Nonstoichiometric Lithium Iron Phosphate for Lithium-Ion Battery Prepared by Supercritical Hydrothermal Synthesis
Received Date: August 21, 2023 Accepted Date: September 21, 2023 Published Date: September 23, 2023
doi: 10.17303/jmsa.2023.7.103
Citation: Kyoo-Seung Han (2023) Nonstoichiometric Lithium Iron Phosphate for Lithium-Ion Battery Prepared by Supercritical Hydrothermal Synthesis. J Mater sci Appl 7: 1-13
Abstract
Lithium iron phosphate is a main cathode material for lithium-ion batteries. The battery performance of LiFePO4 , however, is limited by its small lithium ion diffusivity, which can result in a significant loss of capacity at high currents. To enhance the lithium ion diffusion of LiFePO4 during the charge and discharge of lithium-ion batteries as well as to control in ease the chemical composition, nonstoichiometric lithium iron phosphate, Li1-xFeP1-yO4-z (0 < x ≤ 0.15, 0 < y ≤ 0.05, 0 < z ≤ 0.2), consisting in some atomic vacancies is prepared by supercritical hydrothermal synthesis. It is found that the changes in composition and crystal structure greatly influence the capacity and rate performance of nonstoichiometric lithium iron phosphate due to the expansions of direction and space in lithium ion diffusion path.
Keywords: Lithium Iron Phosphate; Supercritical Hydrothermal Synthesis; Lithium-Ion Battery; Cathode Materials
Introduction
Lithium-ion rechargeable batteries are used as power sources of electronic appliances and instruments such as mobile phones, laptop computers, portable power tools, wireless cleaners, etc. Their application field has been broadening to high capacity batteries for electric vehicles and electrical energy storage system. The emergence of high capacity batteries has created a growing demand for improvements in energy storage devices that are cost effective and smaller in size and weight as well as operate for a longer time. In this way, lithium iron phosphate has received considerable attention as a cathode material for lithium-ion battery because of its low price, reasonable specific capacity (169.89 mAh/g), good capacity retention, as well as structural, chemical, electrochemical and thermal stabilization [1-5]. On the other hand, the battery performance of lithium iron phosphate is limited by its small lithium ion diffusivity, which can result in a significant loss of capacity at high currents [3,6-8]. The poor rate capability of LiFePO4 cathodes makes it difficult to make full use of them in lithium-ion batteries unless modifications are made to improve the slow lithium ion diffusion across the LiFePO4 /FePO4 interface.
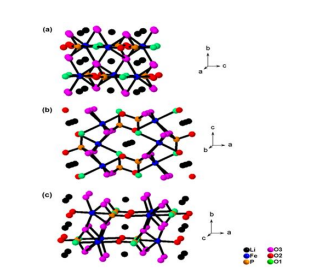
Stoichiometric lithium iron phosphate, LiFePO4 is crystalized in the orthorhombic olivine structure with Pmnb space group. Figure 1 (a), (b) and (c) respectively illustrate the hexagonal close-packed structures of LiFePO4 in the view of a-axis, b-axis and c-axis. Those show that lithium ions form a linear chain of the edge-sharing octahedron in the a-axis, b-axis and c-axis [3,9-11]. Each lithium ion shares an edge with two iron ions and two PO4 tetrahedrons. As shown in Figure 1, the narrow diffusion paths for lithium ions in the direction of aaxis and c-axis result in its small lithium ion diffusivity. Lithium ions are located close to the sites of O3 oxygen in Figure 1. Therefore, it is expected that when one can prepare O3 oxygen less atmosphere in crystal, the crystal structure of lithium iron phosphate can be changed, and thus higher lithium ion diffusivity can be achieved.
To enhance the lithium ion diffusion of LiFePO4 during the charge and discharge of the lithium-ion batteries, a nonstoichiometric lithium iron phosphate consisting in some vacancies of O3 oxygen is considered. However, due to the strong P-O bond in PO4 3-, it is difficult to obtain the desired material using common synthetic methods including a solid-state reaction method, molten salt sintering method, sol-gel method, spray pyrolysis method, coprecipitation-sintering method, wet precipitation, and hydrothermal method [2,9,12-15]. In contrast, a nonstoichiometric lithium iron phosphate can be prepared using supercritical hydrothermal synthesis. Moreover, the indirect effect in the improvement of lithium ion conductivity can be expected with shortening lithium ion diffusion routes by making particles ultrafine. In this work, the material characteristics and the battery performances of nanometer sized and nonstoichiometric lithium iron phosphate are presented.
Experimental
Synthesis of Nonstoichiometric Lithium Iron Phosphate
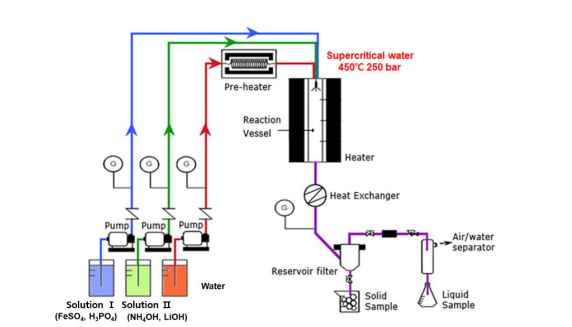
The preparation procedure of nanometer sized and nonstoichiometric lithium iron phosphate consists of three steps: (1) preparation of three water solutions (FeSO4 and H3PO4 water solution (solution Ⅰ), NH4OH and LiOH water solution (solution Ⅱ), supercritical water), (2) supercritical hydrothermal reaction and (3) thermal treatment at 700oC during 10 hours for drying, granulation of nanometer sized primary powders and improvement of crystallinity (Figure 2). Various nonstoichiometric lithium iron phosphates are fabricated at the difference in the molar concentration of FeSO4 , H3PO4 , NH4OH and LiOH solution. The molar concentration of FeSO4 , H3PO4 , NH4OH and LiOH solution is reported in Table 1.
The solution I is prepared by the mixing of 1.0 M FeSO4 water solution and H3PO4 water solution in a fixed molar concentration between 1.0 and 1.2. In addition, the solution II is prepared by the mixing of 2.0 M LiOH water solution and NH4OH water solution in a fixed molar concentration between 1.1 and 2.0. The supercritical water is obtained in the condition of 450oC and 250 bar.
The supercritical hydrothermal reaction is performed in a continuous reactor with the following conditions: 380oC, 250 bar, injection of the solution I at the speed of 8.0 g/min, injection of the solution II at the speed of 8.0 g/min, injection of the supercritical water at the speed of 96.0 g/min, reaction time of 7 seconds.
Characterization
An X-ray diffraction pattern of the products was carefully recorded in accordance with the 2θ step of 0.02o / 40 seconds counting time. In order to improve the atomic positions and the positional quantities of the whole ions in the products, a crystal structure was determined using Rietveld refinement method. In addition, to confirm the molar ratio of Fe and P in the products, inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis was also required.
Micro-battery test was carried out using a granule of the sample I (18 μm in diameter) connected to platinum microelectrode (20 μm in diameter) as cathode, lithium foil as anode and a 1 M LiClO4 solution in ethylene carbonate (EC)/propylene carbonate (PC) (50/50, volumetric %) as electrolyte.
Electrochemical lithium intercalation/deintercalation experiments of the products were realized by charge/discharge of coin type lithium ion batteries using a mixture of the products (90% in weight) with acetylene black (5% in weight) and polyvinylidene fluoride (PVdF, 5% in weight) as cathode, lithium foil as anode and a 1 M LiPF6 solution in ethylene carbonate (EC)/dimethyl carbonate (DMC) (50/50, volumetric %) as electrolyte.
Results and Discussion
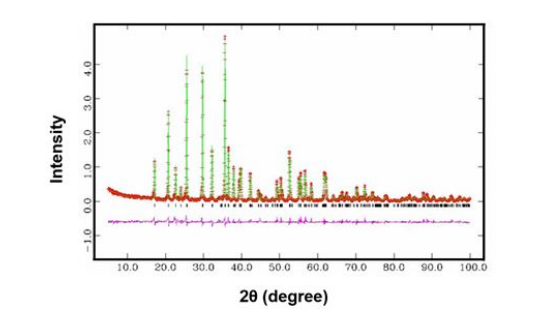
X-ray diffraction profiles both observed and refined patterns for the sample I as well as difference between observed and refined intensities are shown in Figure 3. The measured diffraction pattern overlaps with the calculated diffraction pattern (based on Pnma space group) and the difference is represented by the bottommost line. Rietveld refinement is performed by minimizing Rwp (weighted pattern R factor), which is the difference between the measured and calculated profiles.
The results of Rietveld refinement are considered reliable when Rwp obtained using a least squares approach is found to be less than 10. The refined atomic coordinates and quantities are given in Table 2. Regrettably, in this work, the minimized Rwp values is 11.62. It might result from less uniform distribution of the atomic vacancies as well as small particle size of the ultrafine powders. Nevertheless, the X-ray diffraction pattern for the sample I is well in dexed in the orthorhombic olivine structure with Pnma space group. As given in Table 2, the result of the Rietveld refinement demonstrates the presence of atomic vacancies in the sample I and the chemical formula for the sample I of Li0.87FeP0.96O3.94. The investigation into the Rietveld refinement of X-ray diffraction patterns for all the samples, Li1xFeP1-yO4-z (0 < x ≤ 0.15, 0 < y ≤ 0.05, 0 < z ≤ 0.2), will be reported in forthcoming papers.
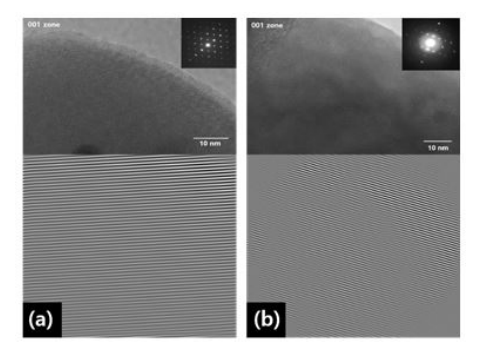
Figure 4 (a) and (b) show high-resolution (HR) transmission electron microscopy (TEM) images and selected area electron diffraction (SAED) patterns of a commercial LiFePO4 and the sample I, Li0.87FeP0.96O3.94, respectively. The HRTEM results of LiFePO4 demonstrate its highly or dered crystal lattices without any dislocations and structural defects [16-18]. However, the Ytype diffraction patterns and the slightly curved diffraction patterns in the HRTEM image as well as the halation in the SAED pattern in Figure 4 (b) indicate that Li0.87FeP0.96O3.94 has some atomic vacancies as point defects that cause a structural deformation [19,20].
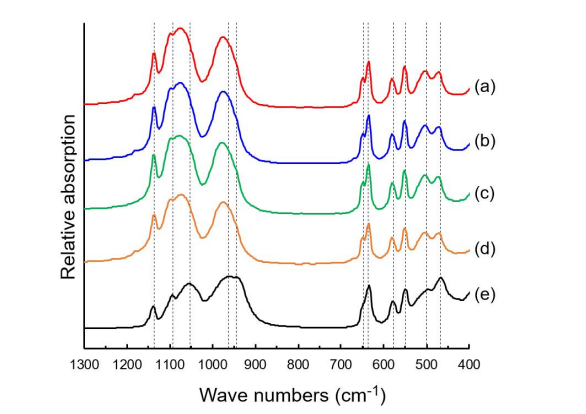
The Fourier-transform infrared (FTIR) spectra of the sample I, the sample Ⅲ, the sample Ⅳ, the sample Ⅵ and commercial LiFePO4 are reported in Figure 5 (a), (b), (c), (d) and (e), respectively. It is clear that the FTIR spectra of all the samples exhibit strong similarities. While the vibration frequencies of all the samples and commercial LiFePO4 in the range of 400 to 700 cm1 are similar, those band shapes are quite different. The bands in the range of 400 to 700 cm-1 correspond to bending vibration modes of O-P-O [9,21,22]. In the range of 900 to 1200 cm1 , both the vibration frequencies and the band shapes between all the samples and commercial LiFePO4 are changed. The bands in the range of 900 to 1200 cm-1 correspond to stretching vibration modes of PO4 [9,21-25]. Especially, the vibration frequencies of all the samples are moved to higher values compared to those of commercial LiFePO4 . These changes mean the structural deformation in PO4 monomer and the reinforcement of P-O band strength due to the atomic vacancies in the samples. In addition, the reinforcement of P-O band strength results in the decrease of P-O bond length and the expansions of direction and space in lithium ion pathway
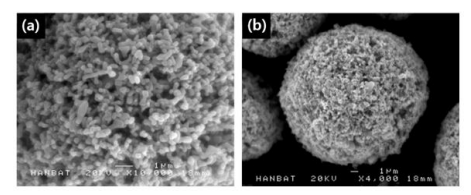
Scanning electron microscopy (SEM) images in Figure 6 directly shows the spherical granules of nanometer sized Li0.87FeP0.96O3.94 powders and also informs of the approximate sizes of the granule and the primary powder. Figure 6 (a) show the primary powder of Li0.87FeP0.96O3.84, and its granules are shown in Figure 6 (b). Mostly the powders are in sphere form.
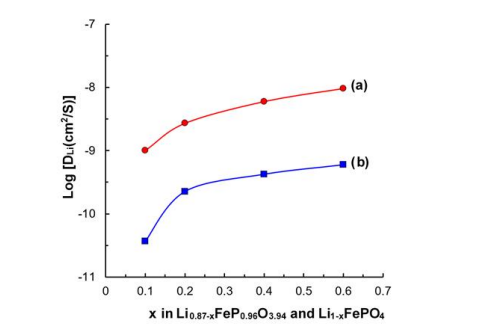
Figure 7 (a) and (b) show the lithium ion diffusion coefficient for Li0.87-xFeP0.96O3.94 and Li1-xFePO4 , respectively. According to x, the highest value of Li0.87-xFeP0.96O3.94 was 9.63x10-9 and the lowest was 1.01x10-9, while the highest value of Li1xFePO4 was 5.96x10-10 and the lowest value was 3.67x10-11 . In the same x, the lithium ion diffusion coefficient for Li0.87xFeP0.96O3.94 is 12~27 times high in comparison with that for Li1-xFePO4 . These differences of lithium ion diffusion mean that the structural change of Li0.87FeP0.96O3.94 expands the lithium ion transfer path [26].
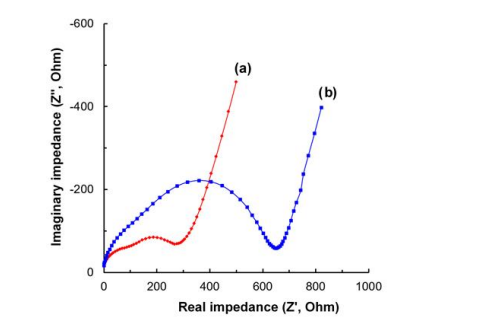
Figure 8 (a) and (b) show the Nyquist plots of impedance for Li0.87FeP0.96O3.94 and LiFePO4 , respectively. The real impedance (Z') for Li0.87FeP0.96O3.94 is under half the Z' for LiFePO4 in solid electrolyte interphase, charge transferelectrochemical double layer and mass transport areas. This difference means the resistance of Li0.87FeP0.96O3.94 is smaller than LiFePO4 at both surface and inner part of powder by reducing amount of oxygen and corresponding structural changes.
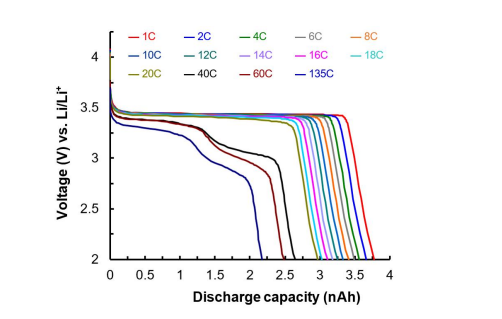
The variations of the cell voltage in the range between 2.0 and 4.0 V at 25 o C vs micro-battery capacity for a granule of Li0.87FeP0.96O3.94 during discharging under various C-rates are given in Figure 9. Extraordinarily, the discharge capacity at 135 C is 58.7% of the discharge capacity at 1 C, which means that the micro-battery can be fully discharged in 27 seconds (1/135 hour). Existing cellular phones require a charge/discharge rate of 0.5 C. Hybrid electric vehicles require output C-rate of 40 C [3,27]. Unfortunately, the Li0.87FeP0.96O3.94//Li microbattery is capable of operating up to 20 C.
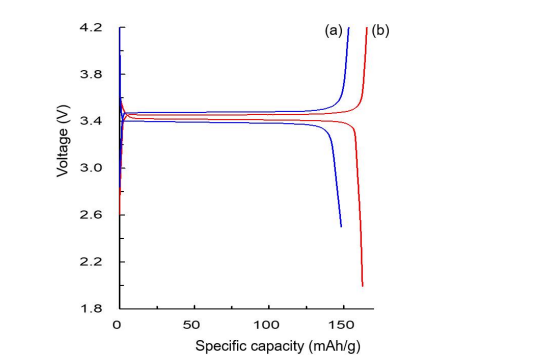
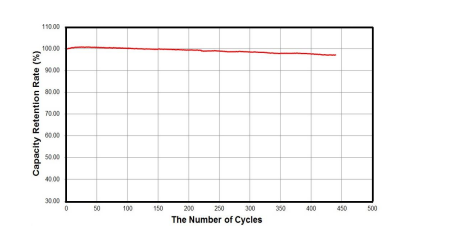
The charge and discharge curves of the LiFePO4 //Li cell and the Li0.87FeP0.96O3.94//Li cell at 0.1 C are shown in Figure 10 (a) and (b), respectively. The Li0.87FeP0.96O3.94//Li cell exhibits the discharge capacity of 162.58 mAh/g, which is 95.7% of the theoretical capacity of LiFePO4 . Due to the improved lithium ion mobility and the lower impedance, the Li0.87FeP0.96O3.94//Li cell has much better battery performances such as the nominal voltage drop (gap between charge and discharge potential) and the discharge capacity in comparison with the LiFePO4 //Li cell. These results represent that the coulomb efficiency and the energy efficiency of the Li0.87FeP0.96O3.94//Li cell are higher than those of the LiFePO4 //Li cell. Figure 11 shows the capacity retention rate vs the number of cycles of the Li0.87FeP0.96O3.94//Li cell at 1 C.
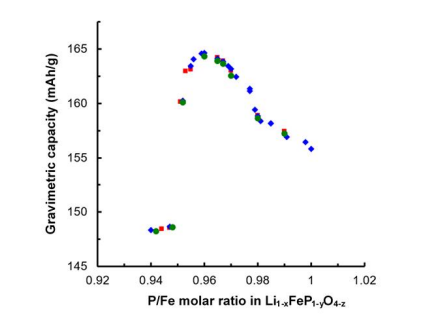
In order to find optimum chemical composition of nonstoichiometric lithium iron phosphate, the variation of the specific capacity vs P/Fe molar ratio in Li1-xFeP1-yO4-z (0 < x ≤ 0.15, 0 < y ≤ 0.06, 0 < z ≤ 0.2) is collected in Figure 12 during the cycles of the Li1-xFeP1-yO4-z //Li cells. As the P/Fe molar ratio decreases from 1.00 to 0.96, the specific capacity gradually increases. Conversely, as the P/Fe molar ratio decreases from 0.96 to 0.95, the specific capacity gradually decreases. Unfortunately, in the case with a P/Fe molar ratio of less than 0.95, the specific capacity rapidly decreases. The capacity at P/Fe molar ratio 0.96 was the highest. According to decreasing the P/Fe molar ratio from 1.00 to 0.96, Li1-xFeP1-yO4-z has the gradual widening of the lithium ion diffusion path while maintain its structural stability. However, Li1-xFeP1-yO4-z with a P/Fe molar ratio of less than 0.95 has too many atomic vacancies, which cause the destruction of the structural stability. The structural unstability of Li1-xFeP1-yO4-z with a P/Fe molar ratio of less than 0.95 makes an irreversible cycle.
Conclusion
To enhance the battery performance of LiFePO4, nanometer sized Li1-xFeP1-yO4-z (0 < x ≤ 0.15, 0 < y ≤ 0.05, 0 < z ≤ 0.2) consisting in some atomic vacancies is fabricated by supercritical hydrothermal synthesis. The Li0.87FeP0.96O3.94//Li micro-battery capacity at 135 C is 58.7% of its discharge capacity at 1 C, which means that the micro-- battery can be fully discharged in 27 seconds. Therefore, the Li0.87FeP0.96O3.94//Li micro-battery has ultrahigh rate capability. It is found that the changes in composition and crystal structure greatly influence the battery capacity and rate performance of Li1-xFeP1-yO4-z due to the expansions of direction and space in lithium ion diffusion path.
Acknowledgement
This work was supported by research fund of Chungnam National University.
- Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive electrode materials for rechargeable lithium batteries, Journal of Electrochemical Society 144:1188-94.
- Yang S, Zavalij PY, Whittingham MS (2001) Hydrothermal synthesis of lithium iron phosphate cathodes, Electrochemistry Communications 3:505-8.
- Park JK, Doh CH, Han KS, Hong YS, Kang KS et al. (2012) Principles and applications of lithium secondary batteries, John Wiley & Sons.
- Jugovic D, Uskokovic D (2009) A review of recent developments in the synthesis produces of lithium iron phosphate powders, Journal of Power Sources 190: 538-44.
- Zhao Q, Zhang S, Li T, Xu C, Yang J et al. (2023) Enhanced electrochemical delithiation of LiFePO4 in a composite aqueous electrolyte for high performance olivine FePO4, Journal of Electrochemical Society 170: 040521.
- Chung SY, Bloking JT, Chiang YM (2002) Electronically conductive phospho-olivines as lithium storage electrodes, Nature Materials 1: 123-8.
- Prosini PP, Lisi M, Zane D, Pasquali M (2002) Determination of the chemical diffusion coefficient of lithium in LiFePO4, Solid State Ionics 148: 45-51.
- Amin R, Balaya P, Maier J (2007) Anisotropy of electronic and ionic transport in LiFePO4 single crystals. Electrochemical and Solid-State Letters 10: 13-6.
- Ait Salah A, Jozwiak P, Garbarczyk J, Benkhouja K, Zaghib K et al. (2005) Local structure and redox energies of lithium phosphates with olivine- and Nasicon-like structures, Journal of Power Sources 140: 370-5.
- Kobayashi S, Kuwabara A, Fisher CAJ, Ukyo Y, Ikuhara Y (2018) Microscopic mechanism of biphasic interface relaxation in lithium iron phosphate after delithiation, Nature Communications 9: 1-10.
- Yang S, Song Y, Zavalij PY, Whittingham MS (2002) Reactivity, stability and electrochemical behavior of lithium iron phosphates, Electrochemistry communications 4: 239-44.
- Kim JK, Choi JW, Chauhan GS, Ahn JH, Hwang GC et al. (2008) Enhancement of electrochemical performance of lithium iron phosphate by controlled sol-gel synthesis, Electrochimica Acta 53: 8258-64.
- Ni JF, Zhou HH, Chen JT, Zhang XX (2007) Molten salt synthesis and electrochemical properties of spherical LiFePO4 particles, Materials Letters 61: 1260-4.
- Wang GX, Bewlay SL, Konstantinov K, Liu HK, Dou SX, Ahn JH (2004) Physical and electrochemical properties of doped lithium iron phosphate electrodes, Electrochimica Acta 50: 443-7.
- Xie H, Zhou Z (2006) Physical and electrochemical properties of mix-doped lithium iron phosphate as cathode material for lithium ion battery, Electrochimica Acta 51: 2063-7.
- Chen G, Song X, Richardson TJ (2006) Electron microscopy study of the LiFePO4 to FePO4 phase transtion, Electrochemical and Solid State Letters 9: 295-8.
- Zhao Q, Zhang Y, Meng Y, Wang Y, Ou J et al. (2017) Phytic acid derived LiFePO4 beyond theoretical capacity as high-energy density cathode for lithium ion battery, Nano Energy 34: 408-20.
- Teng F, Santhanagopalan S, Lemmens R, Geng X, Patel P, Meng DD (2010) In situ growth LiFePO4 nanorod arrays under hydrothermal condition, Solid State Sciences 12: 952-5.
- Kiely CJ, Pond RC, Kenshole G, Rockett A (1991) A TEM study of the crystallography and defect structures of single-crystal and polycrystalline copper indium diselenide, Philosophical Magazine A-Physics of Condensed Matter Structure Defects and Mechanical Properties 63: 1249-73.
- Aidhy DS, Lu C, Jin K, Bei H, Zhang Y et al. (2015) Point defect evolution in Ni, NiFe and NiCr alloys from atomistic simulations and irradiation experiments, Acta Materialia 99: 69-76.
- Burba CM, Frech R (2004) Raman and FTIR spectros copic study of LixFePO4 (0≤x≤1), Journal of the Electrochemical Society 151: 1032-8.
- Ornek A, Bulut E, Ozacar M (2014) The chemical, physical and electrochemical effects of carbon sources on the nano-scale LiFePO4 cathode surface, Ceramics International 40: 15727-36.
- Brochu F, Guerfi A, Trottier J, Kopec M, Mauger A et al. (2012) Structure and electrochemistry of scaling nano CLiFePO4 synthesized by hydrothermal route: complexing agent effect, Journal of Power Sources 214:1-6.
- Miao C, Bai P, Jiang Q, Sun S, Wang X (2014) A novel synthesis and characterization of LiFePO4 and LiFePO4/C as a cathode material for lithium-ion battery, Journal of Power Sources 246:232-8.
- Jozwiak P, Garbarczyk JE, Wasiucionek M, Gorzkowska I, Gendron F et al. (2008) DTA, FTIR and impedance spectroscopy studies on lithium-iron-phosphate glasses with olivine-like local structure, Solid State Ionics 179: 46-50.
- Satyavani TVSL, Ramya Kiran B, Rajesh Kumar V, Srinivas Kumar A, Naidu SV (2016) Effect of particle size on dc conductivity, activation energy and diffusion coefficient of lithium iron phosphate in Li-ion cells, Engineering Science and Technology an International Journal 19: 40-4.
- Nelson P, Amine K, Rousseau A, Yomoto H (2007) presented at Proceedings from the 23rd Electric Vehicle Symposium.

Tables at a glance
Figures at a glance