The Osteogenic Effects of Simvastatin on Normal Human Osteoblasts
Received Date: May 01, 2024 Accepted Date: June 01, 2024 Published Date: June 03, 2024
doi: 10.17303/jmsa.2024.8.103
Citation: K. Maheshwari, K. Singla, V. Maheshwari, L. Chou (2024) The Osteogenic Effects of Simvastatin on Normal Human Osteoblasts. J Mater sci Appl 8: 1-11
Abstract
Statins, hydroxymethylglutaryl-coenzyme-A reductase inhibitors (HMG-Co-A), are known to reduce plasma cholesterol levels. Interestingly, Simvastatin was previously reported to positively affect the proliferation and odontoblastic differentiation of human dental pulp cells. The purpose of this study was to compare the effectiveness of different concentrations of Simvastatin on the differentiation and mineralization of normal human osteoblasts.
Osteoblasts were cultured with Simvastatin at various concentrations of 1, 10, 25, 50, 75, 100 mol/L, and 0 mol/L was used as a control. The differentiation and mineralization were investigated at 7, 14, and 21 days. Statistical analysis was performed using ANOVA. P-values ≤0.05 were considered statistically significant.
The results showed that Simvastatin at all concentrations significantly upregulated ALP activity (P< 0.001), osteocalcin expression (P< 0.001), RUNX2 expression (P< 0.001), and mineralization (P< 0.001) of normal human osteoblasts compared with control.
Keywords: Osteogenic Effects; Statins; Osteoblasts; Simvastatin; Human Dental Pulp Cells
Introduction
Statins, Hydroxymethylglutaryl-coenzyme A inhibitors first identified as fungal extracts of Aspergillus terreus back in 1976 [1], are known to reduce plasma cholesterol levels and cardiovascular morbidity and mortality [2,3]. Statins inhibit the rate-limiting step of cholesterol synthesis by preventing HMG-CoA from being reduced to mevalonate via HMG-CoA reductase [3]. By inhibiting the hepatic cholesterol biosynthesis at the level of HMG-CoA reductase, this drug increases hepatic low-density lipoprotein receptors, resulting in an increased uptake of low-density lipoprotein cholesterol from the blood and the subsequent lowering of circulating cholesterol levels.
In rats, Simvastatin given periorally increased both tibial and vertebral trabecular bone volume and vertebral compressive strength [4]. It also reduced ovariectomy-induced trabecular bone loss in vertebrae [5,6]. In addition, there is evidence that simvastatin delivered in the diet can improve fracture repair [7]. Statins increase bone formation and cancellous bone volume [5,8]. The local application of simvastatin in the tooth extraction socket has been reported to enhance the alveolar bone remodeling [9] and the proliferation and osteoblastic differentiation of human PDL cells [10].
The effect of statins on bone formation has been reported using alternative routes of administration. Orally administered usually produces a deficient concentration of active systemic metabolites and local administration of simvastatin may provide higher tissue dosage [11]. When Simvastatin was administered subcutaneously, it also increased bone formation in calvarial bone in the mouse model [5,12].
Simvastatin has been found to promote osteoblastic activity and inhibit osteoclastic activity [13,14]. Simvastatin enhances the repressive action of FoxO3a on the synthesis of Cyr61 in osteoblasts and subsequently decreases CCL2 production and macrophage recruitment [15]. It inhibits periapical bone resorption by diminishing macrophage chemotaxis to the inflammation site [16].
The anti-inflammatory action of Simvastatin is associated with its effects to induce autophagy and inhibit apoptosis in osteoblasts [17]. The anti-apoptotic effect of simvastatin has been shown in the osteoblasts [17]. Simvastatin inhibited cytokine-stimulated Cyr61 expression in osteoblastic cells and suppressed disease progression and osteoblastic expression of Cyr61 in inflammatory arthritis [18]. Simvastatin alleviates the progression of induced apical periodontitis in rats [16].
In vitro, studies suggest that statins promote osteoblast differentiation and mineralization demonstrated by an increased number of osteoblasts at all stages of differentiation [5]. It is further suggested that these effects lead to the up-regulation of BMP-2 in osteoblast-like cells [19] and osteosarcoma cells [20]. Simvastatin significantly improves the osseointegration of pure titanium implants [21] and enhances fracture healing [22]. This study was designed to further compare the effectiveness of different concentrations of Simvastatin on the differentiation and mineralization of normal human osteoblasts.
Methods
Simvastatin Preparation
Simvastatin was activated by dissolving 25 mg of Simvastatin in 100 uL of Ethanol. 150 uL of 0.1 N NaOH was added to the solution. The mix was incubated at 50o C for 2 hours. The pH was tested and brought down to 7.2 by HCL. The final concentration of the stock solution is 25 mg/ml. The stock solution was kept at -20o C for up to a month [15-17,23].
Cell Culture
Human intra-oral alveolar bone fragments were obtained from healthy patients between the ages of 18 and 50 years old who have no systemic or metabolic bone diseases or acute infections and did not use steroids in the last 6 months before surgery. Bone samples were obtained from discarded bone tissue during dental teeth extraction and other ostectomy procedures under the IRB approval. The procedure to obtain human osteoblast cells was based on a previously published protocol with modifications [24-27]. First, the soft tissue attached to the bone was removed using a sterile surgical blade (Henry Schein). Bone fragments were then cut into 2-4 mm small pieces using a sterile micro dissecting scissor and #11 surgical blade. After the enzymatic digestion of the soft tissue and fibroblasts, bone fragments were cultured in a 12.5 cm2 flask containing 6 mL growth media [10% fetal bovine serum (FBS), 1X Penicillin/Streptomycin antibiotic (100 U/mL), Amphotericin B anti-fungal (2.5 mg/ml) in Dulbecco’s Modified Eagle Medium (DMEM)]. The bone fragments were maintained at 37°C, in a standard CO2 incubator with 5% carbon dioxide, and saturated humidity until the second passage. The culture medium was replaced every three days until the cells reached 80% confluence. The cells were then detached from the flask using 0.05% Trypsin-EDTA (thermos Fisher Scientific, USA) and centrifuged (TJ-6 Beckman Centrifuge) for 5 mins at 1000 rpm. The cells collected in the pellet were counted and utilized for the experiments.
Osteogenic Media Preparation
For differentiation and mineralization studies, growth media was replaced with a pre-osteogenic inductive medium for a total of 48 hours before each predetermined time interval: for Simvastatin it was 7, 14, and 21 days. Preosteogenic media consists of 10% charcoal-stripped fetal bovine serum FBS, 100 U/mL Penicillin G, 100 mg/mL Streptomycin, 10-8 M Menadione, 10 mM β-Glycerophosphate, 1.5 mg/mL L-ascorbic acid, and 2 mM L-glutamine all in a calculated volume of DMEM media.
At 24 hours before each predetermined time interval, the cell culture medium was replaced by a fresh pre-odontogenic inductive medium to which was added 10 nM VitD3. The supernatant fluid was collected on predetermined days. The ALP, OSC, and RUNX2 production were measured from the collected supernatants. The remaining fixed cells in culture plates were used to perform the mineralization assay. The osteogenesis phenotype was confirmed by adding Vitamin D3.
Cell Attachment and Proliferation
To measure the differentiation of the cells on a per million-cell basis, the data on cell attachment and proliferation have been presented in the previous paper [28].
Alkaline Phosphatase Activity Assay
The fluorometric alkaline phosphatase assay kit (Abcam) was employed to measure alkaline phosphatase activity in cell culture supernatants. This kit is an ultra-sensitive, direct, and HTS-ready assay designed to measure ALP activity in serum and bio-samples with a detection sensitivity of 1μU, which is more sensitive than colorimetric assays.
The ALP activity was measured according to the manufacturer’s instructions. For each condition, three samples were employed. In each of a 96-well black plate with a clear bottom, exactly 100 μL culture supernatants were incubated with 20 μL of the non-fluorescent 4-methylumbelliferone phosphatase disodium salt (MUP) substrate; MUP was converted to the fluorescent 4-methylumbelliferone when dephosphorylated by active secreted ALP. After incubation for 30 minutes at 25°C in the dark, the reaction was stopped with 20 μL stop solution by gentle shaking of the plate. The emission was measured at 440 nm by excitation at 360 nm on a microplate reader (TECAN, Infinite 1000 Pro). Three measurements for each value were performed for averaging and alkaline phosphatase activities were calculated by a standard curve and normalized to ALP activity on a per million cell basis.
Osteocalcin ELISA Assay
To measure osteocalcin (OSC) released in osteoblast cell cultures supernatants, a competitive human osteocalcin immunoassay (Quantikine ELISA) was employed. The kit is a 4.5-hour solid-phase ELISA assay and employs a monoclonal antibody that is specific for human osteocalcin and is pre-coated onto a 96-well microplate provided. It also contains an Osteocalcin-HRP conjugate, that binds to human osteocalcin protein, making osteocalcin amounts detectable.
RUNX2 ELISA Assay
This kit was based on sandwich enzyme-linked immune-sorbent assay technology. Anti-RUNX2 antibody was pre-coated onto 96-well plates. The biotin-conjugated anti-RUNX2 antibody was used as the detection antibody. The standards, test samples, and biotin-conjugated detection antibody were added to the wells subsequently and washed with wash buffer. HRP-Streptavidin was added, and unbound conjugates were washed away with a wash buffer. TMB substrates were used to visualize the HRP enzymatic reaction. TMB was catalyzed by HRP to produce a blue color product that changed into yellow after adding an acidic stop solution. The density of yellow is proportional to the RUNX2 amount of sample captured in the plate. The optical density was read at an absorbance of 450nm in a microplate reader, and then the concentration of RUNX2 can be calculated.
Mineralization Assay by Alizarin Red S Staining
Alizarin Red S (ARS), an anthraquinone dye, has been widely used to evaluate calcium deposits in cell culture, which is a sensitive tool for the recovery and semiquantification of ARS in a stained monolayer of cells. Mineralization is assessed by extraction of calcified mineral at low pH, neutralization with ammonium hydroxide, and colorimetric detection at 405 nm. This assay is more sensitive than the cetylpyridinium chloride (CPC) extraction method, improving the detection of weakly mineralizing monolayers.
Statistics
All data were normalized on a per million-cell basis. All experiments were performed in six replicates. Data are presented in means and standard deviations. The means and standard deviations (SD) of osteoblast differentiation and mineralization were tested at 7, 14, and 21 days. Statistical analysis was performed using the software JMP Pro 12 (ver. 12.1.0) in Student’s t-test and ANOVA to detect the statistical differences between the groups. Differences at p ≤ 0.05 were considered statistically significant.
Results
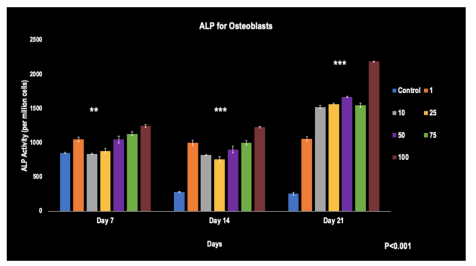
Alkaline Phosphatase Activity
A pattern was observed from days 7, 14, and 21, with a relatively dose-dependent increase in ALP activity from the least to the greatest Simvastatin concentration. Here, the control group's ALP activity decreased from day 7 to 21. By day 21, the 100 umol/L concentration stood out with the highest ALP activity. Statistically significant differences were evident across all groups compared to the control at all time points (p< 0.001).
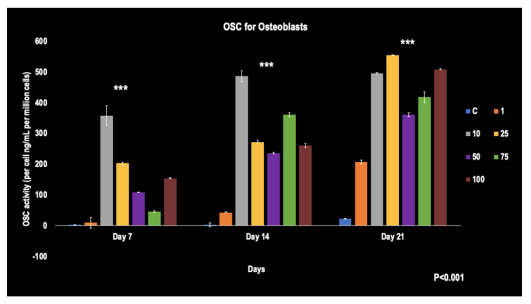
Osteocalcin Levels
As depicted in the figure, all concentrations showed significant increases in osteocalcin expression from day 7 to 21. On days 7 and 14, the 10 umol/L concentration expressed the highest OSC at 375 ng/mL and 475 ng/mL per million cells, respectively. On day 21, the highest OSC level was seen with the 25 umol/L concentration, reaching 550 ng/mL per million cells. Significant differences were observed amongst the groups when compared to the control at each time point (p< 0.001).
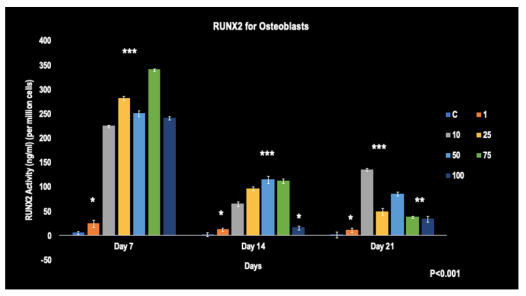
RUNX2 Levels
The figure illustrates a relatively time-dependent decrease in all concentrations over days 7, 14, and 21. Notably, on day 7, the 75 umol/L concentration of Simvastatin showed the highest level of RUNX2 at 347 ng/mL per million cells. By day 21, the 10 umol/L concentration of Simvastatin displayed the highest RUNX2 levels than the rest of the concentrations. All groups showed statistically higher levels of RUNX2 expression compared to the control at each time point (p< 0.001).
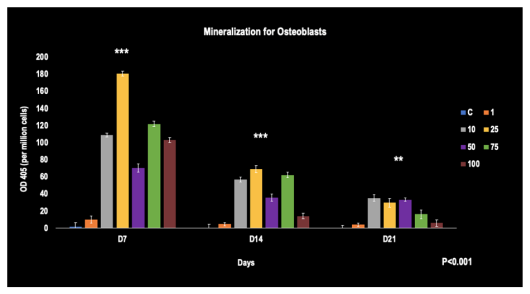
Mineralization
From days 7 to 21, a time-dependent decrease across all concentrations was observed. The 25 umol/L concentration showed the most potent mineralization activity on days 7 and 14. By day 21, most concentrations had significantly decreased. When compared to the control at all time points, statistically significant upregulation of mineralization was identified among all concentrations of Simvastatin (p< 0.001).
Discussion
The effects of statins on bone formation have long been a topic of interest in medical research. One of the first pieces of evidence for this connection came from Mundy et al. in 1999, who reported that statins could stimulate bone formation in rodents [5]. The mechanism was reported to be through the upregulation of bone morphogenic protein-2 (BMP-2) in osteoblasts, which promotes their differentiation into bone-forming cells (29). Furthermore, statins inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-- CoA) reductase, a process that also has a dampening effect on osteoclasts, the cells responsible for bone resorption [30]. More recent studies have also suggested that statins can modulate inflammation, promote osteogenesis and angiogenesis, and inhibit osteoblast apoptosis and osteoclastogenesis [4,5,7,12,31-40]. However, this positive picture [41-43] has been complicated by other studies showing that statins might not always have beneficial effects on bone health [44-46]. In a randomized controlled trial, Simvastatin had no significant effect on bone mineral density and bone turnover in postmenopausal osteopenic women [47]. Some studies have suggested that statins could be harmful to bone healing [14,48-56].
At the in vitro level, the current study found that Simvastatin increased the activity of alkaline phosphatase (ALP), a marker of osteoblast differentiation, in a dose- and time-dependent manner. The results are also in line with previous osteoblast studies [5,19], which reported that statins could increase ALP activity, thus promoting bone formation. However, some caution is required in interpreting these results, as the effects of Simvastatin can be cell-type and context-dependent, and not all studies have found an increase in ALP activity with statin treatment. For example, a study reported that Simvastatin could decrease ALP activity in osteoblasts under inflammatory conditions [57]. The ALP activity of bone marrow stromal cells decreased during the later days of 9 and 12 in cell culture [58]. In the present study, ALP activity was significantly increased at the later time points of cell culture by Simvastatin of all concentrations. While ALP is a marker of osteoblast differentiation, its activity can also increase under pathological conditions such as bone diseases or certain cancers. Therefore, the rise in ALP levels upon Simvastatin treatment should be interpreted in the context of the overall health status and other related biomarkers.
Osteocalcin (OSC) expression in the present study showed significant increases by a few to hundreds of folds in all concentrations of Simvastatin at all-time points when compared to the control. This result showed much more significant increases in OSC expression compared with Baek’s report that showed Simvastatin enhanced the OSC expression of bone marrow stromal cells by approximately 1.5-- fold when compared to the control [58]. Mundy also showed in their study that simvastatin stimulates bone formation in vitro and rodents, increasing the expression of bone morphogenetic protein-2 (BMP-2) and consequently increased osteocalcin levels [5]. Similarly, a study by Maeda showed that simvastatin enhanced bone formation, elevating serum osteocalcin levels in rats [19].
In the present study, RUNX2 levels in normal human osteoblasts decreased over time from day 7 to day 21. This could suggest that differentiation was happening early and then tapers off. Lee found that Simvastatin increases RUNX2 levels in HDPCs at 7 and 14 days [59]. This is like the present study as the results showed an increase in RUNX2 levels at day 7 when compared to the control. Kamada noticed Simvastatin promoted the gene expressions of RUNX2 in the dental pulp cells on day 3, but a suppression on day 16 [60], which is comparable to the present study as RUNX2 levels decreased from day 14 to 21. Simvastatin upregulated the expression of RUNX2 in osteoblasts at day 7 [61], which agrees with the present day 7 results. A particularly prominent finding in the present study was the significant upregulation of RUNX2 expression by all concentra tions of Simvastatin at each time interval in comparison to the control.
The mineralization results showed a time-dependent decrease across all concentrations from days 7 to 21 in culture. At each time point of culture, normal human osteoblasts showed significant upregulation of mineralization by Simvastatin of all concentrations designed in this study compared with control. This result is in line with the previous study showed a significant increase in mineralization in the a-TCP-Sim treated cells when compared with the untreated HDPCs [62]. An increase in mineralization was noticed in PCL/PLLA/HA scaffolds incorporated with Simvastatin in the dental pulp stem cells [23]. Simvastatin was noticed to be able to accelerate mineralized tissue formation in vivo [63]. This enhanced mineralization is likely due to the increased expression of odontoblastic differentiation markers, such as dentin sialophosphoprotein and alkaline phosphatase caused by simvastatin.
Different passages of cells were used. In this present study, experiments were performed on cell cultures at 2nd passage. Min used cell cultures between the 5th and 7th passages [64]. The phenotype of the cells can drastically affect how Simvastatin reacts to it.
Time intervals played a big role in this study. Most studies were short-term [10,58,63,64]. The current study is unique because the long-term effects of Simvastatin on Osteoblasts up to 21 days were studied.
Conclusion
Simvastatin at all concentrations of 1, 10, 25, 50, 75, 100 mol/L tested in this study significantly upregulated ALP activity (P< 0.001), osteocalcin expression (P< 0.001), RUNX2 expression (P< 0.001), and mineralization (P< 0.001) of human osteoblasts compared with the control.
- Endo A, Kuroda M, Tanzawa K (1976) Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett, 72: 323-6.
- Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F (1999) New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther, 84: 413-28.
- Abeles AM, Pillinger MH (2006) Statins as antiinflammatory and immunomodulatory agents: a future in rheumatologic therapy? Arthritis Rheum, 54: 393-407.
- Oxlund H, Dalstra M, Andreassen TT (2001) Statin given perorally to adult rats increases cancellous bone mass and compressive strength. Calcif Tissue Int, 69: 299-304.
- Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. (1999) Stimulation of bone formation in vitro and in rodents by statins. Science, 286: 1946-49.
- Oxlund H, Andreassen TT (2004) Simvastatin treatment partially prevents ovariectomy-induced bone loss while increasing cortical bone formation. Bone, 34: 609-18.
- Skoglund B, Forslund C, Aspenberg P (2002) Simvastatin improves fracture healing in mice. J Bone Miner Res, 17: 2004-8.
- Gutierrez G, Lalka D, Garrett I, Rossini G, Mundy G (2006) Transdermal application of lovastatin to rats causes profound increases in bone formation and plasma concentrations. Osteoporosis international, 17: 1033-42.
- Liu C, Wu Z, Sun HC (2009) The effect of simvastatin on mRNA expression of transforming growth factor-beta1, bone morphogenetic protein-2 and vascular endothelial growth factor in tooth extraction socket. Int J Oral Sci, 1: 90-8.
- Yazawa H, Zimmermann B, Asami Y, Bernimoulin JP (2005) Simvastatin promotes cell metabolism, proliferation, and osteoblastic differentiation in human periodontal ligament cells. J Periodontol, 76: 295-302.
- Jamal SM, Eisenberg MJ, Christopoulos S (2004) Rhabdomyolysis associated with hydroxymethylglutaryl-coenzyme A reductase inhibitors. American heart journal, 147: 956-65.
- Thylin MR, McConnell JC, Schmid MJ, Reckling RR, Ojha J, Bhattacharyya I, et al. (2002) Effects of simvastatin gels on murine calvarial bone. J Periodontol, 73: 1141-8.
- Grasser W, Baumann A, Petras S, Harwood H, Devalaraja R, Renkiewicz R, et al. (2003) Regulation of osteoclast differentiation by statins. Journal of Musculoskeletal and Neuronal Interactions, 3: 53-62.
- Staal A, Frith JC, French MH, Swartz J, Güngör T, Harrity TW, et al. (2003) The ability of statins to inhibit bone resorption is directly related to their inhibitory effect on HMG‐CoA reductase activity. Journal of Bone and Mineral Research, 18: 88-96.
- Lin LD, Lin SK, Chao YL, Kok SH, Hong CY, Hou KL, et al. (2013) Simvastatin suppresses osteoblastic expression of Cyr61 and progression of apical periodontitis through enhancement of the transcription factor Forkhead/winged helix box protein O3a. J Endod, 39: 619-25.
- Lin SK, Kok SH, Lee YL, Hou KL, Lin YT, Chen MH, et al. (2009) Simvastatin as a novel strategy to alleviate periapical lesions. J Endod, 35: 657-62.
- Lai EH, Hong CY, Kok SH, Hou KL, Chao LH, Lin LD, et al. (2012) Simvastatin alleviates the progression of periapical lesions by modulating autophagy and apoptosis in osteoblasts. J Endod, 38: 757-63.
- Kok SH, Hou KL, Hong CY, Wang JS, Liang PC, Chang CC, et al. (2011) Simvastatin inhibits cytokine-stimulated Cyr61 expression in osteoblastic cells: a therapeutic benefit for arthritis. Arthritis Rheum, 63: 1010-20.
- Maeda T, Matsunuma A, Kawane T, Horiuchi N (2001) Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochemical and biophysical research communications, 280: 874-7.
- Sugiyama M, Kodama T, Konishi K, Abe K, Asami S, Oikawa S (2000) Compactin and simvastatin, but not pravastatin, induce bone morphogenetic protein-2 in human os-teosarcoma cells. Biochem Biophys Res Commun, 271: 688-92.
- Du Z, Chen J, Yan F, Xiao Y (2009) Effects of Simvastatin on bone healing around titanium implants in osteoporotic rats. Clin Oral Implants Res, 20: 145-50.
- Sallam MM (2011) The influence of oral administration of simvastatin on delayed non-union facial fractures—- clinical study. J Am Sci, 7: 812-8.
- Samiei M, Aghazadeh M, Alizadeh E, Aslaminabadi N, Davaran S, Shirazi S, et al. (2016) Osteogenic/Odontogenic Bioengineering with co-Administration of Simvastatin and Hydroxyapatite on Poly Caprolactone Based Nanofibrous Scaffold. Adv Pharm Bull, 6: 353-65.
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences, 97: 13625-30.
- Suchanek J, Soukup T, Ivancakova R, Karbanova J, Hubkova V, Pytlik R, et al. (2007) Human dental pulp stem cells--isolation and long term cultivation. Acta Medica (Hradec Kralove) 50: 195-201.
- Hilkens P, Gervois P, Fanton Y, Vanormelingen J, Martens W, Struys T, et al. (2013) Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell and tissue research, 353: 65-78.
- Raoof M, Yaghoobi MM, Derakhshani A, Kamal-Abadi AM, Ebrahimi B, Abbasnejad M, et al. (2014) A modified efficient method for dental pulp stem cell isolation. Dental Research Journal, 11: 244.
- Maheshwari K, Chou L (2021) Cytotoxic Effects of Simvastatin on Normal Human Osteoblasts. Cell; 16: 24.
- Garrett IR, Mundy GR (2002) The role of statins as potential targets for bone formation. Arthritis research & therapy, 4: 1-4.
- Edwards C, Russell R, Spector T (2001) Statins and bone: myth or reality? Calcified tissue international, 69: 63-6.
- Moshiri A, Shahrezaee M, Shekarchi B, Oryan A, Azma K (2015) Three-dimensional porous gelapin–simvastatin scaffolds promoted bone defect healing in rabbits. Calcified tissue international, 96: 552-64.
- Maritz FJ, Conradie MM, Hulley PA, Gopal R, Hough S (2001) Effect of statins on bone mineral density and bone histomorphometry in rodents. Arteriosclerosis, thrombosis, and vascular biology, 21: 1636-41.
- Junqueira JC, Mancini MN, Carvalho YR, Anbinder AL, Balducci I, Rocha RF (2002) Effects of simvastatin on bone regeneration in the mandibles of ovariectomized rats and on blood cholesterol levels. Journal of oral science, 44: 117-24.
- Wong R, Rabie A (2003) Statin collagen grafts used to repair defects in the parietal bone of rabbits. British Journal of Oral and Maxillofacial Surgery, 41: 244-8.
- Saraf S, Singh A, Garbyal R, Singh V (2007) Effect of simvastatin on fracture healing—an experimental study.
- Moriyama Y, Ayukawa Y, Ogino Y, Atsuta I, Koyano K (2008) Topical application of statin affects bone healing around implants. Clinical oral implants research; 19: 600-5.
- Pauly S, Back DA, Kaeppler K, Haas NP, Schmidmaier G, Wildemann B (2012) Influence of statins locally applied from orthopedic implants on osseous integration. BMC musculoskeletal disorders, 13: 1-8.
- Ayukawa Y, Ogino Y, Moriyama Y, Atsuta I, Jinno Y, Kihara M, et al. (2010) Simvastatin enhances bone formation around titanium implants in rat tibiae. Journal of oral rehabilitation, 37: 123-30.
- Goes P, Lima APS, Melo IM, Rêgo ROCC, Lima V (2010) Effect of Atorvastatin in radiographic density on alveolar bone loss in wistar rats. Brazilian Dental Journal, 21: 193-8.
- Yoshii T, Hafeman AE, Nyman JS, Esparza JM, Shinomiya K, Spengler DM, et al. (2010) A sustained release of lovastatin from biodegradable, elastomeric polyurethane scaffolds for enhanced bone regeneration. Tissue Engineering Part A, 16: 2369-79.
- Edwards C, Hart D, Spector T (2000) Oral statins and increased bone-mineral density in postmenopausal women. The Lancet, 355: 2218-19.
- Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H (2000) Statin Drugs and the Risk of Fracture—Reply. Jama, 284: 1921-2.
- Chan KA, Andrade SE, Boles M, Buist DS, Chase GA, Donahue JG, et al. (2000) Inhibitors of hydroxymethylglutaryl-coenzyme A reductase and risk of fracture among older women. The Lancet; 355: 2185-8.
- van Staa T-P, Wegman S, de Vries F, Leufkens B, Cooper C (2001) Use of statins and risk of fractures. Jama, 285: 1850-5.
- Bjarnason N, Riis B, Christiansen C (2001) The effect of fluvastatin on parameters of bone remodeling. Osteoporosis International, 12: 380-4.
- Sirola J, Sirola J, Honkanen R, Kröger H, Jurvelin J, Mäenpää P, et al. (2002) Relation of statin use and bone loss: a prospective population-based cohort study in early postmenopausal women. Osteoporosis International, 13: 537-41.
- Rejnmark L, Buus HN, Vestergaard P, Heickendorff L, Andreasen F, Larsen LM, et al. (2004) Effects of simvastatin on bone turnover and BMD: a 1‐year randomized controlled trial in postmenopausal osteopenic women. Journal of Bone and Mineral Research, 19: 737-44.
- Von Stechow D, Fish S, Yahalom D, Bab I, Chorev M, Müller R, et al. (2003) Does simvastatin stimulate bone formation in vivo? BMC musculoskeletal disorders, 4: 1-10.
- Murray MM, Spindler KP, Devin C, Snyder BS, Muller J, Takahashi M, et al. (2006) Use of a collagen‐platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. Journal of Orthopaedic Research, 24: 820-30.
- Golomb BA, Dimsdale JE, White HL, Ritchie JB, Criqui MH (2008) Reduction in blood pressure with statins: results from the UCSD Statin Study, a randomized trial. Archives of internal medicine, 168: 721-7.
- Mammen AL, Amato AA (2010) Statin myopathy: a review of recent progress. Current opinion in rheumatology, 22: 644-50.
- Godlee F (2014) Adverse effects of statins. In.: British Medical Journal Publishing Group.
- Oryan A, Kamali A, Moshiri A (2015) Potential mechanisms and applications of statins on osteogenesis: Current modalities, conflicts and future directions. Journal of controlled release, 215: 12-24.
- Tomlinson SS, Mangione KK (2005) Potential adverse effects of statins on muscle. Physical therapy, 85: 459-65.
- Mansi I, Frei CR, Pugh MJ, Makris U, Mortensen EM (2013) Statins and musculoskeletal conditions, arthropathies, and injuries. JAMA internal medicine, 173: 1318-26.
- Peeters G, Tett SE, Conaghan PG, Mishra GD, Dobson AJ (2015) Is statin use associated with new joint‐related symptoms, physical function, and quality of life? Results from two population‐based cohorts of women. Arthritis care & research, 67:13-20.
- Sakoda K, Yamamoto M, Negishi Y, Liao J, Node K, Izumi Y (2006) Simvastatin decreases IL-6 and IL-8 production in epithelial cells. Journal of dental research, 85: 520-3.
- Baek KH, Lee WY, Oh KW, Tae HJ, Lee JM, Lee EJ, et al. (2005) The effect of simvastatin on the proliferation and differentiation of human bone marrow stromal cells. Journal of Korean medical science, 20: 438-44.
- Lee SY, Min KS, Choi GW, Park JH, Park SH, Lee SI, et al. (2012) Effects of simvastain and enamel matrix derivative on Portland cement with bismuth oxide-induced growth and odontoblastic differentiation in human dental pulp cells. J Endod, 38: 405-10.
- Kamada A, Yoshikawa Y, Domae E, Shintani T, Kikuchi Y, Yoshimoto H, et al. (2017) Statins Regulate Runx2 Expression in Human Dental Pulp Cells. Journal of Oral Tissue Engineering, 14: 157-63.
- Zhang W, Wu Y, Shiozaki Y, Sugimoto Y, Takigawa T, Tanaka M, et al. (2018) miRNA-133a-5p inhibits the expression of osteoblast differentiation-associated markers by targeting the 3′ UTR of RUNX2. DNA and Cell Biology, 37: 199-209.
- Varalakshmi PR, Kavitha M, Govindan R, Narasimhan S (2013) Effect of statins with alpha-tricalcium phosphate on proliferation, differentiation, and mineralization of human dental pulp cells. J Endod, 39: 806-12.
- Okamoto Y, Sonoyama W, Ono M, Akiyama K, Fujisawa T, Oshima M, et al. (2009) Simvastatin induces the odontogenic differentiation of human dental pulp stem cells in vitro and in vivo. J Endod, 35: 367-72.
- Min KS, Lee YM, Hong SO, Kim EC (2010) Simvastatin promotes odontoblastic differentiation and expression of angiogenic factors via heme oxygenase-1 in primary cultured human dental pulp cells. J Endod, 36: 447-52.

Figures at a glance