Chemical Recycling of Polyester Waste to Synthesize Novel Bis-azo Naphthol Based Disperse Dyes
Received Date: November 08, 2024 Accepted Date: December 08, 2024 Published Date: December 11, 2024
doi: 10.17303/jmsa.2024.8.205
Citation: Vikrant S. Palekar (2024) Chemical Recycling of Polyester Waste to Synthesize Novel Bis-azo Naphthol Based Disperse Dyes. J Mater sci Appl 8: 1-11
Abstract
The paper aims at effective chemical recycling of poly(ethylene terephthalate) (PET) fiber waste into useful value added products, such as synthesis of novel Bis-azo Naphthol based dyes. For this PET in the form of waste fibers and disposable soft drink bottles was subjected to depolymerization through aminolysis using hydrazine hydrate as a monomeric product in good yield. During our continuous efforts to work on depolymerization of polymer waste into useful chemicals & its subsequent application. In the present work we have synthesized bis oxadiazole heterocyclic compounds and its further diazotization followed by coupling with various unsubstituted/ substituted naphthol acid and sulphonic acid derivative into series of novel bis-azo disperse dyes obtained from terephthalic dihydrazide. The different naphthol couplers were used for synthesis of dyes and the study were carried out to check effect of -SO3H group position in naphthol ring, on the colour, dyeing, and fastness properties. The synthesized novel dyes were obtained with good yield from 68 % to 78 % & observed to show excellent spectral and fastness properties. The paper highlights recent advancements in chemical recycling of PET waste into value added dye synthesis with subsequent applications.
Keywords: Poly (Ethylene Terephthalate); Depolymerization; Terephthalic Dihydrazide; Bis-azo Naphthol Dyes; Spectral Properties
Introduction
Presently for the advanced development of science & technology & thus for high standard of living polymers are becoming of increasingly important materials. We can clearly observe that the modern society cannot live or progress without use of polymeric materials. Polymers are replacing the conventional materials like metal, wood and glass because of their lower cost, higher flexibility, manufacturing ease, and better performance [1-2]. Synthetic polymers have become very versatile and useful materials for modern technology. Because polymers are low cost and can be easily fabricated to consumer products by fast automated machines, they have been widely used in form of packaging materials for farm, forest, dairy products and other consumer items. PET is widely used form of plastic to make bottles for mineral water, soft drink, beverages etc. The global production of PET is increasing day by day & also use of PET for drinking bottles has increased from years & it is expected that this growth will continue & consumption will increase gradually over the coming years [3].
Extensive use of polymeric materials, lead to the waste disposal generation of non-degradable solid waste is a serious issue of present days [4]. The possible solution to reduce disposal of PET waste by effectively applying different chemical recycling ways & converting into monomeric units & thereby converting into value-added materials via depolymerization. PET through chemical depolymerization break down into smaller molecules by reacting various reagents in presence of catalyst for effective dissolution of PET. PET is a thermoplastic polyester and solvolytic chain cleavage is possible by reagents, such as water (hydrolysis), alcohols (alcoholysis), amines (aminolysis), acids (acidolysis), leading to a large variety of valuable end products [5]. Hydrolysis, aminolysis, methanolysis and glycolysis are the possible routes to PET depolymerization [6].
In our earlier study, we have worked on effective depolymerization of PET by glycolysis & aminolysis route by using environment-friendly catalyst to produce good yield monomer which has further potential of converting into useful value-added products [7-9]. During our previous study, the aminolysis techniques were effectively applied for depolymerization polyester bottle waste to obtain monomer terephthalic dihydrazide using hydrazine hydrate as a reagent. The terepthalic dihydrazide obtained then reacted with series of reactive compound to obtain novel disazo disperse dyes which were evaluated for spectral study & also effectively applied on fabrics [10]. Azo compounds has emerged as versatile class of synthetic dyes due to their versatile applications including in textile fibres, polymers, medical studies and laser discs [11-14].
It has also been reported in literature that certain compounds bearing 1,3,4-oxadiazole nucleus possess significant anti-inflammatory activity [15]. In our earlier efforts of chemicals recycling PET waste into synthesis of value-added compounds, we have prepared terephthalic dihydrazide from PET waste by reacting with hydrazine hydrate. Synthesis of heterocycles such as bis-1,3,4-oxadiazole, bis-1,2,4-triazole and 4-thiazolidinone derivatives are synthesized from terephthalic dihydrazide. The synthesized diverse novel heterocyclic compounds were tested for their antibacterial activity [16]. Around the globe there is increasing demand & use of PET material & hence the pollution of waste PET to the environment has become increasingly prominent. Thus there is serious need of attention to be provided for chemical recycling to reduce PET waste disposal problem by carrying out depolymerization and conversion of the products of the reaction into value added materials having effective applications in the diverse area.
In literature it was observed that the bis-azo dyes are valuable for providing high intensity of color and good technical properties such as fastness to light, heat, water and other solvents [17-19]. Since efforts are done for azo coupling with 3-hydroxy-2-naphthoic acid precursor to synthesize bis-azo naphthol dyes to address the issue of PET waste management and the sustainable dye synthesis.
In the present work our aim was to synthesis and study the dyeing properties of the symmetrical bis azo reactive dyes obtained by diazotization of 4,4’-(5,5’-(1,4-phenylene)bis(1,3,4-oxadiazole-5,2-diyl)dianiline and coupling with unsubstituted/ substituted naphthol acid and sulphonic acid derivative. The effects of -SO3H group position in naphthol ring, on the colour, dyeing, and fastness properties of the dyes are studied. The synthesized dyes were applied on cotton and wool fabrics and evaluated for the spectral properties.
Experimental
Materials
Discarded PET soft drink bottles were obtained from a local market, cut into small pieces of approximate size 5x5 mm after separating from the non-PET components such as labels and caps. The pieces were boiled with a solution for removal of dirt present with subsequent washing with water and drying of PET. Cotton fabric (52/40 GSM 93) and wool fabric (100D) were used for dyeing purpose. The fabrics were boiled with a solution of non-ionic detergent followed by washing thoroughly with water and dried.
Equipments
Melting point: Melting points were determined in an open capillary. The adsorbent used for thin layer chromatography (TLC) was silica gel procured from S. D. Fine Chemicals Ltd. FTIR spectra were recorded on Shimadzu FTIR-470 spectrophotometer and are reported in wave numbers (cm-1). 1H NMR spectra were recorded on Bruker DRX-400 spectrometer at 400 MHz in deuterated dimethylsulphoxide (DMSO-d6). Mass spectra were measured on Bruker mass spectrometer by electron ionization. Ultraviolet–visible double beam spectrophotometer was used for measurement of absorption spectra in various solvents. The wash fastness study of the dyed samples were carried out on the automatic Wash Fastness Tester (Rossari Labtech, India). The sublimation fastness was evaluated using Sublimation Fastness Tester (R. B. Electronics and Engg. Pvt. Ltd., India).The light fastness testing was performed using Q-SUN Xenon test chamber. The study of Colourimetric data of dyed samples were evaluated from Datacolour computer colour matching system Spectraflash.
Polyester Waste Cleaning
PET bottle pieces were boiled with a solution containing 2 g/L of non-ionic detergent for 1 h to remove any surface finish and dirt present. It was then washed thoroughly with water and dried in an oven at 80οC.
Synthesis of Terephthalic Dihydrazide from Polyester Bottle Waste
The PET bottle waste was treated with hydrazine hydrate under reflux at 110 οC in the presence of certain amount of ionic liquid for appropriate time periods. The ionic liquid, [HMim.TfO] were used as a catalyst for the reaction. After reaction is over the distilled water was added in excess to the reaction mixture with vigorous agitation to precipitate out the product terephthalic dihydrazide. The filtrate contained mainly excess hydrazine hydrate and little quantities of a few water-soluble PET degradation products. The precipitate obtained was filtered and dissolved in distilled water by boiling for about 30 min. White crystalline powder of terephthalic dihydrazide was obtained by first concentrating the filtrate by boiling and then chilling it. It was further purified by recrystallization in water. It was then dried in an oven at 800C and weighed for estimating the yield. Different techniques of analysis were used for its characterization & it was observed in good agreement with those reported in the literature [20].
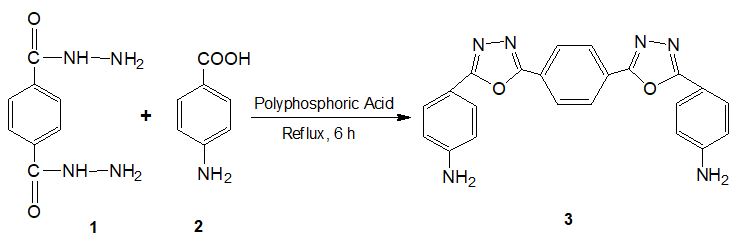
Synthesis of 4,4’-(5,5’-(1,4-phenylene)bis(1,3,4-oxadiazole-5,2-diyl)dianiline (4)
The terephthalic dihydrazide (0.38g, 2.0 mmol) and 4-amino benzoic acid (0.54g, 4.0 mmol) was reacted with polyphosphoric acid (5 mL) & refluxed at 110 οC for 6 h. Reaction progress was monitored with TLC (Ethyl acetate: Hexane). After reaction completion, the reaction mass was slowly added on crushed ice. The obtained solid was then separated by neutralization by sodium bicarbonate solution, filtered, washed with water. The recrystallized was carried with from ethanol to five compound 4 in 70% yield.
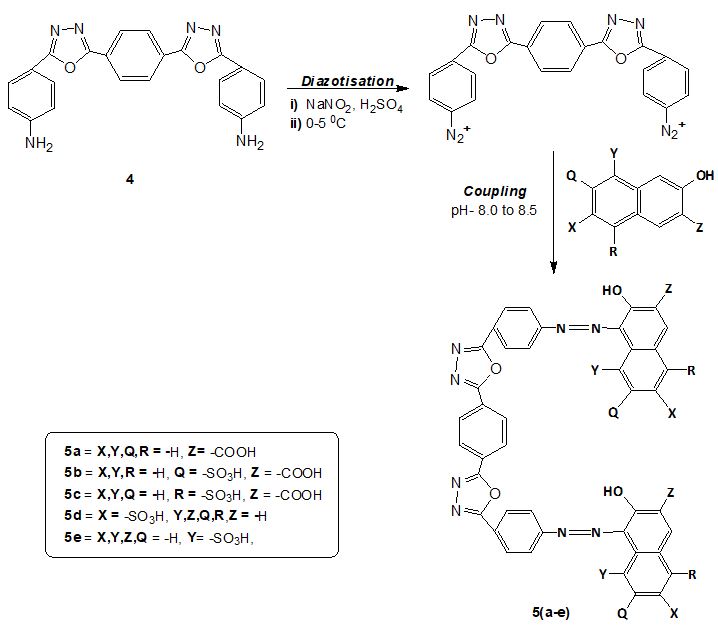
Synthesis procedure of Dye (5a-5e)
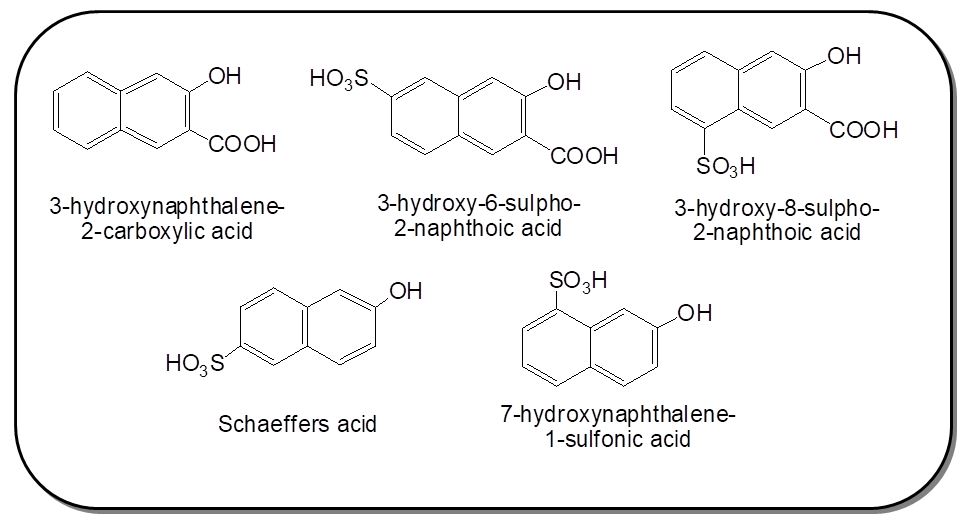
The sodium nitrite (0.27g, 4.0 mmol) solution was slowly added to concentrated sulphuric acid (5.0 ml, 98 % w/w) below 10 οC to get nitrosylsulphuric acid. The reaction temperature was then increased upto 65οC to completely dissolve sodium nitrate. The solution was then allowed to cool below 10οC. To this compound 4 (0.79 g, 2.0 mmol) prepared in earlier stage was then added gradually over a 15 min period. The mixture was then stirred for about 4 h below 100C. The prepared diazonium salt solution was then utilized for coupling reaction with the following coupling components: 3-hydroxynaphthalene-2-carboxylic acid (BON acid), 3-hydroxy-6-sulfo-2-naphthoic acid, 3-hydroxy-8-sulpho-2-naphthoic acid, 6-hydroxynaphthalene-2-sulphonic acid, 7-hydroxy naphthalene-1-sulphonic acid. In naphthoic acid derivatives coupling takes place always at α-position, the coupling position is influenced by the position of sulphonic acid group.
The naphthol coupler (2 mmol) was taken in another flask, water (10ml) was added to it with temperature below 10 οC and diluted with Na2CO3 solution to maintain pH~8.5 with dissolution of naphthol coupler. The above prepared diazonium salt was slowly added to it, the pH of the solution was maintained between 8-8.5 by simultaneous addition of dilute Na2CO3. The addition was continued for around 20 min, thereafter stirring was continued for 2 h. The pH of the solution was maintained to pH~4.0 for precipitation of dye and the dye obtained was then filtered, washed with water and dried. The purification of product was carried out.
The Dyes synthesized were analyzed for their physical and spectral characterization.
Spectroscopic Characterization
Compound 3:Yield- 70%; M.P.: 276-278 0C;
FTIR:(cm-1): 3310 (-NH), 2990 (-CH), 1665 (-C=N);
1H-NMR (DMSO-d6M) δ: 11.4 (br, 4H, -NH, D2O exchangeable), 8.0 (s, 2H, Ar-H), 7.9 (d, J=8.2 Hz, 2H, Ar-H), 7.8 (d, J=8.0 Hz, 2H, Ar-H);
MS(m ⁄ z): 397 [M+].
Dye 5a: 4,4'-(4,4'-(5,5'-(1,4-phenylene)bis(1,3,4-oxadiazole-5,2-diyl))bis(4,1-phenylene)-bis-(diazene -2,1-diyl) bis(3-hydroxy-2-naphthoic acid)
Yield- 76%; M.P.: 333-337 oC; Particle size (average) = 14.68
FTIR (KBr) spectrum (cm-1): 3282 (-NH), 3055 (-OH), 2858 (-CH), 1712 (-CO group), 1645(-C=N), 1553 (N=N).
1H-NMR (DMSO-d6, 300 MHz) spectrum ( δ ppm): δ 8.51 (s, 2H, Ar-H), 7.96 (d, 2H, J=8.4 Hz, naphthol Ar-H), 7.76 (d, 2H, J=8.1 Hz, naphthol Ar-H), 7.54 (d, 2H, Ar-H), 7.35-7.27 (m, 4H, Ar-H).
Mass spectrum: m/z = 795 [M+1].
Dye 5b: 4,4'-(4,4'-(5,5'-(1,4-phenylene)bis(1,3,4-oxadiazole-5,2-diyl))bis(4,1-phenylene))-bis-(diazene-2,1-diyl)-bis(3-hydroxy-7-sulpho-2-naphthoic acid)
Yield- 73%; M.P.: 342-346 oC; Particle size (average) = 13.63.
FTIR (KBr) spectrum (cm-1): 3413 (-NH), 3353 (-OH), 2997 (-CH), 1724 (-CO group), 1665(-C=N), 1563 (N=N).
1H-NMR (DMSO-d6, 300 MHz) spectrum (δ ppm): δ 8.51 (s, 2H, Ar-H), 7.96 (d, 2H, J=8.4 Hz, naphthol Ar-H), 7.76 (d, 2H, J=8.1 Hz, naphthol Ar-H), 7.54 (d, 2H, Ar-H), 7.35-7.27 (m, 4H, Ar-H)
Mass spectrum:m/z= 955 [M+1].
Dye 5c : 4,4'-(4,4'-(5,5'-(1,4-phenylene)bis(1,3,4-oxadiazole-5,2-diyl))bis(4,1-phenylene)) bis- (diazene-2,1-diyl)-bis(3-hydroxy-5-sulpho-2-naphthoic acid)
Yield- 69%;M.P.: 342-346 oC; Particle size (average) = 14.20 µm.
FTIR (KBr) spectrum (cm-1): 3491 (-NH), 3450 (-OH), 3093 (-CH), 1631 (-C=N), 1510 (N=N).
1H-NMR (DMSO-d6, 300 MHz) spectrum (δ ppm): δ 8.59 (s, 2H, naphthol Ar-H), 8.23 (d, 2H, J=8.6 Hz, Ar-H), 8.16 (d, 2H, J=8.2 Hz, Ar-H), 7.78 (s, 4H, Ar-H), 7.7-7.4 (m, 4H, naphthol Ar-H).
Massspectrum: m/z= 954 [M+1].
Dye 5d : 5,5'-(4,4'-(5,5'-(1,4-phenylene)bis(1,3,4-oxadiazole-5,2-diyl))bis(4,1-phenylene))-bis- (diazene-2,1-diyl)bis(6-hydroxynaphthalene-2-sulphonic acid)
Yield- 76%; M.P.: 380oC; Particle size (average) = 22.59 µm.
FTIR(KBr) spectrum (cm-1); 3491 (-NH), 3450 (-OH), 3093 (-CH), 1631 (-C=N), 1510 (N=N)
1H-NMR (DMSO-d6, 300 MHz) spectrum (δ ppm): δ 9.77 (s, 2H, naphthol Ar-H), 7.98 (s, 2H, Ar-H), 7.78 (d, 2H, J=8.4 Hz, Ar-H), 7.58 (d, 2H, J=8.8 Hz, naphthol Ar-H), 7.09 (d, 4H, J=9.0 Hz, naphthol Ar-H).
Dye 5e: 8,8'-(4,4'-(5,5'-(1,4-phenylene)bis(1,3,4-oxadiazole-5,2-diyl))bis(4,1-phenylene))-bis- (diazene-2,1-diyl)bis(7-hydroxynaphthalene-1-sulphonic acid)
Yield- 78%; M.P.: 250-254 0C; Particle size (average)=16.90 µm.
FTIR (KBr) spectrum (cm-1): 3427 (-NH), 3412 (-OH), 2879 (-CH), 1686 (-C=N), 1548 (N=N).
1H-NMR (DMSO-d6, 300 MHz) spectrum (δ ppm): δ 8.50(d, 2H, J=8.2 Hz, Ar-H), 8.41(s, 2H, Ar-H),8.20 (d, J=7.2 Hz, naphthol Ar-H), 7.93 (d, 2H, J=9.6 Hz, naphthol Ar-H), 7.74(d, 2H, J=7.8 Hz, naphthol Ar-H), 7.42 (d, 4H, J=9.0 Hz, naphthol Ar-H); 6.7 (d, 2H, J=9.8 Hz, naphthol Ar-H)
Preparation of Dye Dispersion
To prepare dispersion of Dye (1.0 g) added to water (5 mL) which is then taken in a mortar and milled for 60 min time & then diluted with 20 mL water. Further it was diluted upto 100 mL with the water.
Cotton Dyeing
Direct dyeing of cotton fabric piece (1 g) was carried out in Rotadyer closed stainless steel beakers at around 80 οC for 60 min with a liquor ratio upto 40. The dyed samples were washed with water and then dried.
Wool Dyeing
The wool fabric pieces (1 g) were used for acid dyeing in a Rotadyer at 90 οC for 60 min at a liquor ratio upto 40 & pH 4-5 by adjusting with acetic acid. The dyed samples was washed with water and then dried.
Fastness Properties Evaluation
The fastness analysis of the dyed cotton & wool fabric samples were evaluated as per standard tests as follows: Wash fastness: ISO-III; Light fastness: ISO-III Test method.
Results and Discussion
Aminolysis with hydrazine hydrate is been explored to obtained terephthalic dihydrazide monomer as a crystalline solid for synthesis of some potent heterocyclic derivatives having varied application from PET waste. Post-consumer PET wastes from soft drink bottles in the form of scrap flakes were subjected to aminolysis at 110 οC using hydrazine hydrate in the presence of zinc acetate as a catalyst for 4 h. The dianiline compound was then prepared by the reaction of terephthalic dihydrazide (1) with 4-amino benzoic acid (2) in polyphosphoric acid to form compound (3) having terminal free amine group (Scheme 1). The IR data of 4,4’-(5,5’-(1,4-phenylene)bis(1,3,4-oxadiazole-5,2-diyl)dianiline (compound 3) shows sharp peak at 1676 cm-1 due to presence of C=N stretching. The oxadiazole ring formation is confirmed by absence of carbonyl peak at 1630 cm-1. Its 1H-NMR of compound 4 shows a multiplet peaks at δ 7.7-7.9 ppm due to presence of aromatic protons.
The series of new disazo dyes 5a-e were prepared by diazotizing compound 4 using nitrosylsulphuric acid and coupling with 3-hydroxynaphthalene-2-carboxylic acid, 3-hydroxy-6-sulpho-2-naphthoic acid, 3-hydroxy-8-sulpho-2-naphthoic acid, 6-hydroxynaphthalene-2-sulphonic acid, 7-hydroxy naphthalene-1-sulphonic acid at mild alkaline pH. The coupling component used in the preparation of bis azo reactive dyes were shown in Figure 1. The structures of synthesized dyes were verified with different spectroscopic techniques (Scheme 2).
Spectral properties of the synthesized dyes
The infrared spectra of these dyes showed a weak and broad band above 3400cm-1 due to -NH, the broad band around 3200 cm 1 due to -OH, peak at 2980-2855 cm-1 is due to -C-H stretching & 1530-1550 cm 1 indicates presence of -N=N. The infrared spectra of dyes showed a strong and broad band within the range 3412-3055 cm-1 as a result of intermolecular hydrogen bonding due to the α-hydroxyl group in naphthol ring and azo group. In case of dye 5a-5c, there is presence of carbonyl group shown by the sharp band between 1712-1726 cm-1. The 1H NMR spectrum of all the dyes showed the peaks in the aromatic region due to the different protons in benzene ring and in naphthol ring. The 1H NMR spectrum of compound 5a-e was recorded in DMSO-d6. The mass spectrum of dye 5a revealed a molecular ion peak (m+1) (m/z) at 795. Analytical and spectral data fully supported the structures of all compounds.
Study of solvent effects
UV-vis absorption spectra were recorded using spectrophotometer in the wavelength range 300-700 nm. Absorption spectra of dyes were recorded in various solvents at a concentration of 10-6 to 10-8 M and these are all run at different concentrations. The results are summarized in Table 2. The dyes showed single or two absorbance in all used solvents. It can be suggested that the dyes may exist as a mixture of tautomeric forms in various solvents. The deep colour of these products was attributed to the presence of extended conjugation and the introduction of strong electron-donating substituents with effect of sulphonic group in naphthol ring.
The electronic spectra of these dyes showed absorption bands in the 330-360 nm in UV region and 490-525 nm in visible region in dimethyl formamide (DMF), 315-355 nm in UV region and 500-520 nm in visible region in methanol, and for ethanol in UV region between 320-360 nm and in visible region between 495-530 nm. The electronic transitions associated in conjugate system (both of the phenyl ring & in oxadiazole heterocycles & in naphthol ring are due to π-π* transitions). The dyes 5a-5c show comparatively higher wavelength due to presence of carboxylic group in naphthol ring than the dye 5d and 5e. The increase in visible absorption band were also observed on increasing the solvent polarity.
Dyeing properties
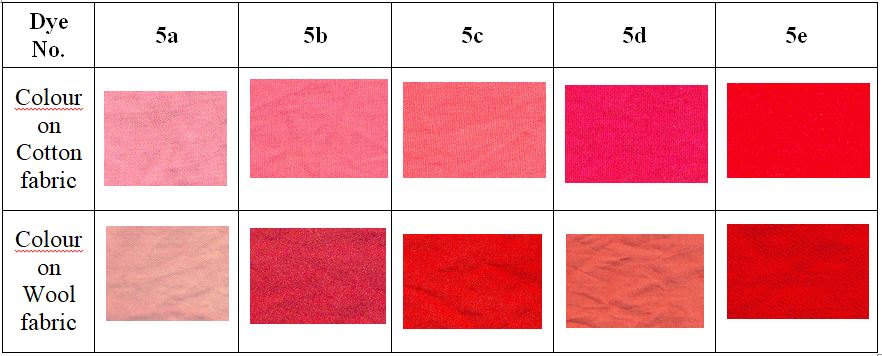
Spectral data of dyed samples is given for dyed cotton samples in Table 3 and for dyed wool samples in Table 4. The Kubelka Munk function, K/S shows the depth of colour onto the fabric. The colour coordinates values of L*, a* and b* shows lightness-darkness, red-green and yellow-blue tones of colours. The positive values it is expected that dyes have reddish tone whereas higher L* values confirm the colour brilliancy [21]. K/S data of dyed samples shows high values for cotton & wool fabrics, indicating good colour depth of synthesized dyes.
The wash fastness properties of the dyed fabrics were evaluated according to the ISO-III test procedures using standard grey scale for colour change and staining. The wash fastness study of the dyed cotton & wool fabrics was found to be good to excellent (rating 4-5 on the scale of 5, where 5 is excellent). Also the light fastness study shows to be good to moderate for both the dyeings (rating 5-6 on the scale of 8, where 8 is excellent). The results are summarized in the Table 5.
The cotton and wool samples dyed with dye 5a showed very light colour as there was no sulphonic group present in dye imparting very little affinity towards the fibre. For rest all dyes 5b-5e the dyeing on cotton and wool was found to give good to excellent colour depth.
Conclusion
Aminolysis revealed that it is possible to obtain complete conversion of PET waste into value added monomeric product terephthalic dihydrazide having potential for being recycled into useful products through various chemical reactions. The terephthalic dihydrazide obtained was successfully converted into novel bis-azo dyes based on naphthol derivatives having carboxyl and sulphonyl group. These dyes show good colouration characteristics on cotton and wool fabrics with satisfactory performance properties. Substituent present in naphthol ring plays important role for imparting reddish yellow hues on textile fabrics. The results obtained show that the synthesized dyes have excellent spectral and fastness properties. It is attributed that PET waste has been successfully chemically recycled through synthesizing ranges of novel dyestuffs with properties analogous to commercial dyes.
- F Gu, J Guo, W Zhang, P A Summers, P Hall (2017) Sci Total Environ. 601: 1192.
- D Civancik-Uslu, L Ferrer, R Puig, P Fullana-i-Palmer (2018) Sci Total Environ. 626: 927.
- P T Benavides, J B Dunn, J Han, M Biddy, J Markham (2018) ACS Sustain Chem Eng. 6: 9725.
- J Scheirs (2008) Polymer Recycling: Science, Technology and Applications. Chichester: Wiley.
- D Paszun, T Spychaj (1997) Ind Eng Chem Res. 36:1373.
- M Xanthos, S H Patel (1998) Frontiers Sci Technol Polym Recycl. 351: 425.
- S R Shukla, K S Kulkarni (2002) J Appl Polym Sci, 85: 1765.
- S R Shukla, A M Harad (2005) J Appl Polym Sci. 97:513.
- S R Shukla, V S Palekar, N D Pingale (2008) J Appl Polym Sci. 110: 501.
- V S Palekar, N D Pingale and S R Shukla (2010) Coloration Technology, 126: 86.
- K Tanaka, K Matsuo, A Nakanishi, J H Shiota, M Yamaguchi and S Yoshino (1984) Chem Pharm Bull. 32: 3291.
- A A Fadda, H A Etmen, F A Amer, M Barghout & K S Mohammed (1994) J Chem Technol Biotechnol, 61: 343.
- G Hallas, J Soc (1979) Dyers Colour, 95: 285.
- J Griffiths (1981) Rev Prog Color, 11: 37.
- M D Mullican, M W Wilson, D T Connor, C R Kostlan, D J Schrier, R D Dyer (1993) J Med Chem, 36: 1090.
- V S Palekar, A J Damle, S R Shukla (2009) European Journal of Medicinal Chemistry, 44: 5112.
- H Zollinger (1991) Color Chemistry, 2nd ed., WileyVCH, Weinheim.
- R M Christie (2001) Colour Chemistry, Royal Society of Chemistry, Cambridge.
- H S Freeman, A T Peters (2000) Colorants for Non Textile Applications, Elsevier, Amsterdam.
- A S Goje, S A Thakur, T M Patil, S Mishra, (2003) J Appl Polym Sci, 90: 3437.
- R McDonald (1997) Colour Physics for Industry, 2nd Ed. UK: Society of Dyer and Colourists.

Tables at a glance
Figures at a glance