Fabrication of Layered Crystal Structure Li1-xNi1-x-yMnxCoyO2 (0.1 ≤ x+y ≤ 0.5) with Homogeneous Arrangement of Ni3+, Mn4+, and Co3+, as well as its Battery Performances
Received Date: August 21, 2023 Accepted Date: September 21, 2023 Published Date: September 23, 2023
doi: 10.17303/jmsa.2023.7.104
Citation: Dong-Heon Lee, Huan-Hee Yoo, Kyoo-Seung Han (2023) Fabrication of Layered Crystal Structure Li1-xNi1-x-yMnxCoyO2 (0.1 ≤ x+y ≤ 0.5) with Homogeneous Arrangement of Ni3+, Mn4+, and Co3+, as well as its Battery Performances. J Mater sci Appl 7: 1-15
Abstract
Intrinsic disadvantages of LiNi1-xNi1-x-yMnxCoyO2 such as an inappropriate but stable oxidation state of Ni2+, as well as a heterogeneous arrangement of Ni, Mn, and Co within LiNi1-xNi1-x-yMnxCoyO2 are overcome by the solubility control according to coprecipitation temperature for homogeneous arrangement of Ni, Mn, and Co, as well as the selective oxidation control from Ni2+ to Ni3+ without changing the states of Co3+ and Mn4+ . The novel LiNi1-xNi1-x-yMnxCoyO2 (0.1 ≤ x + y ≤ 0.5) prepared through the selective oxidation has a single-phase layered crystal structure with a space group R3m. The battery performance of the Li LiNi1-xNi1-x-yMnxCoyO2 with homogeneous arrangement of Ni3+, Mn4+, and Co3+ shows a maximum available capacity of 262 mAh/g and a capacity retention rate of 99.7% in 40 charging and discharging cycles.
Keywords: Lithium Secondary Battery; Cathode Materials; Lithium Transition Metal Oxide; Layered Crystal Structure; Selective Oxidation
Introduction
Humans pursue goals endlessly, and efforts to achieve them are expressed as energy conversion in any form. The representative ability of mankind is that mankind knows how to use tools, and mankind is making efforts to use tools as the most effective way to achieve its goals. Naturally, energy conversion is also necessary for the operation of the tool. Energy is neither created nor dissipated, just convertible. Efficient tool operation is realized through the highest efficiency energy conversion, and the energy associated with the highest conversion efficiency is electrical energy. Therefore, since the most efficient tool is electronic devices, electronic devices are indispensable to mankind for efficient and convenient life. It is generally overlooked or misunderstood that an electronic device without a battery, whether direct or indirect, may not exist. A battery is a device that converts various types of energy into electrical energy using chemical reactions, radiation, temperature differences, light activity, power, etc.
Because it is easy to design and manufacture lithium secondary batteries to best suit properties such as energy density, capacity, power, safety, life cycle, price, etc. depending on the unique demands of various electronic devices, they can be applied as a power source for a wide variety of electronic devices that require a wide range of energy density and power density.
Chemical formulas of commercially representative cathode materials, such as a layered crystal structure lithium transition metal oxide, a spinel-type crystal structure lithium transition metal oxide, and an olivine-type crystal structure lithium transition metal phosphate, can be abbreviated as LiMO2 (M = Ni, Mn, Co, etc.), LiM'2O4 (M' = Mn, Ni, Co, etc.), LiM"PO4 (M" = Fe, Mn, Ni, Co, etc.), respectively. Here, all of the M, M', and M" are 3d transition metals. This is because 3d transition metals have higher electrode potential than 4d and 5d transition metals, are relatively lightweight and small in size, which are advantageous in terms of battery capacity per unit weight and per unit volume [1].
Layered crystal structure LiNi1-xNi1-x-yMnxCoyO2 (0.1 ≤ x + y ≤ 0.5) may exhibit a theoretical and also available capacity of up to 280 mAh/g as well as sustainable battery performances [1,2]. To obtain such LiNi1-xNi1-x-yMnxCoyO2 , two significant challenges need to be addressed.
The first challenge is to ensure homogeneity in the arrangement of Ni, Mn, and Co within LiNi1-xNi1-x-yMnxCoyO2 . A liquid reaction producing method can be easily and homogeneously mixed Ni, Mn, and Co in atomic units. As a solid reaction producing method, Ni, Mn, and Co cannot be homogeneously mixed in atomic units, and as a vapor phase reaction producing method, Ni, Mn, and Co can be homogeneously mixed in atomic units, but it is impossible to produce a powder-type cathode active material, and a film-type cathode active material can be produced. During charging and discharging of a lithium secondary battery made of LiNi1-xNi1-x-yMnxCoyO2 as a cathode active material, Ni, Mn, and Co are oxidized and reduced in different sequences. When the arrangement of Ni, Mn, and Co within LiNi1-xNi1-x-yMnxCoyO2 is heterogeneous, it can decrease the available capacity and shorten the overall battery lifespan. To overcome this issue, many efforts have been attempted, such as developments of particles achieving a concentration-gradient Ni, Mn, and Co as well as a hybridized shape of Ni-rich core and Mn-rich shell [3,4]. Regrettably, a cathode active material with a concentration-gradient Ni, Mn, and Co has not registered a noticeable market share.
The second challenge is the selective oxidation number control of nickel. Only when the average oxidation number of Ni, Mn, and Co of LiNi1-x-yMnxCoyO2 is +3 or slightly higher than +3, LiNi1-x-yMnxCoyO2 may have a layered crystal structure and maintain charge neutrality. Cobalt ion prefers the electron configuration of Co3+, while nickel and manganese ion prefers the electron configuration of Ni2+ and Mn4+ to Ni3+ and Mn3+ [1,5]. In the case of LiNi1-x-yMnxCoyO2 with high nickel ratio, single-phase layered crystal structure LiNi1-x-yMnxCoyO2 containing Ni2+ cannot be generated. Thus, three-dimensional crystal structure Li1-zNi1-x-y+zMnxCoyO2 in which Li+ and Ni2+ are mixed in a non-stoichiometric ratio in each other, not in their own position, and/or impurities such as Li1+zNi1-x-y-zMnxCoyO2 are inevitably mixed [1,6,7]. Therefore, only selective oxidation from Ni2+ to Ni3+ are required without altering the oxidation number of cobalt and manganese ions. Although it is known that one of the fundamental disadvantages of LiNi1-x-yMnxCoyO2 is an inappropriate but stable oxidation state of Ni2+, many indirect efforts have been made to solve this disadvantage, but unfortunately, there is no direct effort to solve this disadvantage [8-12]. It's important to note that the layered crystal structure LiNi1-x-yMnxCoyO2 is composed of a stacked combination of Ni1-x-yMnxCoyO2 layers and LiO2 layers.
In this study, the solubility control depending temperature of coprecipitation conditions for homogeneous arrangement of Ni, Mn, and Co and the process conditions for only selective oxidation from Ni2+ to Ni3+ without altering the oxidation number of cobalt and manganese ions were established at the same time.
Experimental
A fabrication of a layered crystal structure Li1-xNi1- x-yMnxCoyO2 (0.1 ≤ x + y ≤ 0.5) with homogeneous arrangement of Ni3+, Mn4+, and C3+ may be summarized into three ways.
Homogeneous Arrangement of Ni, Mn, and Co in the Ni1-x-yMnxCoy Coprecipitation Granules Through a Rapid Hydroxide Coprecipitation Method in 580o C Aqueous Solution of Nickel Sulfate, Manganese Sulfate, and Cobalt Sulfate
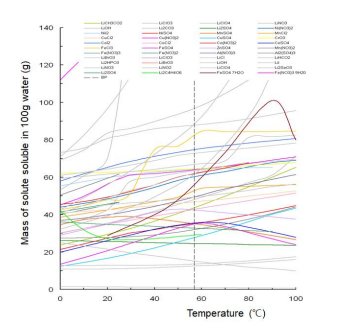
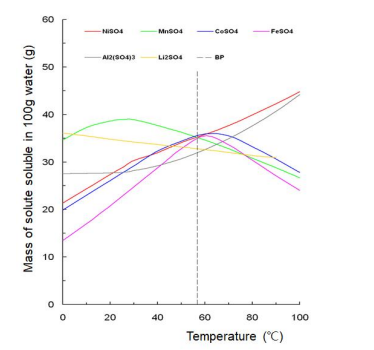
The solubility of various Li salts and transition metal salts was evaluated according to temperature. For the production of Li1-xNi1-x-yMnxCoyO2 (0.1 ≤ x + y ≤ 0.5) with homogeneous arrangement of Ni, Mn, and Co using a liquid reaction method, the evaluated solubilities were relatively compared to determine Ni salt, Mn salt, and Co salt having the same solubility at a specific temperature. These results are shown in Figure 1 and 2.
As shown in Figure 2, nickel sulfate, manganese sulfate, cobalt sulfate, and iron sulfate have the same solubility in 58o C water. However, lithium sulfate and aluminium sulfate have a slightly lower solubility than nickel sulfate, manganese sulfate, cobalt sulfate, and iron sulfate in 58o C water. The small difference in solubility between lithium sulfate and nickel/manganese/cobalt sulfates in 58o C water indicates that aqueous ionization solution solgel synthesis method cannot be applied to the preparation method of single-phase layered crystal structure Li1-xNi1-x-yMnxCoyO2 . The same solubility of nickel sulfate, manganese sulfate, and cobalt sulfate in 58o C water represents that a precursor for producing Li1-xNi1-x-yMnxCoyO2 with homogeneous arrangement of Ni, Mn, and Co can be produced through a rapid hydroxide coprecipitation method in 58o C aqueous solution of nickel sulfate, manganese sulfate, and cobalt sulfate.
A mixed aqueous solution of potassium hydroxide (KOH), ammonium hydroxide (NH4OH), and sodium hydroxide (NaOH) was selected as alkaline aqueous solutions for rapid hydroxide coprecipitation. Mixing molar ratio of potassium hydroxide (KOH), ammonium hydroxide (NH4OH), and sodium hydroxide (NaOH) depends on the molar ratio of Ni, Mn, and Co in Li1-xNi1-x-yMnxCoyO2 , as well as a fabrication amount. Here, it should be considered that the following three risk factors must be overcome. Since sodium ions have a smaller ionic radius than potassium ions, they may remain even after several filtrations and washings in the process of recovering secondary coprecipitation granules (result of collection, occlusion, and adsorption of primary coprecipitation particles). Because the remained sodium ions in secondary coprecipitation granules evidently remain in Li1-xNi1-x-yMnxCoyO2 as cathode active material, they adversely affect lithium ion battery performances. Therefore, it is recommended to minimize the content of sodium hydroxide. Potassium hydroxide aqueous solution has the disadvantage of being highly toxic. In addition, ammonium hydroxide helps to uniformize particle size, collect primary particles, and spheroidize granules, but when its amount is excessive, it has a negative effect.
Selective Oxidation of Ni2+ up to Ni3+ without Changing the States of Co3+ and Mn4+ in Secondary Coprecipitation Granules
According to the characteristics of nickel ion and cobalt ion in the layered crystal structure Li1-xNi1-x-yMnxCoyO2, both nickel ion and cobalt ion are located in the octahedral sites surrounded by six oxygen ions, have a low spin electron configuration, as well as an electronpairing energy (P) and an energy difference between t2g and eg orbital energy levels noted as 10Dq or Δoct is in a 6Dq < P < 10Dq relationship for nickel ion, and in a P < 6Dq relationship for cobalt ion. The crystal field stabilization energy is -18Dq + P for Co2+, 24Dq + 2P for Co3+, and -20Dq + 2P for Co4+. Therefore, a cobalt ion prefers the most stable electron configuration of Co3+. By the way, a nickel ion has the crystal field stabilization energy of 12Dq for Ni2+, 18Dq + P for Ni3+, and -24Dq + 2P for Ni4+, so it prefers the most stable electron configuration of Ni2+ [1]. A manganese ion prefers the most stable electron configuration of Mn4+.
In order that the average oxidation number of Ni, Mn, and Co of LiNi1-x-yMnxCoyO2 (0.1 ≤ x + y ≤ 0.5) is +3 or slightly higher than +3, the oxidation number of Ni in the LiNi1-x-yMnxCoyO2 should be +3.
Oxidants capable of increasing the oxidation state of transition metal ions such as Ni, Mn, and Co include potassium permanganate (KMnO4 ), sodium perborate (NaBO3 ), sodium perchlorate (NaClO4 ), sodium chlorate (NaClO3 ), sodium chlorite (NaClO2 ), and sodium hypochlorite (NaClO). However, none of these oxidants selectively oxidized Ni2+ to Ni3+ without changing the states of Co3+ and Mn4+. These oxidants oxidized Co3+ to Co4+ and/or Ni2+ even further to Ni4+. Therefore, in order to control their oxidation power, various oxidizing agent-reducing agent hybrid composites were prepared by adding agent lower than their oxidation power with a different amount and then evaluated. As a result, a mixed aqueous solution of NaClO and hydrogen peroxide (H2O2 ) could selectively oxidize Ni2+ to Ni3+ without changing the states of Co3+ and Mn4+ of the secondary coprecipitation granules. The optimal mixing ratio and each concentration of the NaClO and H2O2 aqueous solutions vary depending on the composition mole ratio of Ni, Mn, and Co, as well as the amount of the secondary coprecipitation granules. However, if the concentration of the mixed aqueous solution of NaClO and H2O2 is higher than 0.05M, dissolution of the secondary coprecipitation granules may occur.
Fabrication of Li1-xNi1-x-yMnxCoyO2 with Homogeneous Arrangement of Ni3+, Mn4+, and Co3+
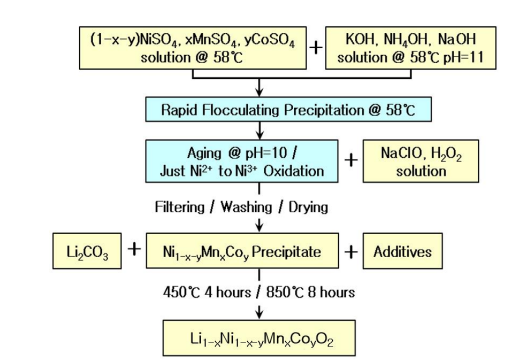
Figure 3 shows the fabrication process diagram of Li1-xNi1-x-yMnxCoyO2 (0.1 ≤ x + y ≤ 0.5) with homogeneous arrangement of Ni3+, Mn4+, and Co3+. During the mixing of the secondary coprecipitation granules (Ni1-x-yMnxCoy coprecipitate) and Li2CO3 , sucrose is added as an additive to prevent the agglomeration of the Li1-xNi1-x-yMnxCoyO2 powders. The mixed molar ratio of Li2CO3 , secondary coprecipitation granules, and sucrose is 0.525 : 1 : 0.1.
The prepared Li1-xNi1-x-yMnxCoyO2 (0.1 ≤ x + y ≤ 0.5) is characterized by means of Xray diffraction (XRD) spectroscopy, inductively coupled plasma-mass spectroscopy (ICP-MS), X-ray absorption near edge structure (XANES) spectroscopy, extended X-ray absorption fine structure (EXAFS) spectroscopy, and Li magic angle spin nuclear magnetic resonance (Li MAS NMR) spectroscopy.
Electrochemical lithium intercalation and deintercalation experiments of the prepared Li1-xNi1-x-yMnxCoyO2 (0.1 ≤ x + y ≤ 0.5) were realized by charge and discharge of coin type lithium secondary batteries using a mixture of the products (94% in weight) with SuperP/VGCF (3% in weight) and polyvinylidene fluoride (PVdF, 3% in weight) as cathode, lithium foil as anode and a 1 M LiPF6 solution in ethylene carbonate (EC) and ethyl methyl carbonate (EMC) (1 : 2, volumetric ratio) as electrolyte.
Results and Discussion
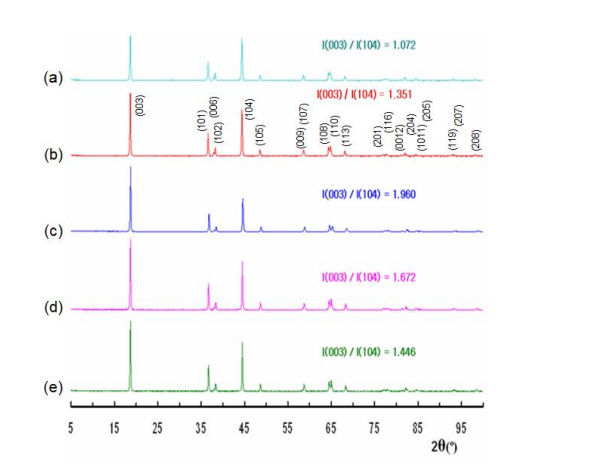
Figure 4 illustrates the X-ray diffraction (XRD) pattern of commercialized LiNi0.8Mn0.1Co0.1O2 and Li0.9Ni0.8Mn0.1Co0.1O2manufactured according to the change in the mixing ratio and concentration of NaClO and H2O2 aqueous solution under the conditions for keeping the states of Ni3+, Mn4+, and Co3+.
The intensity ratio of the (003)/(104) diffraction peaks, symbolized as I(003)/I(104), for commercialized LiNi0.8Mn0.1Co0.1O2 is lower than the cation mixing recognition criterion of 1.2, but I(003)/I(104) for all four Li0.9Ni0.8Mn0.1Co0.1O2 is higher than 1.2. The results of I(003)/I(104) and [I(006)+I(102)]/I(101) Bragg peak intensity analysis, as well as peak splits in the (006)/(102) and (108)/(110) doublets, and show that all four Li0.9Ni0.8Mn0.1Co0.1O2 do not contain a representative impurity with a space group P3112 structure as well as the cation mixing phenomenon do not occur in the all four Li0.9Ni0.8Mn0.1Co0.1O2 [13-15]. Thus, the XRD analysis reveals that all four Li0.9Ni0.8Mn0.1Co0.1O2 have a single-phase layered crystal structure with a space group R-3m despite changes in the mixing ratio and concentration. These results indirectly show the successful preparation of Li0.9Ni0.8Mn0.1Co0.1O2 with Ni3+ , Mn4+, and Co3+.
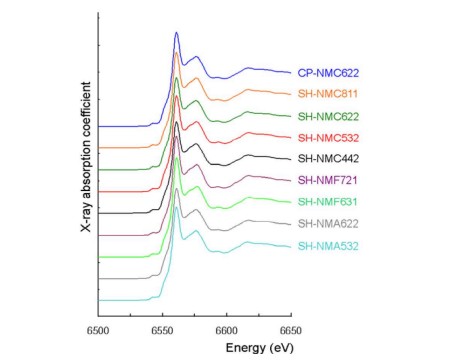
Figure 5 illustrates the Ni X-ray absorption near edge structure (XANES) spectroscopic results of NiO, commercialized LiNi1-x-yMnxCoyO2 manufactured by the general coprecipitation-heat treatment method (CP-NMC), and Li1- xNi1-x-yMnxCoyO2 prepared by this study (SH-NMC). In addition, CP-NMC622 is an abbreviation for commercialized LiNi0.6Mn0.2Co0.2O2 produced by the general coprecipitation-heat treatment method. For consult, SH-NMC, SHNMF and SH-NMA stand for Li1-xNi1-x-yMnxCoyO2 , Li1-xNi1-x-yMnxFeyO2 and Li1-xNi1-x-yMnxAlyO2 prepared by this study, respectively. According to Coulomb's law, as the positive and negative charges increase, the attraction between the positive and negative charges strengthens. Therefore, as the Ni oxidation number increases, the electron binding energy required to separate electrons from Ni ion increases. According to the results of the Ni XANES spectroscopy analysis in Figure 5, the Ni oxidation numbers of NiO, CP-NMC, and SH-NMC are +2, value between +2 and +3 (indicating the presence of both Ni2+ and Ni3+), and +3, respectively
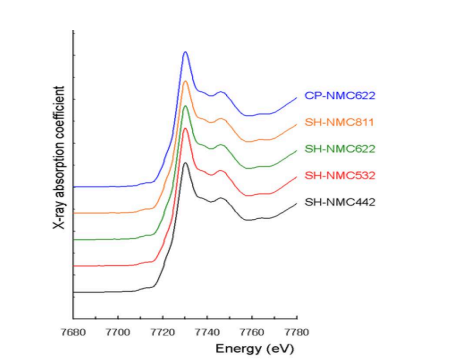
Mn XANES spectroscopy results and Co XANES spectroscopy results of CPNMC and SH-NMC are summarized in Figs. 6 and 7, respectively. According to the results of Mn and Co XANES spectroscopy, the Mn and Co oxidation numbers of CPNMC are +4 and +3, respectively, as well as the Mn and Co oxidation numbers of SHNMC are also +4 and +3.
Moreover, the Ni, Mn, and Co XANES spectroscopy results of SH-NMC show that Ni3+, Mn4+, and Co3+ are all located in octahedral sites through their pre-edge absence and white line shapes, as well as there is no hybridization of d electron orbits and p electron orbits
Consequently, it is confirmed that layered crystal structure Li1-xNi1-x-yMnxCoyO2 (0.1 ≤ x + y ≤ 0.5) with Ni3+ , Mn4+, and Co3+ may be prepared.
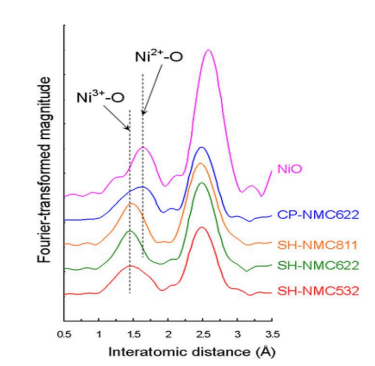
Figure 8 is the results of Fourier transform into r-space of k3 -weighted Ni K-edge extended X-ray absorption fine structure (EXAFS) spectroscopy information of NiO, CP-NMC, and SH-NMC. Figure 8 also represents the indirect support for Ni oxidation number of Li1-xNi1-x-yMnxCoyO2 through the results of Ni-O bond length.
As the difference in oxidation number between cations and anions intensifies, the attraction between cations and anions becomes stronger, and the bond length between cations and anions becomes shorter. Thus, the fact that the Ni-O bond lengths of NiO, CP-NMC, and SH-NMC are gradually shortened in order means that the Ni oxidation numbers of NiO, CP-NMC, and SH-NMC are gradually increasing in order.
Ni, Mn, and Co have nuclear spin quantum numbers of 7/2, 5/2, and 3/2, respectively. Their nuclear proton spins have magnetic spin-spin interactions not only with neighboring proton spins but also with electron spins. Here, the difference between substances occurs. Ni3+ has six paired 3d electrons and one unpaired 3d electron, while Mn4+ has three unpaired 3d electrons. As Ni3+ and Mn4+ have unpaired electrons, both Ni3+ and Mn4+ exhibit paramagnetism. Therefore, magnetic spin-spin interaction can be performed. On the other hand, Co3+ and Ni2+ have diamagnetism because they have only six paired 3d electrons. The difference is shown in Figure 9 as single signal peak and multiple signal peaks, which are the Li magic angle spin nuclear magnetic resonance (Li MAS NMR) microstructure information.
As Li1-xNi1-x-yMnxCoyO2 was prepared through a rapid hydroxide coprecipitation at the same solubility of nickel sulfate, manganese sulfate, and cobalt sulfate in 580 C aqueous solution as well as a selective oxidation process of Ni2+ to Ni3+ without changing the states of Co3+ and Mn4+, it is expected that Li1-xNi1-x-yMnxCoyO2 was made of homogeneous arrangement of Ni3+, Mn4+, and Co3+. By the way, LiNi1-x-yMnxCoyO2 produced through general hydroxide coprecipitation at room temperature has a concentration gradient characteristic of each component due to the precipitation of each component in a different order depending on the difference in solubility of each component. Strictly, LiNi1-x-yMnxCoyO2 with a concentration gradient is a mixture, not a solid solution capable of controlling material properties. For this reason, paramagnetic components and dimagnetic components are distinguished. In this case, the diamagnetic Co3+, the diamagnetic Ni2+ , the paramagnetic Ni3+ and the paramagnetic Mn4+ are individually and locally concentrated, so the Li MAS NMR spectrum of LiNi1-x-yMnxCoyO2 produced through general hydroxide coprecipitation at room temperature might show a combination of broad single signal peak and sharp multiple signal peaks. Because the Li MAS NMR spectrum of Li1-xNi1-x-yMnxCoyO2 prepared by this study does not show such the combination, it is considered that Li1-xNi1-x-yMnxCoyO2 prepared by this study was made of homogeneous arrangement of Ni3+ , Mn4+, and Co3+.
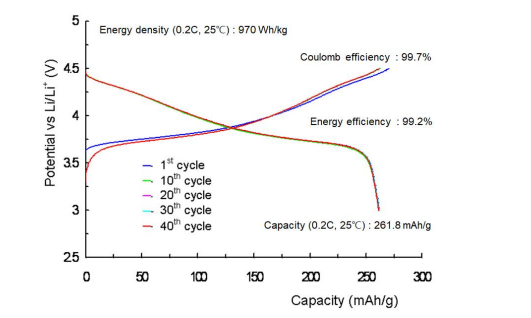
Figure 10 illustrates charge and discharge curves of a coin cell lithium secondary battery manufactured using SH-NMC811 as a cathode active material at 0.2C rate and 25o C. The theoretical discharge capacity of a lithium secondary battery manufactured with LiNi0.8Mn0.1Co0.1O2 is 275.47 mAh/g, and the actual discharge capacity of a lithium secondary battery manufactured with SHNMC811 is 261.84 mAh/g. This is 95.1% of the theoretical discharge capacity per weight. Considering the partially irreversible energy conversion that always occurs in the first charging and discharging cycle, the coulombic efficiency ((discharge capacity)/(charge capacity)) and energy efficiency ([(coulombic efficiency)x(average discharge potential)]/(average charge potential)) of the lithium secondary battery manufactured using SH-NMC811, evaluated from second charging and discharging cycle to 40 charging and discharging cycles, are 99.7% and 99.2%, respectively, which represent very high energy conversion efficiency and also very high battery capacity retention rates. The partially irreversible conversion caused by the formation of solid electrolyte interphase (SEI) films is a necessary evil that inevitably contributes to the stabilization, safety and capacity decrease of a lithium secondary battery [1].
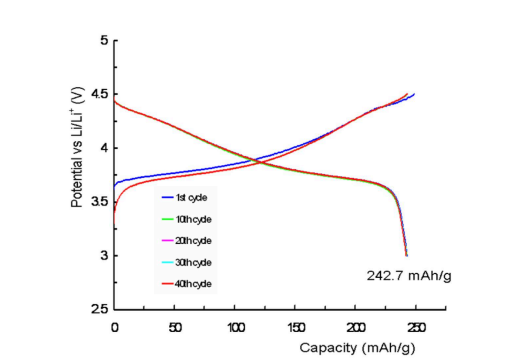
Figure 11 shows charge and discharge curves of a coin cell lithium secondary battery manufactured using SHNMC622 as a cathode active material at 0.2C rate and 250 C. The theoretical discharge capacity of a lithium secondary battery manufactured with LiNi0.6Mn0.2Co0.2O2 is 276.48 mAh/g, and the actual discharge capacity of a lithium secondary battery manufactured with SH-NMC811 is 242.73 mAh/g. This is 87.8% of the theoretical discharge capacity per weight. The lithium secondary battery produced with SH-NMC622 also experiences typical irreversible conversion in the first charging and discharging cycle. From the second charging and discharging cycle onwards, it demonstrates good stability, with high energy conversion efficiency and a nice battery capacity maintenance rate.
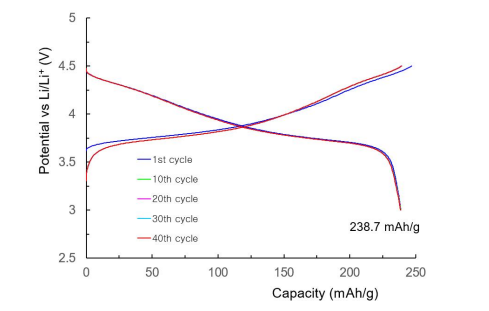
Figure 12 illustrates charge and discharge curves of a coin cell lithium secondary battery manufactured using SH-NMC523 as a cathode active material at 0.2C rate and 250 C. The theoretical discharge capacity of a lithium secondary battery manufactured with LiNi0.5Mn0.2Co0.3O2 is 276.42 mAh/g, and the actual discharge capacity of a lithium secondary battery manufactured with SH-NMC523 is 238.69 mAh/g. This is 86.3% of the theoretical discharge capacity per weight. The lithium secondary battery produced with SH-NMC523 also experiences typical irreversible conversion in the first charging and discharging cycle. From second charging and discharging cycle to 40 charging and discharging cycles, it demonstrates a remarkable stability, a superior energy conversion efficiency, and an amazing battery capacity maintenance rate of 99.8%.
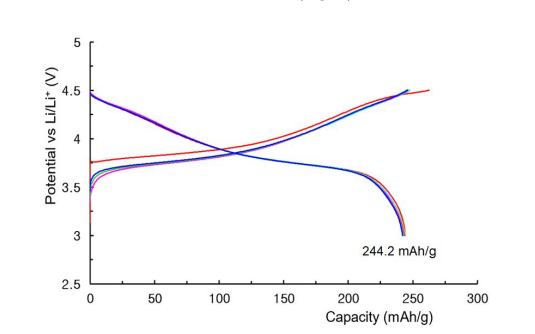
Figure 13 shows charge and discharge curves of a coin cell lithium secondary battery manufactured using SHNMC523 as a cathode active material at 0.2C rate and 600 C. The theoretical discharge capacity of a lithium secondary battery manufactured with LiNi0.5Mn0.2Co0.3O2 is 276.42 mAh/g, and the actual 600 C discharge capacity of a lithium secondary battery manufactured with SH-NMC523 is 244.22 mAh/g. This is 88.4% of the theoretical discharge capacity per weight. Unlike charging and discharging at 250 C, the lithium secondary battery manufactured with SHNMC523 exhibits very slight decrease battery capacity from the second charging and discharging cycle onwards at 600 C.
Conclusion
An application of a lithium secondary battery manufactured with LiNi1-x-yMnxCoyO2 is limited by intrinsic disadvantages of LiNi1-x-yMnxCoyO2 . To overcome the intrinsic disadvantages of LiNi1-x-yMnxCoyO2 such as inappropriate but stable oxidation state of Ni2+ as well as uneven arrangement of Ni, Mn, and Co within LiNi1-x-yMnxCoyO2 , the solubility control according to coprecipitation temperature for homogeneous arrangement of Ni, Mn, and Co, as well as the selective oxidation control from Ni2+ to Ni3+ without changing the states of Co3+ and Mn4+ are established. In this way, single-phase layered crystal structure Li1-x-yMnxCoyO2 (0.1 ≤ x + y ≤ 0.5) with homogeneous arrangement of Ni3+, Mn4+, and Co3+ has been prepared and characterized by means of XRD, XANES, EXAFS, and Li MAS NMR.
The battery performance of the Li1-xNi1-x-yMnxCoyO2 (0.1 ≤ x + y ≤ 0.5) obtained by the developed preparation method shows a maximum available capacity of 262mAh/g and a capacity retention rate of 99.7% in 40 charging and discharging cycles. Additionally, the battery capacity of the lithium secondary battery made with Li1-αNi1-x-yMnxCoyO2 increases as the ratio of Ni increases.
Acknowledgement
This work was supported by research fund of Chungnam National University.
- Park JK, Doh CH, Han KS, Hong YS, Kang KS et al. (2012) Principles and applications of lithium secondary batteries, John Wiley & Sons.
- Min K, Kim K, Jung C, Seo SW, Song YY et al. (2016) A Comparative Study of Structural Changes in Lithium Nickel Cobalt Manganese Oxide as a Function of Ni Content during Delithiation Process, J. Power Sources 315: 111-9.
- Li W, Erickson EM, Manthiram A (2020) High-nickel Layered Oxide Cathodes for Lithium-based Automotive Batteries, Nature Energy 5: 26-34.
- Sun YK, Myung ST, Kim MH, Prakash J, Amine K (2005) Synthesis and Characterization of Li[(Ni0.8Co0.1Mn0.1)0.8(Ni0.5Mn0.5)0.2]O2 with the Microscale Core-shell Structure as the Positive Electrode Material for Lithium Batteries, J. Electrochem. Soc 127: 13411-8.
- Koyama Y, Yabuuchi N, Tanaka I, Adachi H, Ohzuku T (2004) Solid-State Chemisty and Electrochemistry of LiCo1/3Ni1/3Mn1/3O2 for Advanced Lithium-Ion Batteries I. First-Principles Calculation on the Crystal and Electronic Structures, J. Electrochem. Soc 151: 1545-51.
- Ohzuku T, Ueda A, Nagayama M (1994) Electrochemistry and Structural Chemistry of LiNiO2 (R-3m) for 4V Secondary Lithium Cells, J. Electrochem. Soc 140: 1862-70.
- Thackeray MM, Kang SH, Johnson CS, Vaughey JT, Benedek R, Hackney A (2007) Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) Electrodes for Lithium-Ion Batteries, J. Mater. Chem 17: 3112-25.
- Anansuksawat N, Chiochan P, Homlamai K, Jo raleechanchai N, Tejangkura W, Sawangphruk M (2023) Reducing Intrinsic Drawbacks of Ni-rich Layered Oxide with a Multifunctional Materials Dry-coating Strategy, J. Power Sources 554: 232324.
- Han E, Li Y, Zhu L, Zhao L (2014) The Effect of MgO Coating on Li1.17Mn0.48Ni0.23Co0.12O2 Cathode Material for Lithium Ion Batteries, Solid State Ionics 255: 113-9.
- Chen Y, Zhang Y, Chen B, Wang Z, Lu C (2014) An Approach to Application for LiNi0.6Co0.2Mn0.2O2 Cathode Material at High Cutoff Voltage by TiO2 Coating, J. Power Sources 256: 20-7.
- Hu SK, Cheng GH, Cheng MY, Hwang BJ, Santhanam R (2009) Cycle Life Improvement of ZrO2-coated Spherical LiNi1/3Co1/3Mn1/3O2 Cathode Material for Lithium Ion Batteries, J. Power Sources 188: 564-9.
- Kim UH, Lee SB, Ryu JH, Yoon CS, Sun YK (2023) Optimization of Ni-rich Li[Ni0.92 xCo0.04Mn0.04Alx]O2 Cathodes for Lithium-ion Batteries, J. Power Sources 564: 232850.
- Guilmard M, Rougier A, Grüne M, Croguennec L, Delmas C (2003) Effects of Aluminum on the Structural and Electrochemical Properties of LiNiO2, J. Power Sources 115: 305-14.
- Vadivel S, Srimanon K, Sawangphruk M (2022) Comparison of the Structural and Electrochemical Properties of Layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85), J. Power Sources 537: 231526.
- Noh HJ, Youn SJ, Yoon CS, Sun YK (2013) Comparison of the Structural and Electrochemical Properties of Layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85), J. Power Sources 233: 121-30.



Figures at a glance