New Metallomesogen Chemical Mixtures by In-Situ One-Pot Chemical Solution Synthesis Method
Received Date: February 11, 2024 Accepted Date: March 11, 2024 Published Date: March 14, 2024
doi: 10.17303/jmsa.2024.8.102
Citation: H. Hakemi (2024) New Metallomesogen Chemical Mixtures by In-Situ One-Pot Chemical Solution Synthesis Method. J Mater sci Appl 8: 1-7
Abstract
We provide new chemical mixtures of metallomesogens (MOM) by novel one-pot solution synthesis method as alternative approach to conventional physical mixing technique in order to resolve the single-component MOM shortcomings such as high temperature mesogenic range and low solubility in commercial liquid crystal hosts. In this work, we provide the transition temperatures, mesomorphism and eutectic behavior of MOM chemical mixtures based on Salicylal-diaminate Ni2+ and VO2+ metal-complex model structures. The solubility of a MOM mixture in a commercial liquid crystal TNO623 host demonstrates that MOMs could be utilized as advanced materials for potential applications in guest-host display devices.
Keywords: Metallomesogen; Liquid Crystal; Chemical Mixing; One-Pot Synthesis; Eutectic Mixture
Introduction
It is known that presence of metal center in chemical structures of organic liquid crystalline materials could have a dramatic effect on their thermal, physical, mesomorphism and electro- optical properties. The metal-containing liquid crystals known as "metallomesogens" (MOM) have been considered as promising materials for application. This quest has been fulfilled to some extent at the molecular scale by introducing high coordination geometries, lowering of symmetry, reducing of lateral interactions, use of different terminal chains and correlated structures [1-7].
Although the structure-property relations have been found for some MOM chemical structures, they are not yet conclusive for overall perspective of their application. Aside from purely scientific ciuriosity, MOMs can be potentially utilized as dyes, UV absorbers, voltage regulators, optical filters and electrooptical modifiers in liquid crystal devices. In spite of extensive studies on chemistry of MOMs, few attempts have been made on their commercial applications [1,8,9]. Recently, few attempts have been proposed in the literatue on potential utilization of MOMs in photo-luminescence [10-13], electro-luminescence [9,14], magnetic [15,16] and electric [17-20] for few single-component MOMs. However, until now MOMs have not been yet commerciallized even in guest-host liquid crystal materials. Among the major draw backs of MOMs are their high transition temperatures, decomposition at high temperature, inaccesible mesophase range and low solubility in liquid crystal hosts.
The real challenge for commercial utilization of MOMs is not much in parallel to those found in organic liquid crystals or the coordination chemistry, but is to other aspects that are not found in the organic mesogens. In addition to proper molecular engineering and synthesis, in order to overcome these drawbacks, an alternative approach for commercial application of MOMs is the development of multi-component MOM mixtures with eutectic behavior to facilitate their solubility in commercial liquid crystal materials. Consequently, in addition to the synthesis of proper MOM chemical structure, the major factor to their application will be MOM miscible mixtures. Recently, by conventional physical mixing method, we studied eutectic MOM mixtures with wide transition temperatures and mesophases and showed their miscible solutions in commercial liquid crystal hosts [21-25].
In this study, we provide the first study of chemical mixing approach by one-pot solution synthesis method on some model MOM structures of salicylal-diaminate Ni2+ and VO2+ metal-complexes. Accordingly, we studied the tramsition temperatures, mesomorphism and and eutectic behavior of synthesized MOM chemical mixtures, as well as the solubility of one Eutectic MOM mixture in commercial TNO623 liquid crystal host to provide their potential application in liquid crystal devices in the following sections.
Materials and Methods
Materials
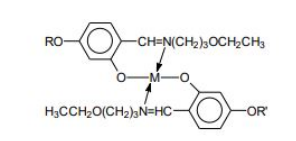
The "one-pot" solution synthesis of multi-component MOM mixtures based on Salicylal- diaminates Ni2+ and VO2+ metal complexes were carried out by simultaneous reaction of appropriate precursors. Each "in-situ" MOM mixture consisted of a three-component system corresponding to the following general chemical structure:
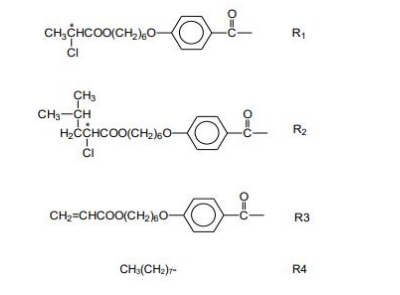
We prepared six three-component "in-situ" MOM mixtures at various combinations of metals and ligands. The structural variations are obtained by changing the structure of ligand's terminal R and R’groups. Each component of the mixture designated with the short formula L-MII-L, consisted of either MII = Ni2+ and VO2+ complexed to different combinations of ligands with aliphatic (L), chiral (L*) or acrylic (L') chains shown in the following structures:
The details of synthetic procedure and preparation of three-component MOM mixtures is chemically analogous to the solution synthesis method mentioned elsewhere [26-28]. The corresponding Ni2+ and VO2+ MOM chemical mixtures carried out by one-pot solution synthesis method are presented in Table-1.
Methods:
The transition temperatures and mesomorphisms of MOM mixtures were determined by indium calibrated Perkin Elmer DSC7 Differential Scanning Calorimeter (DSC) and Nikon Eclipse-50i polarizing optical microscope (POM) equipped with a temperature- controlled Mettler FP5 microscopic hot-stage. The thermo grams of MOM mixtures were carried in DSC pan through repeated heating and cooling modes with scanning rates of 10° C/min and 5° C/min, respectively, until there was no change in their thermograms. The phase behaviour of all synthesized MOM chemical mixtures were investigated by both DSC and POM techniques.
Results and Discussion
The new series of MOM chemical mixtures based on Salicylal-diaminate Ni2+ and VO2+ metal-complexes were prepared by one-pot solution synthesis technique, as presented in Table-1. In Table-2, we present the results of crystal-mesogen (Tcm) and mesogen-isotropic (Tmi) phase transitions on heating mode, the isotropic-mesogen (Tim) and mesogen-crystal (Tmc) phase transitions on cooling mode, as well as the mesomorphisms of MOM chemical mixture complexes. A brief description of the results regarding the transition temperatures and mesomorphisms of MOM chemical mixtures and MOM/TNO623 mixture are described as follows:
MOM Chemical Mixtures:
The overall crystal-mesophase transition temperature (Tcm) ranges of MOM-Ni2+ complexes are higher than those of MOM-VO2+ complex pairs. More specifically, the Snia1-Ni and Snia2-VO complexes exhibit the highest Tcm with two crystalline states. On the other hand, the Snia8-Ni and Snia10-VO complex pairs do not exhibit any crystalline states and show truly eutectic behavior.
Moreover, in the Snia9-Ni and Snia11-VO complex pairs, whereas the former exhibits a Tcm transition, the latter does not exhibit crytalline state and is a eutectic type mixture. Also the crystal-mesophase (Tcm) transition temperatures on heating of MOM-Ni2+ mixtures are within 70-120 °C range whereas those of MOM-VO2+ mixtures are within 75-85 °C range, except those of Snia8-Ni, Snia10-VO and Snia11-VO complexes. It should be noted that the average transition temperatures of Ni2+ and VO2+ MOM chemical mixtures are lower and their mesogenic ranges are wider than those of single-component Ni2+ and VO2+ MOM complexes reported previously [21,29].
Furthermore, the overall mesophase-isotropic (Tmi) transition temperature ranges of MOM- Ni2+ complexes on heating are higher than those of MOM-VO2+ complexes. For example, the Snia5-Ni complex exhibits the largest mesophase range of 37°C, whereas the Snia2-VO and Snia7-VO complexes exhibit the smallest mesophase ranges of 9°C, respectively. On the other hand, the mesophase-isotropic (Tmi) transition temperatures of MOM-Ni2+mixtures are within 95-157 °C range, whereas those of MOMVO2+ mixtures are within 41-106 °C range. It is noticed that, the unexpected low Tmi (41 °C) of Snia11-VO suggests the direct use of this MOM complex in commercial liquid crystal materials for potential application.
Also according to Table-2, the Tim and Tmi mesophase transition temperatures of all studied MOM chemical mixtures on repeated cooling and heating modes exhibit complete reversibility within 2-3°C. In addition, their mesomorphic phases do not crystallized by super cooling even below 0°C and their mesophase-crystal (Tmc) transition temperatures are not accessible. These phase behaviour is another indication of eutectic behaviour of the studied MOM-Ni2+ and MOM-VO2+ complexes, which in addition to physical mixing, chemical mixing is another valid approach to prepare materials for application.
According to the last column of Table-2 and depending on the types of organic components (see Table-1), the synthesized eutectic MOM mixtures exhibit the three nematic, cholesteric and smectic mesophases. In more details, the Snia1-Ni / Snia2-VO couple exhibit nematic phase; the Snia8-Ni / Snia10-VO couple exhibit cholesteric phase and the Snia9-Ni / Snia11- VO couple exhibit smectic phase. On the other hand, the remaining two Snia4-Ni / Snia6-VO and Snia5-Ni / Snia7-VO couple complexes show mixed cholesteric and smectic mesophases.
MOM / TNO623 Guest-Host Mixture:
An example of MOM chemical mixture as potential material for application, Ii Table-3, we present the transition temperature and mesomorphism of a mixture of Snia8-Ni guest with commercial TNO623 liquid crystal host at 1/9 weight percent composition. In comparison with Snia8-Ni and TNO623 components, the Snia8-Ni/TNO623 mixture exhibit reproducable mesogenic-isotropic (Tmi=100-101°C) and isotropic-mesogenic (Tim=99-96°C) transitions within those of Snia8-Ni and TNO623 components. Other MOM chemical mixtures that have not been reported here also showed similar transition behaviors. Furthermore, the Snia8- Ni/TNO623 phase transition clearly indicates the complete miscibility of the guest-host components and the mixture shows an expected cholesteric phase contributed by Snia8-Ni guest. The anticipated supper cooling of Snia8-Ni/TNO623 mixture also confirms the eutectic nature of the mixture, which is further stabilized by eutectic TNO623 host.
Conclusion
In this study, for the first time we utilized the in-situ one-pot solution synthesis method as alternative to conventional physical mixing and prepared a series of model MOM chemical mixtures consisting of MOM-Ni2+ and MOM-VO2+ complex structures. Through this novel approach, we developed eutectic type MOM mixtures with wide range mesogenic phases. All studied MOM chemical mixtures exhibited eutectic behavior, did not crystallize upon cooling and maintained their liquid crystalline phases well below 0°C. The eutectic MOM mixtures exhibited nematic, cholesteric and smectic phases with larger mesomorphisms than those of corresponding single-component MOM materials.
We also studied a Snia8-Ni / TNO623 guest-host mixture and demonstrated their complete miscibility with large eutectic behavior. This example provides the potential utilization of eutectic MOM mixtures in commercial liquid crystal hosts for application in a wide-range of optical and electro-optical devices. The present work was carried on few MOM model structures and their commercial application requires further systematic molecular engineering, characterization and final qualification.
Acknowledgment
The author would like to acknowledge the Electro-Optical Film Group of Snia Riceche, Snia BPD (Fiat Group), Via Pomarico, Pisticci Scalo (MT), Italy, who sponsored and financed the research and development projects on Metallomesogens under collaboration contracts with University of Napoli during 1993-1996 period.
- JL Serrano (1996) Metallomesogens: Synthesis, Properties and Applications.
- Donnio B, Bruce DW (1995) Liquid Crystals II Metallomesogens. Mingos D M P, editor, 95.
- Donnio B, Guillon D, Deschenaux R, Bruce DW, Metallomesogens, Comprehensive Coordination Chemistry II, 6.
- RW Date, EF Iglesias, KE Rowe, JM Elliott & Duncan W. Bruce, Dalton Trans, 2003, 1914.
- Bruce DW, Deschenaux R, Donnio B, Guillon D (2006) In Comprehensive Organometallic Chemistry III, Chapter 12: 05.
- Porta B, Khamsi J, Noveron JC (2008) Current Organic Chemistry, 12: 1298.
- A Crispini, I Aiello N. Godbert, M Ghedini, M La Deda (2021) Comprehensive Coordination Chemistry III: 241.
- Y Wang, J Shi, J Chen, W Zhu and E. Baranoff (2015) J. Mater. Chem. C, 3: 7993.
- Y Wang, J Fan, J Shi, H Qi, E Baranoff, et al. (2016) Dyes Pigments, 133: 238.
- M Krikorian, S Liu and TM Swager, (2014) J. Am. Chem. Soc, 136: 2952.
- H Geng, K Luo, H Cheng, S Zhang, H Ni, et al. (2017) RSC Adv, 7: 11389.
- Materials Cristián Cuerva de Alaíz, PhD thesis, (2018) Deprtment of Inorganic Chemistry, University of Madrid.
- K Rajendiran, T Yoganandham S Arumugam, D Arumugam, K Thananjeyan (2021) Journal of Molecular Liquids, 321: 1-114793.
- Cristián Cuerva, Mercedes Cano & Carlos Lodeiro (2021) Chem. Rev, 121: 20-12966.
- SH Liu, MS Lin, LY Chen, YH Hong, CH Tsai et al. (2011) Organic Electronics 12: 15.
- M Seredyuk, M Muñoz, V Ksenofontov, P Gütlich, Y Galyametdinov, JA Real (2014) Inorg. Chem, 53: 8442.
- AJ Fitzpatrick, PN Martinho, BJ Gildea, JD Holbrey, GG Morgan (2016) Eur. J. Inorg. Chem, 2025.
- A Ionescu, N Godbert, A Crispini, R Termine, A Golemme (2012) J. Mater. Chem, 22: 23617.
- PYS Su, JCW Tseng, KM Lee, JC Wang and IJB Lin (2014) Inorganic Chemistry, 53: 5902.
- PYS Su, SJ Hsu, JCW Tseng, HF Hsu, WJ Wang and IJB Lin (2016) Chem. Eur. J, 22: 323.
- H Hakemi, A Roviello, A Sirigu, B Panunzi & M Ghedini (2002) Proceedings of 19th ILCC, Edinburgh, UK.
- H Hakemi (2022) Journal of Materials & Polymer Science, 2: 1-6.
- H Hakemi (2022) Journal of Materials & Polymer Science, 2: 1-5.
- H Hakemi (2023) Nanotechnology & Advanced Material Science, 6: 1-5.
- H Hakemi, (2023) Intern. Mat. Sci. & Eng. Intern. J, 7: 108.
- U Caruso, A Roviello, A Sirigu (1990) Liquid Crystal, 7: 421.
- U Caruso, A Roviello, A Sirigu (1991) Macromolecules, 24: 2606.
- H Hakemi, V Roviello, U Caruso (2023) Inorganics, 11: 32.
- A Sirigu, A Roviello, H Hakemi & S Pane (1993) SniaRicerche Internal Report.

Tables at a glance
Figures at a glance