-rabbit IgG) were added followed by the addition of 1 ml polyethylene glycol. After gentle mixing, mixture was incubated at room temperature for 20 min and then centrifuged at 1500 X g for 20 min at room temperature. After decanting the supernatant, traces of liquid were removed with the help of filter paper wicks without disturbing the precipitate. Finally the tubes were subjected to radioactivity counting for one minute (CPM) using a 125I gamma counter. A set of quality control sera of rats was also used with each assay.
Estimation of polyphenols and flavonoids
Total polyphenol was measured by using the protocol as followed earlier by [5]. In brief, 0.125 ml of peel extract of known concentration (100 mg/ml) was diluted with 0.5 ml distilled water to which 0.125 ml of Folin–Ciocalteu reagent was added and incubated at RT for 6 min. Then 1.25 ml of 7% sodium carbonate was added to the mixture and total volume was made up to 3.0 ml with distilled water. The whole mixture was incubated at RT for 90 min. Finally the absorbance was measured against the prepared blank at 765 nm. Polyphenol contents of the peel extracts were determined using the standard curve prepared with known concentration of gallic acid (r2 = 0.9748). The results are expressed in mg/100 g dry weight of gallic acid equivalent (GAE). Total flavonoid was also estimated by spectrophotometer method, following the protocol of [5]. Briefly, 0.25 ml of the peel extract of known concentration (100 mg/ml) was diluted with 1.25 ml of distilled water. Then 75 µl of 5% sodium nitrite (NaNO2) and 150 µl of 10% aluminium chloride (AlCl3.6H20) solutions were added to the mixture. After incubation for 5 min at room temperature, 0.5 ml of 1 M NaOH was added. Total volume was made up to 2.5 ml with distilled water. At the end the absorbance was measured against the prepared blank at 510 nm. Standard curve was prepared with known concentrations of quercetin (r2 = 0.9541). Finally the results are expressed as mg of quercetin equivalents/100 g dry weight of the peel extract.
Statistical analysis
Results are expressed as mean ± standard error of mean (SEM) deviation from 07 animals per group. Statistical evaluation was analyzed by analysis of variance (ANOVA) followed by a post hoc Newman-Keuls multiple comparison test using a trial version of Prism 5 software for Windows (Graph Pad Software, Inc., La Jolla, CA, USA). Differences were considered as significant at P < 0.05.
Results
Following the administration of the lower doses of IB and DC peel extracts (240 and 200 respectively), a significant reduction in serum glucose concentration was observed (P<0.001 for the former & P<0.05 for the latter). However, in case of BV, PS and RS peel extracts, although not significant, a decreasing trend was noticed. In hepatic LPO also, a significant decrease was noticed with respect to the administration of 240 and 200 mg/kg of IB and DC peel extracts respectively (P<0.001 & P<0.01) and in rest three peels also a decreasing trend was observed (Table 1 A and B). Considering these observations, low doses of all the peels were taken for the pilot experiment.
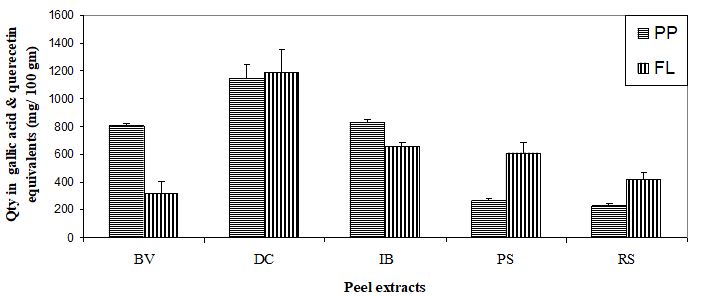
In the second, study while in alloxan treated animals a significant increase in serum glucose concentration was exhibited (P<0.001), following the administration of IB and DC peel extracts there was a marked reduction in the same (P<0.001 for both,).On serum insulin, these two peel extracts could significantly enhance its level (P<0.001 for both). With respect to hepatic LPO, peel extracts of IB and DC significantly decreased it (P<0.05 & P<0.01 respectively) with a simultaneous increase in the level of endogenous antioxidants such as SOD (P<0.001 for both) and CAT (P<0.01 for both, Table 2). While RS also significantly decreased LPO (P<0.01) and increased SOD (P<0.001), but it could not alter the CAT activity significantly. Although hepatic SOD and CAT activities were significantly enhanced (P<0.001 for both) by BV extract administration, no significant alterations in serum glucose and hepatic LPO values were observed (Table 2). Percent changes were also reflected more or less similar efficacies of the test peel extracts (Table 3). While maximum inhibition in serum glucose concentration and in hepatic LPO was found in DC and RS respectively, the percent increase in SOD and CAT activity was more in case of DC. However, with respect to serum insulin, BV showed the highest elevation. The polyphenolic and flavonoids content of the peels of IB and DC were also comparatively high (Figure 1).
Discussion
Results of the first experiment (effects of the test peel extracts in normoglycemic animals) indicated their hypoglycemic nature either in one or both the doses of each peel. With respect to the hepatic lipid peroxidation also, one concentration of each peel extract exhibited a better effect, while the other one was either non-effective or peroxidative in nature. These observations not only indicated a dose specific glucose lowering efficacy, but also the anti peroxidative nature of all five test peels, suggesting their possible anti hyperglycemic potential without any toxic effect in liver, the major target organ of a drug. These findings are somewhat similar to the earlier reports on some other vegetable peel extracts in which their antioxidant properties have been reported [18-20]. However, on the glucose altering property of vegetable peels, so far only two reports are available [23,24].
In the pilot experiment when their potential was evaluated in alloxan induced hyperglycemic animals, although all the test peels exhibited glucose lowering trend, peel extracts of Ipoema batatas and Daucus carota could markedly lower the alloxan induced increase in serum glucose concentration without any hepatotoxic effects, suggesting their possible therapeutic use in ameliorating diabetes mellitus. As similar to these findings only potato peel powder was reported earlier to regulate streptozotocin induced hyperglycemia [24], the present findings appear to be significant. Moreover, for the first time changes in serum glucose have been correlated with the alterations in serum insulin concentration induced by some vegetable peels. In fact, insulin concentration was also significantly enhanced following the administration of IB and DC peel extracts, further supporting their beneficial role in regulating hyperglycemia. Since the administration of IB and DC extracts in alloxan treated animals decreased the serum levels of glucose with a concomitant increase in insulin, it appears that observed antidiabetic activity of IB and DC might have been mediated either through their direct insulin synthesizing potential or through a correction of secretary defects in B-cells of alloxan treated animals [37]. These two peel extracts were also found to be hepatoprotective as they could significantly lower the alloxan induced LPO in this tissue.
Very often alloxan induced increase in LPO is believed to be the result of the generation of reactive oxygen species. After the treatment with peel extracts hepatic LPO was reduced suggesting their antioxidative properties. This antiperoxidative nature of the extracts could be the result of the activation of enzymatic antioxidant defense machinery as there was an increase in SOD and CAT activities following the administration of the peel extracts. This property could also be attributed to the amount as well as type of phenolic compounds present in these two peels. The total polyphenols present in IB was found to be 830.08 ± 22.85 mg/100 gm, while in DC it was 1147 ± 104.00 mg /100 gm. Similarly, amount of flavonoids were also comparatively high in both the peels (65.38 ± 2.72 and 119.12 ± 15.96 mg/100 gm respectively). Similar to our findings, the phenolic acids in commercially important sweet potato cultivars grown in the United States was reported to be very high [38]. It appears that the antiperoxidative and hypoglycemic properties of the two highly effective test peels are related to their phenolic contents.
Conclusion
In conclusion, our findings clearly reveal the hitherto unknown antidiabetic potential of the two test peel extracts without any hepatotoxic effect. The observed hepatoprotective and hypoglycemic action is possibly mediated through their antioxidative properties which may be correlated with their high polyphenolic and flavonoids contents. Thus, at least two test peels (that of Ipoema batatas and Daucus carota) appear to be promising source of antihyperglycemic agents. However, for therapeutic use, further investigations are required.
Significance
There are many allopathic drugs available in the Pharmaceutical Industries for Diabetes mellitus which are effective as suggested by Physicians, but these drugs have so many side effects while taking for long time as taken for such lifestyle disorders. They affect an individual’s liver and kidneys which are vital organs and there by produces other related disorders. Therefore the present study focuses on consumption of vegetables along with peels. Firstly they have no side effects rather provide antioxidants and phytochemicals, and Secondly usually peels are removed and thrown away as kitchen wastes so using them will be cost effective too. In short they may serve as new generation therapeutics to treat diabetes mellitus.
Acknowledgements
Financial support from the University Grant Commission, N. Delhi, India to Ms. Yamini Dixit in the form of a research fellowship is gratefully acknowledged. Some help from Dr. S. Panda and Ms. N. Sharma is also appreciated.
- Rajendra Pradeepa, Viswanathan Mohan (2021) Epidemiology of type 2 diabetes in India, Indian Journal of Opthalmology, 69: 2932-8.
- Skyler JS (2004) Diabetes mellitus: pathogenesis and treatment strategies, Chemistry, 47: 4113-7.
- Akhtar FM, Ali MR (1984) Study of antidiabetic effect of a compound medicinal plant prescription in normal and diabetic rabbits, Journal of Pakistan Medical Association, 34: 239-44.
- Rang HP, Dale MM (1991) The endocrine system. Pharmacology, second ed., Harlow, Longman, United kingdom.
- Leontowicz M, Gorinstein S, Leontowicz H, Krzeminski R, Lojek A, Katrich E, Ciz M, et al. (2003) Apple and pear peel and pulp and their influence on plasma lipids and antioxidant potentials in rats fed with cholesterol-containing diets, Journal of Agriculture and Food Chemistry, 51: 5780-5.
- Khaled F Mahmoud, Amal Z Hammouda, Hatem S Ali, Azza A Amin (2021) Efficiency of Red Onion Peel Extract Capsules on Obesity and Blood Sugar. Pakistan. Journal of Biological Sciences, 24: 99-111.
- Phung Lam Toi, Thunyarat Anothaisintawee, Usa Chaikledkaew, Jamaica Roanne Briones Sirimon Reutrakul, Ammarin Thakkinstian (2020) Preventive Role of Diet Interventions and Dietary Factors in Type 2 Diabetes Mellitus: An Umbrella Review, Nutrients, 12: 2722.
- Kanazawa K, Sakakibara H (2000) High content of dopamine, a strong antioxidant in Cavendish banana, Journal of Agriculture and Food Chemistry, 48: 844-8.
- Higashi-Okai K, Kamimoto K, Yoshioka A, Okai Y (2002) Potent suppressive activity of fresh and dried peels from Satsuma mandarin (Citrus unshiu Marcorv.) on hydroperoxide generation from oxidized linoleic acid, Phytotherapy Research, 16: 781-4.
- Lata B (2007) Relationship between apple peel and the whole fruit antioxidant content: Year and cultivar variation, Journal of Agriculture and Food Chemistry, 55: 663-71.
- Kar A, Panda S (2004) Ayurvedic therapies for thyroid dysfunction. In L. Mishra (Ed.), Scientific Basis of Ayurvedic Therapies, 8: 133-48.
- Kar A, Panda S (2005) Plant extracts in the regulation of hypothyroidism. In SK Sharma, JN Govil, VK Singh (Eds.), Recent Progress in Medicinal Plants, 10: 419-26.
- Parmar HS, Panda S, Jatwa R, Kar A (2006) Cardio-protective role of Terminalia arjuna bark extract is possibly mediated through alterations in thyroid hormones. Pharmazie, 61: 793-5.
- Wolfe K, Wu X, Liu RH (2003) Antioxidant activity of apple peels, Journal of Agriculture and Food Chemistry, 51: 609-14.
- Gorinstein S, Leontowicz H, Leontowicz M, Drzewieckji J, Jastrzebski Z, Tapia MS, et al. (2005) Red star Ruby (Sunrise) and blond qualities of Jaffa grape fruits and their influence on plasma lipid levels and plasma antioxidant activity in rats fed with cholesterol containing and cholesterol free diets, Life Science, 77: 2384-97.
- Parmar HS, Kar A (2007) Atherogenic diet induced daibetes mellitus: involvement of thyroid hormones, European Journal of Pharmacology, 570: 244-8.
- Parmar HS, Kar A (2008) Antiperoxidative, antithyroidal, antihyperglycemic and cardioprotective role of Citrus sinensis peel extract in male mice, Phytotherapy Research, 22: 791-5.
- Bhandari PR (1966) Detection of kämpferol in onion peels (Allium cepa Linn.), Naturwissenschaften, 53: 82-3.
- Ichikawa M, Ryu MK, Yoshida J, Ide N, Kodera Y, Sasaoka T, Rosen RT (2003) Identification of six phenylpropanoids from garlic skin as major antioxidants, Journal of Agriculture and Food Chemistry, 51: 7313-7.
- Sadilova E, Stintzing FC, Carle R (2006) Anthocyanins, colour and antioxidant properties of eggplant (Solanum melongena L.) and violet pepper (Capsicum annuum L.) peel extracts, Z Naturforsch C, 527-35
- Park J, Kim J, Kim MK (2007) Onion flesh and onion peel enhance antioxidant status in aged rats, Journal of Nutrition Science and Vitaminology, 53: 21-9
- Agha A. El, Makris DP, Kefalas P (2008) Peroxidase-active cell free extract from onion solid wastes: biocatalytic properties and putative pathway of ferulic acid oxidation. Journal of Bioscience and Bioengineering, 106: 279-85.
- Dixit Y, Panda S, Kar A (2008) Lagenaria siceraria peel extract in the regulation of hyperthyroidism, hyperglycemia and lipid peroxidation in mice. Interernational Journal of Biomedical and Pharmaceutical Sciences, 2: 79-83.
- Singh N, Rajini PS (2005) Protective effect of potato peel powder in ameliorating oxidative stress in streptozotocin diabetic rats, Plant Foods for Human Nutrition, 60: 49-54.
- Dixit Y, Kar A (2009) Antioxidative activity of some vegetable peels determined in vitro by inducing liver lipid peroxidation. Food Research International, 42: 1351-4.
- Raskovic A, Gavrilovic M, Jakovljevic V, Sabo J (2004) Glucose concentration in the blood of intact and alloxan-treated mice after pretreatment with commercial preparations of Stevia rebaudiana (Bertoni), European Journal of Drug Metabolism and Pharmacokinetics, 29: 87-90.
- Panda S, Kar A (2007) Antidiabetic and antioxidative effects of Annona squamosa leaves are possibly mediated through quercetin-3-O-glucoside, Biofactors, 31: 201-10.
- Jatwa R, Parmar HS, Panda S, Kar A (2007) Amelioration of corticosteroid- induced type 2 diabetes mellitus by rosiglitazone is possibly mediated through stimulation of thyroid function and inhibition of tissue lipid peroxidation in mice, Basic and Clinical Pharmacology and Toxicology, 101: 177-80.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric reaction, Analytical Biochemistry, 95: 351-8.
- Jamall IS, Smith JC (1985) Effects of cadmium on glutathione peroxidase, superoxide dismutase and lipid peroxidation in the rat heart: A possible mechanism of cadmium cardiotoxicity. Toxicology and Applied Pharmacology, 80: 33-42.
- Marklund S, Marklund G (1974) Involvement of superoxide anion radical in antioxidation of pyrogallol and a convenient assay of superoxide dismutase, European Journal of Biochemistry, 47: 469-74.
- Aebi HE (1983) Catalase, In H.U. Bergmeyer (Ed.), Methods in Enzymatic Analysis, 2: 276-86.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin- phenol reagent ,The Journal of Biological Chemistry, 193: 265-75.
- Trinder P (1969) Practical clinical Biochemistry. Vol 10 fifth ed. William Heinamman Medical books LTD, New York.
- Parmar HS, Kar A (2007) Antidiabetic potential of Citrus sinensis and Punica granatum peel extracts in alloxan treated male mice. Biofactors, 31: 17-24.
- Parmar HS, Kar A (2009) Protective role of Mangifera indica, Cucumis melo and Citrullus vulgaris peel extracts in chemically induced hypothyroidism. Chemico- Biological Interactions, 177: 254-8.
- Hellman B, Berne C, Grapengiesser E, Grill V, Gylfe E, Lund PE (1990) The cytoplasmic Ca2+ response to glucose as an indicator of impairment of the pancreatic beta-cell function. European Journal of clinical investigations, 20: S10-7.
- Truong VD, Mc Feeters, RF Thompson, RT Dean LL, Shofran B (2007) Phenolic acid content and composition in leaves and roots of common commercial sweet potato (Ipomea batatas L.) cultivars in the United States, Journal of Food Science, 72: 343-9.
- Agrawal M, Srivastava VK, Saxena KK, Kumar A (2006) Hepatoprotective activity of Beta vulgaris against CCl4 induced hepatic injury in rats, Fitoterapia, 77: 91-3.
- Vasudevan M, Gunnam KK, Parle M (2006) Antinociceptive and anti-inflammatory properties of Daucus carota seeds extract, Journal of Health Science, 52: 598-606.
- Ludvik B, Waldhaus IW, Prager R, Kantzkywiller A, Pacini G (2003) Mode of action of Ipoema batatas (caicapo) in type 2 diabetic patients, Metabolism, 52: 875-80.
- Abatan MO, Makinde MJ (1986) Screening Azadirachta indica and Pisum sativum for possible antimalarial activities, Journal of Ethnopharmacology, 17: 85-93.
- Chaturvedi P, Machacha CN (2007) Efficacy of Raphanus sativus in the treatment of paracetamol induced hepatotoxicity in albino rats, British Journal of Biomedical Science, 64: 105-8.

Tables at a glance
Figures at a glance