DHA/Arachidonic Acid Ratio Contribute to Lipid Peroxidation in Social Behaviors of Individuals with Autism Spectrum Disorder
Received Date: January 07, 2023 Accepted Date: February 07, 2023 Published Date: February 10, 2023
doi: 10.17303/jnnd.2023.11.101
Citation: Kunio Yui, George Imataka, Tomoyo Hayashi, Yuki Shiko (2023) DHA/Arachidonic Acid Ratio Contribute to Lipid Peroxidation in Social Behaviors of Individuals with Autism Spectrum Disorder. J Neurophysiol Neurol Disord 11: 1-16.
Abstract
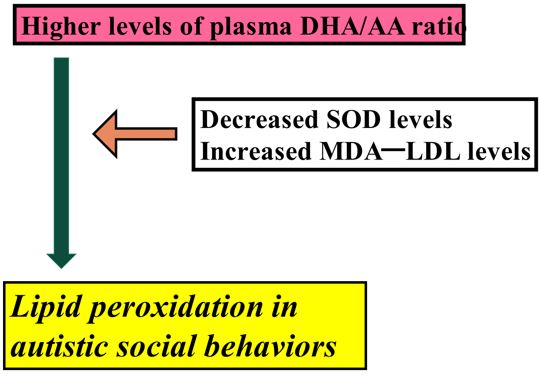
Lipid peroxidation contributes to the development of autism spectrum disorder (ASD). Polyunsaturated fatty acids (PUFAs)undergo lipid peroxidation, and conversion to malondialdehyde (MDA). MDA reacts with acetaldehyde to form malondialdehyde-modified low-density lipoprotein (MDA-LDL). MDA-LDL is known as a marker of lipid peroxidation.However, the association between PUFAs and MDA-LDL in the pathophysiology of ASD is unclear. We studied this association in 18 young individuals with ASD and 8 age- and sex-matched normal healthy controls. Social behaviors were assessed using the Social Responsiveness Scale (SRS). To overcome the small sample size, three measures were conducted: firstly,adaptive Lasso was used to enhance the accuracy of interpretability; second, the coefficient of variation was estimated for appropriatevariable selection; and finally, appropriate variables were selected. Plasma MDA levels and DHA/omega 6 PUFA arachidonic acid ratio were significantly higher, whereas plasma levels of superoxide dismutase were significantly lower in the ASD group than in the control group. The total SRS scores in the ASD group were significantly higher than those in the control group. Multiple linear regression analysis and the adaptive Lasso revealed association of increased plasma DHA/ARA ratio with the SRS total scores and increased plasma MDA-LDL levels. These associations between plasma DHA/ARA ratio and increased plasma MDA-LDL levels contributed to autistic social behaviors.
Keywords: Lipid Peroxidation; Autism Spectrum Disorder; Social Autistic Behaviour; Malondialdehyde-Modified Low-Density Lipoprotein (MDA-LDL); Docosahexaenoic Acid (DHA); Arachidonic Acid
List of Abbreviations
ASD = Autism Specrum Disorder
DHA = Docosahexaenoic acid
ARA = Arachidonic acid
PUFA = Polyunsaturated Fatty acid
MDA = Malondialdehyde
MDA-LDL = Malondialdehyde-Modified Low-density Lipoprotein
4-HNE = 4-hydroxy-2-nonenal (4-HNE)
SOD = Supeoxide dismutase
Introduction
Lipid peroxidation is a process under which oxidants attack lipids containing carbon-carbon double bonds, especially polyunsaturated fatty acids (PUFAs), and play an important role in cell biology and human health [1]. Polyunsaturated acids are sensitive to lipid peroxidation, inducing numerous physiological processes [2]. The oxidative degradation of lipids preferentially induces two main lipid peroxidation products: malondialdehyde (MDA) and 4-hydroxy-2-nonenal (4-HNE) [2]. Oxidative stress has been implicated as an important mechanism that is associated with lipid peroxidation in humans [3]. Many neurological studies have revealed an important role of lipid peroxidation in the pathophysiology of autism spectrum disorder (ASD) [4]. However, there have been few studies on the relationship between omega-3 PUFA docosahexaenoic acid (DHA) and omega-6 PUFA arachidonic acid (ARA), and lipid peroxidation in ASD.
With respect to plasma MDA levels in ASD, previous studies reported that plasma MDA levels were significantly higher in 20 children with ASD than in 20 age-- matched controls [5], and that blood MDA levels in 45 autistic children (age 3–11 years) were higher than those in 42 age-matched controls [6]. However, between PUFA and MDA-LDL, which variable is the main contributor to the development of autistic social symptoms remains unclear.
Numerous clinical and animal studies have indicated an association between lipid peroxidation and major antioxidant protein superoxide dismutase (SOD), Of reference, a previous study revealed that the reduction of hyperglycemia by sodium tungstate reduced lipid peroxidation and caused alteration in the antioxidant system in the salivary glands of streptozotocin -induced diabetic rats [7], and that vitamin C-induced nephrotoxicity decreased lipid peroxidation and increased SOD in a human kidney cell line [8].The major antioxidant protein superoxide dismutase (SOD) might be associated with MDA in erythrocytes. This enzyme plays an important role in protecting tissues by scavenging oxidative stress-related reactive oxygen species (ROS) [9]. Serum levels of SOD were increased in 20 children with ASD in comparison to 25 age-matched controls, suggesting that the activity of the antioxidant defense mechanism was reduced [5]. Serum SOD levels were significantly lower in autistic children of <6 years of age in comparison to age-matched controls, while the MDA levels of these children were significantly higher in comparison to controls [10]). These studies indicate a close association between lipid peroxidation markers and SOD. However, definitive blood SOD levels in children with ASD remain unclear.
ASD group (n = 27) exhibited increased erythrocyte SOD activity as compared with normal group (n = 26) in related to lipid peroxidation [11]. Additionally, ASD model mice injected with VPA exhibited neurobehavioral deficits typical of ASD exhibited that changes in the activity of SOD increased lipid peroxidation [12]. These findings indicated that a close association between lipid peroxidation and total antioxidant of SOD.
One of the components of the lipid profile, low-density lipoprotein (LDL), plays an important role in brain development. A previous clinical study reported significantly higher blood levels of LDL in 22 adult subjects with Asperger’s syndrome (mean age, 40.8 ± 10.8 years) in comparison to 22 age-matched controls (44.6 ± 14.8 years), indicating abnormal cholesterol metabolism [13]. Thus, blood LDL levels may contribute to the pathophysiology of ASD.
MDA easily reacts with acetaldehyde to form malondialdehyde-modified low-density lipoprotein (MDA-LDL) [14]. MDA-LDL is a good marker of oxidative stress and lipid peroxidation [15]. However, the role of MDA-LDL in the pathophysiology of ASD has not been investigated in detail. This is the first study to examine the role of MDA-LDA in ASD. Collectively, the product of lipid peroxidation MDA is combined with LDL, inducing MDL-LDL
Taken together, the following avenues of investigation were proposed: (a) the type of PUFA (omega-3 or omega-6) that mainly contributes to MDA formation; (b) between PUFA and MDA-LDL, which variable is the main contributor to the development of autistic social symptoms; (c) the role of MDA-LDL in the pathophysiology of ASD, and (d) the association between lipid peroxidation and the antioxidant SOD with respect to autistic social impairment. This study mainly addressed to the role of PUFAs and MDA-LDL in the social impairment of ASD. We employed the adaptive Lasso technique to emphasize the accuracy of prediction. This offers consistent variable selection and is essential for identifying important variables [16] in small samples [17].
Subjects and Methods
Ethics Approval
All participants gave their written informed consent before voluntary enrollment. This study was performed with the approval of the Ethics Committee of Dokkyo Medical University, Japan. Written informed consent was obtained from the respective participants and/or their parents.
Subjects
Eighteen individuals with high-functioning ASD and 8 age-matched and healthy male and female control individuals were included in this study. Each of the 18 individuals with ASD had received an independent clinical diagnosis of ASD. The 18 patients with ASD included 12 males and 6 females (mean age: 10.9 ± 5.6 years. The 8 healthy controls included 5 males and 3 females (mean age: 9.6 ± 4.0 years; age range: 5-21 years) (Table 1). One member each of the ASD and control groups was 21 years of age because the two subjects hoped to participate in this study to learn their plasma levels of lipid and omega-3 and omega-6 PUFAs metabolites, including DHA. All participants were Japanese, and were born and lived in Hyogo, or Osaka Prefecture (central Japan). Based on interviews and, the diagnosis of ASD was made based on the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). The Autism Diagnostic Interview-Revised (ADI-R) was also conducted by one of the authors (KY), who was already experienced and reliable in the diagnosis of autism using the Japanese version of the ADI-R. The ADI-R is a semi-structured interview that is conducted with a parent, usually the mother, and is used to confirm the diagnosis and evaluate the core symptoms of ASD. These 18 individuals with ASD had the core symptoms of the DSM-5 diagnostic criteria for ASD, without any abnormal neurological symptoms. The 18 individuals with ASD and the 8 normal controls were matched with respect to feeding habits, age and full intelligence quotient (IQ) scores (Table 1). No participants had any abnormalities in the results of their physical examinations and laboratory findings. We used the Wechsler Intelligence Scale for Children, third edition (WISC-III), to exclude subjects with a full-scale IQ of <70. We excluded comorbid psychiatric illnesses using the Structured Clinical Interview for DSM-5. Individuals were excluded from the study if they had epileptic seizures, obsessive-compulsive disorder, or were diagnosed with any additional psychiatric or neurological conditions. All individuals with ASD were drug-naïve and no individuals with ASD were taking dietary supplements.
The healthy control subjects were recruited locally by advertisement. All control individuals underwent a comprehensive assessment of their medical history to exclude individuals with neurological or other medical disorders. The SCID was conducted to exclude any personal history of past or present psychiatric illness.
The IQ was assessed using the Wechsler Intelligence Scale for children and adolescents of 6-16 years of age (Wechsler intelligence scale for children [WISC-V] or the respective scale for adults (Wechsler Adult Intelligence Scale, WAIC-R).
Precautions for mitigating the effects of small sample sizes
As the small sample size in this study may limit the interpretation of the results, we employed three measures. First, the importance of selecting the most appropriate method is needed [18]. A modified least absolute shrinkage and selection operator (adaptive Lasso) is useful for selecting appropriate covariates to account for confounding bias and thereby maintain statistical efficiency [19]. Therefore, we used adaptive Lasso. Second, standard deviations (SDs) are useful for expressing variability. Therefore, the data reliability was evaluated using the coefficient of variation (CV, %), which is defined as the SD/the mean value [20] to measure the relative variation of a random variable. The CV was used to determine between- and within-subject reliability [21]. Finally, appropriate and valid variables were chosen by selecting good candidates for small-sample studies [22].
Assessment of Social Behaviors
Social behaviors were assessed using the social responsiveness scale (SRS), which is used to distinguish ASD from other psychiatric disorders. The SRS is a 65-item questionnaire completed by the parents of subjects for the quantitative assessment of autistic traits to distinguish ASD from other psychiatric conditions [23] and is used to assess the severity of autism symptoms [24].
Controlling for dietary intake and assessment of nutrient intake
As plasma fatty acid levels may be confounded by prior dietary intake [25], all 18 participants received the “Japanese Food Guide” (Ministry of Health, Labour and Welfare, and Ministry of Agriculture, Forestry and Fishers, Japanese Food Guide. 2012), which outlines the recommended daily intake of nutrients and food based on the “Overview of Dietary Reference Intake for Japanese (2010) (Ministry of Health, Labour, and Welfare. The National Nutrition Survey in Japan., 2010). All individuals’ parents were provided with a sample of the diet meal plan and menu (KAWASAKI FOODMODEL) (http://item.rakuten.co.jp/ foodmodel/751741/), which was edited according to the “Japanese Food Guide” (Ministry of Health, Labour and Welfare, and Ministry of Agriculture, Forestry and Fishers, Japanese Food Guide. Ministry of Health, Labour and Welfare, and Ministry of Agriculture, Forestry and Fishers, Tokyo, 2012). Moreover, to assess the daily food and nutrient intake, a semi-constructive questionnaire for the Japanese (DHQ) was conducted (DHQ Support Center, http://www.ebnjapan.org/). The DHQ15 consisted of 72 questions on the frequency of intake of 150 food and beverage items and cooking methods. The DHQ15 was conducted one month before the study with randomly selected subsamples of the 18 individuals with ASD and 8 normal controls according of the order of the submission of the questionnaires to our medical consultation during January 2020 and June 2021. The validity of the DHQ15 has been verified [26].
Measurement of plasma levels of PUFAs, Cp, SOD and Tf
Blood Sampling Procedures
Whole-blood samples were collected by venipuncture into EDTA tubes after 3-hour fasting and thenplaced on ice. Nine to 12 hours of fasting before triglyceride measurement has been considered appropriate [27]. Non-fasting triglyceride levels may replace fasting levels in evaluating cardiovascular disease risk [28]. Thus, the fasting time of 3 hours after breakfast, which was applied in the present study, should be reasonable. The blood samples were frozen at - 80°C until the plasma levels of variables were analyzed at a clinical laboratory (SRL Inc., Tokyo, Japan).
Plasma levels of PUFAs
Blood samples were drawn from study participants after at least 12 h of fasting. The serum specimens were separated, frozen, and stored at -80°C until use. Plasma fatty acid levels of the samples were measured using the gas chromatography method (SRL, Tokyo). Twenty-four polyunsaturated fatty acid fractionations, including EPA, DHA, and AA concentrations, were measured. The intra- and inter-assay coefficients of ARA were 110.14 μg/ml (standard deviation [SD], 3.87; coefficient of variation [CV], 5.28%) and 100.63 μg/ml (SD, 5.51; CV, 5.48%), respectively, while those of DHA were 73.87 μg/ml (SD, 2.30; CV, 3.11%) and 68.07 μg/ml (SD, 2.30; CV, 3.33%). The plasma levels were expressed as the mean ± SD weight (%) of the total PUFAs.
Plasma Levels of SOD
Plasma SOD was estimated by the cytochrome c method using an SOD Assay Kit (Takara Bio Inc., Inc., Kusatsu, Japan) by a clinical analytical laboratory (SRL Inc., Tokyo, Japan). The assay sensitivity was 0.3 U/mL The intra-and inter-assay coefficients were 2.11 and 2.10 U/mL, respectively.
Plasma levels of Cp
For estimation of plasma CP levels, a Bering BN ⅡNephelometer (Siemens Healthcare Diagnostics K.K., USA) was used. The assay sensitivity was 3.0 mg/dl.
Plasma levels of Tf
A standard turbidimetric assay and an automated biochemical analyzer (JCA-BM8000 series, JEOL Ltd., Tokyo, Japan) were utilized to estimate plasma Tf levels
Plasma levels of MDA-LDL
An enzyme-linked immunosorbent assay (ELISA) was used to measure plasma levels of MDA-LDL; the measurement was performed by a clinical analytical laboratory (SRL Inc., Tokyo, Japan). The detection limit was 6.3 U/L, and the intra- and inter-assay coefficients were <5.6% and <9.4% respectively [29].
Statistical analyses
Relationships between plasma variables and SRS scores in the two groups were confirmed by a multiple linear regression analysis (Table 2). To identify the most effective variables for the interpretation of small sample data, an adaptive Lasso was used. The adaptive Lasso is useful for consistent variable selection and identifying important variables [18] in small-sized samples [19]. All of the statistical analyses were performed using SPSS version 27.0.
Results
Characteristics of the individuals with ASD
The results of the Mann-Whitney U Test revealed that the age of the ASD and control groups did not differ to a statistically significant extent (U = 63.00, p = 0.664). The 18 individuals with ASD were characterized by impaired social communication behaviors (n = 18). Their mean total SRS score was 83.39 ± 35.44 (Table 1). As the total SRS scores of 150 participants of 5-21 years of age with a diagnosis according to the DSM-5 criteria was 119 ± 27 (121.5-180) [30] (Table 1), the total SRS scores in the present study indicated mild impairment of social communication behaviors
Dietary nutrients
There were no significant differences between the ASD and control groups in weight, height, energy intake, or in the intake of protein (p = 0.91), cholesterol (p = 0.55), vitamin B2 (p = 0.97), vitamin B12 (p = 0.97), vitamin C (p = 0.18), omega-6 (p = .44), omega-3 (p = 0.50), iron (p= 0.55) or copper (p = 0.55) (Table 2).
Plasma levels of MDA-LDL, SOD and PUFAS
In the ASD group, plasma MDA-LDL levels were significantly higher and plasma SOD levels were significantly lower in comparison to the control group. The plasma level of DHA, and the plasma DHA/ARA ratio were significantly higher and the plasma level of adrenic acid (AdA), which is an omega 6 PUFA, was significantly lower than that in the control group (Table 1).
Results of the multiple linear regression analysis
The multiple linear regression analysis revealed that the plasma DHA level (R2 = 0.997, p = 0.000), and plasma DHA/ARA ratio (R2 = 0.972, p = 0.001) were significantly associated with adjustment in the plasma variables and the total SRS scores in the two subject groups (Table 3). These findings revealed that the plasma DHA level, and plasma DHA/ARA ratio may predict these variables in the two groups. The use of plasma alpha-linolenic acid levels as a dependent variable showed the significant contribution of the plasma DHA/ARA level (unstandardized coefficients, B= -0.267 ± 0.102, β = -0.316, p = 0.04) (Table 3). Therefore, the plasma DHA level and plasma DHA/ARA ratio fit the models that distinguished the ASD group from the control group.
Results of the adaptive Lasso analysis
The plasma DHA/ARA ratio (standardized coefficient=61.15; 95%CI, 7.544 to 114.8; p=0.0254) was selected for the SRS total score and MDA-LDL levels (standardized coefficient= 5.63, 95% CI, -25.72 to 36.87; p=0.723) (Table 3). For plasma MDA-LDL levels, plasma levels of SOD (standardized coefficient=40.66, 95%CI, -60.13 to 21.19, p<0.0001) were selected (Table 4). Collectively, these statistical findings indicated that plasma DHA/ARA levels were more significantly associated with total SRS scores and plasma MDA-LDL levels.
Coefficients of variation
The mean CVs for plasma PUFAs in the ASD and control groups were 0.03 (3.0 %) and 0.096 (9.6%), respectively.
Discussion
Due to the small sample size, three measures were employed to overcome the limitations of the data interpretation. First, the adaptive Lasso technique was used to select appropriate covariates in order to maintain statistical efficiency [19]. Second, data reliability was evaluated using coefficients of variation (SD/mean values, CV, %) [20]. Finally, appropriate and valid variables were chosen by selecting good candidates for small-sample studies [22]. In the present study, the mean CV for plasma PUFA was 0.03 (3.0 %) in the 18 individuals with ASD and 0.096 (9.6%) in the control group. The mean CVs of the pharmacokinetics and tolerability of an oral evening dose of HLD 200 (54 mg), which is a psychostimulant used for the treatment of attention-deficit/hyperactivity disorder (ADHD), in 18 subjects with ADHD and 11 healthy adults was 7.8–17.7% [31]. The CVs of plasma prostaglandin J2 in human plasma samples of diabetic patients with an HbA1C > 9% were 11.8% (intra-day) and 14.7% (inter-day) in 25 diabetic patients [32]. Taking these previous reported findings into account, the CV in the present study was appropriate.
Previous studies suggested that lipid peroxidation was induced by the downregulation of endogenous antioxidant defense [33] and that the antioxidant defense was not sufficient to prevent oxidative stress damage [34]. Examination of confocal microscopy images showed significantly higher levels of neuronal lipid peroxidation products MDA in idiopathic autism [35]. Previous animal studies indicated that DHA attenuates lipid peroxidation [36], and that DHA reduced lipid peroxidation [37].
Therefore, vulnerability of antioxidant capacity and DHA may be related to lipid peroxidation. In the present study, a dietary assessment revealed no significant differences in dietary intake between the ASD and control groups. Considering the above described findings on lipid peroxidation, the present finding suggested that the 18 subjects with ASD may have endogenous vulnerability to oxidative stress, inducing lipid peroxidation. Of reference, imbalance between oxidative stress and antioxidant capacity or vulnerable antioxidant capacity may have contributed to lipid peroxidation. Moreover, the decreased DHA/ARA ratio (0.57); as a result, DHA showed less potent antioxidant capacity.
The plasma levels of MDA-LDL and the plasma DHA/ARA ratio were significantly higher, whereas those of SOD and the omega-6 PUFA fraction, AdA, were significantly lower in the ASD group than in the control group (Table 1). Notably, a multiple linear regression analysis identified that the plasma DHA/ARA ratio fit the models for distinguishing the ASD group from the control group. Of reference, DHA plays an important role in lipid mediator production [38]. Synaptic connectivity and cortical maturation are promoted by DHA [38]. Furthermore, DHA has an important role in synaptogenesis and the synaptic expression of synapsin, which has positive effects on neuronal plasticity [39]. DHA is a substrate of cyclooxygenases and lipoxygenases, resulting in an array of lipid mediators and is susceptible to peroxidation by lipid peroxidation [40]. A higher DHA ratio in the liver of NASH rats might regulate the inflammatory response through a low n-6 ratio and diminished oxidative stress [41]. These previous reported findings indicated an important role of DHA and ARA in the peroxidation [40]. Of reference, the DHA/ARA balance is important for cognitive and behavioral development in infants [42]. We previously showed that a higher plasma omega-3/omega-6 ratio, reflecting a lower plasma ARA level, reducing the plasma level of the signaling protein ceruloplasmin, and plasma DHA/ARA ratios were significantly associated with total ABC scores and plasma levels of MDA-LDL [43]. Fish oil containing 80% DHA might be a protecting mechanism,due to the general improvement of antioxidant defense observed in those rats [44]. Values of 34 or 25 mg/100 kcal for ARA and 17 mg/100 kcal for DHA (DHA/ARA ratio (0.68) have been recommended for nervous system development in infants [45] As clinical evidence suggests that an ARA/DHA ratio greater than 1/1 is associated with improved cognitive outcomes [46]. Additionally, a higher ARA/DHA ratio induces greater ROS effects [47]. In the present study, the DHA/ARA ratio was 0.57. This ratio indicated decreased plasma DHA, resulting in less potent antioxidant capacity. Importantly, this lower DHA/ARA ratio may be related to the increased plasma levels of the final lipid peroxidation product, MDA-LDL, and the subsequent reduction in plasma SOD.
With respect to increased plasma MDA-LDL levels and decreased plasma SOD levels in lipid peroxidation, former clinical studies indicated that a total of 50 diabetic patients had significantly higher levels of MDA and significant decreases in levels of SOD as compared with controls in patients with diabetes [48], and that plasma MDA levels were elevated and plasma SOD levels were decreased in 17 patients with head and neck squamous cell carcinoma due to elevated lipid peroxidation [49]. Collectively, the relationship between increased plasma MDA and decreased plasma SOD was induced in association with oxidative stress-related lipid peroxidation.
ARA is metabolized by two pathways, leading to the formation of prostaglandins, among which prostaglandin E2 (PGE2) is an important mediator of synaptic plasticity [50]. Since the Wnt pathway is crucial to brain development and organization, cross-talk between PGE2 and Wnt signaling in neuronal cells contributes to the development of ASD [51]. A previous study reported that erythrocyte ARA-derived 4-HNE levels in 20 autistic patients were significantly increased in comparison to 18 controls, indicating impairment of the redox status [52]. Therefore, ARA may be related ASD via ARA-derived 4-HNE. A metabolic link between DHA and/or the DHA-derived 4-HHE pathway may provide a therapeutic strategy against oxidative damage due to cerebral ischemia, and other brain injuries [40]. Thus, ARA may contribute to neurodegeneration, while DHA may have therapeutic potential.
The plasma level of AdA (a member of the n-6 PUFA family) in the ASD group was significantly lower in comparison to the control group. AdA could play a role in resolution of inflammation in vivo [53], and induce lipid proxidation [54]. As neuroinflammation has been suggested in pathophysiology of ASD [55], AdA may contribute to lipid peroxidaion in neuroinflammation related ASD.
MDA is an endogenous material that is a product of enzymatic and oxygen radical-induced lipid peroxidation [56] and plays a role as a signaling molecule [40] (Yang). The production of MDA is elicited by DHA [57]. MDA is including in the brain during a specific form of oxidative stress, such as following daily activities and sleep deprivation [58] via breaking the homeostasis between excitatory and inhibitory neurons. MDA-modified low-density lipoprotein (MDA-LDL) is related to oxidative stress [59], but not cell proliferation [60]. Therefore, plasma MDA-LDL increase oxidative stress, inducing lipid peroxidation.
The results of adaptive Lasso revealed that the association between increased plasma MDA-LDA levels and decreased plasma SOD levels. Early studies revealed that serum MDA levels were increased in association with decreased serum SOD levels in neurodegeneration [61, 62]. Reduced SOD levels in children with ASD may indicate the involvement of mitochondrial (Mn) SOD in pathogenesis of ASD [63]. We previously reported that reduced plasma SOD levels may be related to decreased endogenous antioxidant capacity [64], the present finding that elevated plasma levels of MDA-LDL are associated with decreased plasma levels of SOD may be reasonable.
Taken together, the findings of the present study suggest that lower plasma SOD levels may be involved in neuronal deficitsin in social impairment in the 18 individuals with ASD. However, further detailed studies are needed.
This present study was associated with some limitations. First, the most prominent products are 4-hydroxyhexenal (4-HHE) from DHA and 4-hydroxynonenal (4-HNE) from ARA [40]. Of reference, MDA-LDL appears to be the most mutagenic final product of lipid peroxidation, whereas 4-HHE and 4-HNE are recently considered to be a bioactive marker of lipid peroxidation [40]. However, MDA-LDL is well known as a biomarker of lipid peroxidation of omega-3 and omega-6 PUFAs [1]. Therefore, we used plasma MDA-LDL as a biomarker of lipid peroxidation in this study.
The small sample size may induce the likelihood of a false null hypothesis (type II and type I errors), and skews the results and reduce the power of the study. The adaptive Lasso is very competitive in terms of variable selection, estimation accuracy, and high efficiency when small sample sizes are used [16, 17]. Thus, adaptive Lasso in statistical analyses may improve the reproducibility and sensitivity of the findings and reduce the likelihood of type II and type I errors. Moreover, the present findings suggested a significant correlation between increased plasma MDA-LDL and decreased plasma SOD. A previous review article reported similar results [9, 65, 66] ngs regarding the importance of the plasma DHA/ARA ratio are consistent with those of previous studies [40, 66-69], we applied three measures to ameliorate the difficulty in drawing significant conclusions from a small sample. Third, the ratio of case-to-control control subjects in the present study was small (2.5: 1). In a case-control evaluation of pulmonary and extrapulmonary findings of incidental asymptomatic COVID-19 infection 4:1 control:case ratio on the PET-CT scanning [70]. Moreover, in a previous case-control genomic study, ratios of up to 3:1 to 4:1 induced significant results in comparison to ratios of 1:1 or 2:1 [71]. Therefore, this ratio of our study (2.2: 1) may be reasonable.
Conclusion
The present findings might provide useful information the association between increased plasma DHA/ARA ratio and increased plasma MDA-LDL levels, which may counteract plasma SOD levels, reducing plasma SOD levels. This neurobiological phenomenon may induce neuronal deficit related to autistic social behavior in individuals with ASD. Importantly, imbalance in metabolism between omega -3 and omega 6 PUFAs might induce increased plasma DHA/ARA ratio.
Acknowledgment
Declared none.
Funding
This work was financially supported by a Grant-in-Aid for Scientific Research (C) (2018-2021, NO. 26461777) (Kunio Yui), Ministry of Education, Culture, Sports, Science and Technology, Japan.
Contribution of Authors
Kunio Yui and George Imataka contributed substantially to the design and performance, Shiko Yui conducted statistical analysis, or reporting of the work and are required to indicate their specific contribution. Tomyo Hayashi and Kunio Yui have substantially contributed to the study for important intellectual content, or who was involved in the article’s drafting the manuscript and revising. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
The authors have no conflict of interests concerning the materials or methods used in the present study or findings presented in this study.
For this manuscript reporting data from studies involving human participants, the present study has been performed with the approval of the ethical standards of Dokkyo Medical University (Tochigi, Japan, NO. 27014) and with appropriate participants’ informed consent in compliance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from participants.
p>Human and Animal RightsHuman Subjects were used for studies that are base of this research.
Consent for Publication
We made sure to seek consent from individuals to publish their data prior to submitting their paper to a journal. Consent for publication was obtained from the all subjects including children, and/or their parents.
Availability of Data and Materials
The data that support the findings of the present study are available from rthe corresponding author upon reasonable request
Conflict of Interest
The authors declare that they have no competing interests.
- Ayala A. Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell Longev 360438.
- Lawrence GD (2021) Perspective: The Saturated fat-unsaturated oil dilemma: relations of dietary fatty acids and serum cholesterol, atherosclerosis, inflammation, iancer, and all-cause mortality. Adv. Nutr 12: 647-56.
- Ambroz A, Vlkova V, Rossner Jr P, Rossnerova A, Svecova V et al. (2016) Impact of air pollution on oxidative DNA damage and lipid peroxidation in mothers and their newborns. Int. J. Hyg. Environ. Health 219: 545-56.
- Cortelazzo A, De Felice C, Guerranti R, Signorini C, Leoncini S et al. (2016) Expression and oxidative modifications of plasma proteins in autism spectrum disorders: Interplay between inflammatory response and lipid peroxidation. Proteomics Clin. App 1103-12
- Meguid N, Dardir AA, Abdel-Raouf ER, Hashish A (2011) Evaluation of oxidative stress in autism: defective antioxidant enzymes and increased lipid peroxidation. Biol. Trace Elem. Res 143: 58-65.
- González-Fraguela ME, Hung MD, Vera H, Maragoto C, Noris E et al. (2013) Oxidative stress markers in children with autism spectrum disorders. Br. J. Med. Med. Res 3: 307-17.
- de Souza DN, de Souza EMN, da Silva Pedrosa M, Nogueira FN, Simões A (2021) Effect of Tungstate administration on the lipid peroxidation and antioxidant parameters in salivary glands of streptozotocin -induced diabetic rats, J. Biol. Trace Elem. Res 199: 1525-33.
- Xu W, Mao Z, Zhao B, Ni T, Deng S et al. (2021) Vitamin C attenuates vancomycin induced nephrotoxicity through the reduction of oxidative stress and inflammation in HK-2 cells Ann. Pallia.t Med 10: 1748-54.
- Wang Y, Branicky R, Noë A, Hekimi S (2017) Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol 217: 1915-28.
- Padalkar PK, Shinde AV, Patil SM (2012) Superoxide dismutases: dual roles in controlling ROS damage and regulating ROS sige 2 diabetes mellitus. Biomed. Res 23: 207-10.
- Zoroglu SS, Armutcu F, Ozen S, Gurel A, Sivasli E et al. (2004) Increased oxidative stress and altered activities of erythrocyte free radical scavenging enzymes in autism. Eur. Arch. Psychiatry Clin. Neurosci 254: 143-7.
- Ornoy A, Weinstein-Fudim L, Tfilin M, Ergaz Z, Yanai J et al. (2019) S-adenosyl methionine prevents ASD like behaviors triggered by early postnatal valproic acid exposure in very young mice. Neurotoxicol Teratol 71: 64-74.
- Dziobekm I, Gold SM, Wolf OT, Convit A (2007) Hypercholesterolemia in Asperger syndrome: independence from lifestyle, obsessive-compulsive behavior, and social anxiety. Psychiatry Res 149: 321-4.
- Liu Q, Liu Y, Shi J, Gao M, Liu Y et al. (2018) Entire peroxidation reaction system of myeloperoxidase correlates with progressive low-density lipoprotein modifications via reactive aldehydes in atherosclerotic patients with hypertension. Cell Physiol. Biochem 50: 1245-54.
- Ogawa K, Tanaka T, Nagoshi T, Sekiyama H, Arase S et al. (2015) Increase in the oxidised low-density lipoprotein level by smoking and the possible inhibitory effect of statin therapy in patients with cardiovascular disease: a retrospective study. BMJ Open 5: e005455.
- Wahid A, Khan DM, Hussain L (2017) Robust Adaptive Lasso method for parameter's estimation and variable selection in high-dimensional sparse models. PLoS One 12: e0183518.
- Abdul-Razik Ismail E (2015) Behavior of lasso quantile regression with small sample sizes. J. Multidiscip. Eng. Sci. Technol 2: 388-94.
- Morgan CJ (2017) Use of proper statistical techniques for research studies with small samples. Am. J. Physiol. Lung Cell Mol. Physiol 313: L873-7.
- Shortreed SM, Ertefaie A (2017) Outcome-adaptive lasso: Variable selection for causal inference. Biometrics 73: 1111-22.
- Packiasabapathy S, Prasad V, Rangasamy V, Popok D, Xu X et al. (2020) Cardiac surgical outcome prediction by blood pressure variability indices Poincaré plot and coefficient of variation: a retrospective study. BMC Anesthesiol 20: 56.
- Clubb J, Towlson C, Barrett S (2022) Measurement properties of external training load variables during standardised games in soccer: Implications for training and monitoring strategies. PLoS One 202217: e0262274.
- Lichou F, Orazio S, Dulucq S, Etienne G, Longy M et al. (2019) Novel analytical methods to interpret large sequencing data from small sample sizes. Hum. Genomics 13: 41.
- Constantino JH, Gruber CP (2012) Social Responsiveness Scale-Second Edition (SRS-2). Western Psychological Services, 2012, Torrance, CA.
- Sipsock D, Tokadjian H, Righi G, Morrow EM, Sheinkopf SJ (2021) Rhode Island Consortium for Autism Research and Treatment (RI-CART). Autism severity aggregates with family psychiatric history in a community-based autism sample. Autism Res 14: 2524-32.
- Holtenius K, Agenäs S, Delavaud C, Chilliard Y (2003) Effects of feeding intensity during the dry period. 2. Metabolic and hormonal responses. J. Dairy. Sci 86: 883-91.
- Okuda M, Sasaki S, Bando N, Hashimoto M, Kunitsugu I et al. (2009) Carotenoid, tocopherol, and fatty acid biomarkers and dietary intake estimated by using a brief self-administered diet history questionnaire for older Japanese children and adolescents. J. Nutr. Sci Vitaminol (Tokyo) 55: 231-41.
- Simundi AM, Cornes M, Grankvis K, Lippi G, Nybo M (2014) Standardization of collection requirements for fasting samples for the Working Group on Preanalytical Phase (WG-PA) of the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). Clin. Chimca Acta 432: 33037.
- Stalenhoef AF, de Graaf J (2008) Association of fasting and nonfasting serum triglycerides with cardiovascular disease and the role of remnant-like lipoproteins and small dense LDL. Curr. Opin Lipidol 19: 355-61.
- Kitano S, Kanno T, Maekawa M, Sakiurabayashi I, Kotani K et al. (2004) Improved method for the immunological detection of malondialdehyde-modified low-density lipoproteins in human serum. Anal. Chim. Acta 509: 229-35.
- Aran A, Harel M, Cassuto H, Polyansky L, Schnapp A et al. (2016) Castellanos, Cannabinoid treatment for autism: a proof-of-concept randomized trial. Mol. Autism 6.
- Childress AC. Stark JG (2018) Diagnosis and Treatment of Attention-Deficit/Hyperactivity Disorder in Preschool-Aged Children. J. Child. Adolesc. Psychopharmacol 28: 606-14.
- Morgenstern J, Fleming T, Kadiyska I, Brings S, Groener JB et al. (2018) Nawroth, P; Hecker, M; Brune, M. Sensitive mass spectrometric assay for determination of 15-deoxy-Δ12,14-prostaglandin J2 and its application in human plasma samples of patients with diabetes. Anal. Bioanal. Chem 410: 521-8.
- Che Y, Zhou Z, Shu Y, Zhai C, Zhu Yl et al. (2015) Chronic unpredictable stress impairs endogenous antioxidant defense in rat brain. Neurosci. Lett 584: 208-13.
- Ozturk OH, Oktar S, Aydin M, Kucukatay V (2010) Effect of sulfite on antioxidant enzymes and lipid peroxidation in normal and sulfite oxidase-deficient rat erythrocytes. J. Physiol. Biochem 66: 205-12.
- Frackowiak Jl, Mazur-Kolecka B, Schanen NC, Brown WT, Wegiel J et al. (2013) The link between intraneuronal N-truncated amyloid-β peptide and oxidatively modified lipids in idiopathic autism and dup (15.q11.2-q13)/autism. Acta Neuropathol. Commun 1: 61.
- White-Springer SH, Vineyard KR, Kivipelto J, Warren IK (2021) Dietary. omega-3 fatty acid supplementation does not impair vitamin E status or promote lipid peroxidation in growing horses. J. Anim. Sci 99: skab177.
- Huun MU, Garberg HT, Escobar J, Chafer C, Vento M et al. (2018) A reduces oxidative stress following hypoxia-ischemia in newborn piglets: a study of lipid peroxidation products in urine and plasma. J. Perinat Med 46: 209-17.
- Calder PC (2016) Docosahexaenoic acid. Ann. Nutr. Metab 69: 7-21.
- Dyall SC (2015) Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci 7: 52.
- Yang B, Li R, Michael Greenlief C, Fritsche KL, Gu Z et al. (2019) Yin-Yang mechanisms regulating lipid peroxidation of docosahexaenoic acid and arachidonic acid in the central nervous system. Front. Neurol. Actions 10: 642.
- Takayama F, Nakamoto K, Totani N, Yamanushi T, Kabuto H et al. (2010) Effects of docosahexaenoic acid in an experimental rat model of nonalcoholic steatohepatitis J Oleo. Sci 59: 407-14.
- Colombo J, Shaddy DJ, Kerling FH, Gustafson KM, Carlson SE (2017) Docosahexaenoic acid (DHA) and arachidonic acid (ARA) balance in developmental outcomes. Prostaglandins Leukot. Essent. Fatty Acids 121: 52-6.
- Yui K, Imataka G, Kawasak Y, Yamada H (2016) Increased ω-3 polyunsaturated fatty acid/arachidonic acid ratios and upregulation of signaling mediator in individuals with autism spectrum disorders. Life Sci 145: 205-12.
- Miralles-Pérez B, Méndez L, Nogués MR, Sánchez-Martos V, Fortuño-Mar À et al. (2021) Ramos-Romero, S.; Hereu, M.; Medina, I.; Romeu, M. Effects of a fish oil rich in docosahexaenoic acid on cardiometabolic risk factors and oxidative stress in healthy rats. Mar Drugs 19: 555
- Hoffman DR, Harris CL, Wampler JL, Patterson AC, Berseth CL (2019) Growth, tolerance, and DHA and ARA status of healthy term infants receiving formula with two different ARA concentrations: Double-blind, randomized, controlled trial. Prostaglandins Leukot. Essent. Fatty Acids 146: 19-27.
- Hoffman DR, Boettcher JA, Diersen-Schade DA (2009) Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: a review of randomized controlled trials. Prostaglandins Leukot Essent. Fatty Acids 81: 151-8.
- Ghazali R, Mehta KJ, Bligh SA, Tewfik I, Clemens D et al. (2020) Patel, V.B.; (2020) High omega arachidonic acid/docosahexaenoic acid ratio induces mitochondrial dysfunction and altered lipid metabolism in human hepatoma cells. World J. Hepatol 12: 84-98
- Arif M, Islam MR, Waise TM, Hassa F, Mondal SI et al. (2010) Kabir, Y. DNA damage and plasma antioxidant indices in Bangladeshi type 2 diabetic patients. Diabetes Metab 36: 51-7.
- Gupta A, Bhatt ML, Misra K (2009) Lipid peroxidation and antioxidant status in head and neck squamous cell carcinoma patients. Oxid. Med. Cell Longev 291: 68-72.
- El-Ansary AL, Ayadhi L (2012) Lipid mediators in plasma of autism spectrum disorders. Lipid Health Dis 11: 160.
- Wong CT, Ahmad E, Li H, Crawford DA (2014) Prostaglandin E2 alters Wnt- dependent migration and proliferation in neuroectodermal stem cells: implications for autism spectrum disorders. Cell Commun. Signal 12: 19.
- Gelen V, Şengül E, Yıldırım S, Senturk E, Tekin S et al. (2021) Kükürt, A. The protective effects of hesperidin and curcumin on 5-fluorouracil-induced nephrotoxicity in mice Environ. Sci. Pollut. Res. Int 28: 47046-55.
- Jana T, Tzveta S, Zlatina N, Natasha I, Dimitrinka A et al. (2020) Effect of endurance training on diurnal rhythms of superoxide dismutase activity, glutathione and lipid peroxidation in plasma of pinealectomized rats. Neurosci. Lett 16: 134637.
- Pecorelli A, Leoncini S, De Felice C, Signorini C, Cerrone C et al. (2013) Non-protein-bound iron and 4-hydroxynonenal protein adducts in classic autism. Brain Dev 35: 146-54.
- Brouwers H, Jónasdóttir HS, Kuipers ME, Kwekkeboom JC, Auger JL et al. (2020) Anti-Inflammatory and proresolving effects of the omega-6 polyunsaturated fatty acid adrenic acid. J Immunol 205: 2840-9.
- Sánchez-Illana Á, Shah V, Piñeiro-Ramos JD, Di Fiore JM, Quintás G et al. (2019) Adrenic acid non-enzymatic peroxidation products in biofluids of moderate preterm infants. Free Radic. Biol. Med 142: 107-12.
- Liu W, Li L, Xia X, Zhou X, Du Y et al. (2022) Integration of Urine Proteomic and Metabolomic Profiling Reveals Novel Insights into Neuroinflammation in Autism Spectrum Disorder. Front Psychiatry 13: 780747.
- Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ (2003) Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J. Biol. Chem 278: 31426-33.
- Mahéo K, Vibet S, Steghens JP, Dartigeas C, Lehman M et al. (2005) Differential sensitization of cancer cells to doxorubicin by DHA: a role for lipoperoxidation. Free Radic. Biol. Med 39: 742-51.
- Li F, Yang Z, Lu Y, Wei Y, Wang J et al. (2010) Malondialdehyde suppresses cerebral function by breaking homeostasis between excitation and inhibition in turtle Trachemys scripta. PLoS One 5: e15325.
- Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K et al. (2006) Tetrahydrobiopterin improves aging-related impairment of endothelium-dependent vasodilation through increase in nitric oxide production. Atherosclerosis 186: 390-5.
- Lopes-Virella MF, Virella G (2019) Modified, LDL Immune Complexes and Cardiovascular Disease. Curr Med Chem 26: 1680-92.
- Prasad DKV, Satyanarayana U, Shaheen U, Prabha TS, Munshi A (2017) Oxidative Stress in the Development of Genetic Generalised Epilepsy: An observational Study in southern indian population. J. Clin. Diagn. Res 11: BC05-8
- Huang L, He Z, Guo L, Wang H (2008) Improvement of cognitive deficit and neuronal damage in rats with chronic cerebral ischemia via relative long-term inhibition of rho-kinase. Cell Mol. Neurobiol 28: 757-68.
- Yenkoyan K, Harutyunya H, Harutyunyan A (2018) A certain role of SOD/CAT imbalance in pathogenesis of autism spectrum disorders. Free Radic. Biol. Med 123: 85-95.
- Yui K, Tanuma N, Yamada H, Kawasaki Y (2017) Decreased total antioxidant capacity has a larger effect size than increased oxidant levels in urine in individuals with autism spectrum disorder. Environ. Sci. Pollut. Res. Int 24: 9635-44.
- Jiménez-Fernández S, Gurpegui M, Díaz-Atienza F, Pérez-Costillas L, Gerstenberg M et al. Oxidative stress and antioxidant parameters in patients with major depressive disorder compared to healthy controls before and after antidepressant treatment: results from a meta-analysis. J. Clin. Psychiatry 76: 1658-67
- Prie BE, Iosif L, Tivig I, Stoian Giurcaneanu C (2016) Oxidative stress in androgenetic alopecia. J. Med. Life 9: 79-83.
- Oborna I, Wojewodka G, De Sanctis JB, Fingerova H, Svobodova M et al. (2010) Increased lipid peroxidation and abnormal fatty acid profiles in seminal and blood plasma of normozoospermic males from infertile couples. Hum. Reprod 25: 308-16.
- Hadley KB, Ryan AS, Forsyth S, Gautier S, Jr Salem N (2016) The Essentiality of arachidonic acid in infant development. Nutrients 8: 216
- Starčević K, Roškarić P, Šperanda M, Đidara M, Kurilj AG et al. (2019) High dietary n6/n3 ratio decreases eicosapentaenoic to arachidonic acid ratios and upregulates NFκB/p50 expression in short-term low dose streptozotocin and high-fructose rat model of diabetes. Prostaglandins Leukot Essent. Fatty Acids 149.
- Subesinghe M, Bhuva S, Dunn JT, Hammers A, Cook GJ et al. (2022) Barrington, S.F.; Fischer, B.M. A case-control evaluation of pulmonary and extrapulmonary findings of incidental asymptomatic COVID-19 infection on FDG PET-CT. Br. J. Radiol 95: 20211079.
- Kang M, Choi S, Koh IS (2009) The effect of increasing control-to-case ratio on statistical power in a simulated case-control SNP association study. Genomics & informatics 7: 148-51.

Tables at a glance
Figures at a glance