Hemiplegic Migraine with Hemibody Dysautonomia: First Presentation of Hemiplegic Migraine with CT Perfusion and Electrographic Abnormalities Triggered by Influenza B Infection
Received Date: September 08, 2024 Accepted Date: October 08, 2024 Published Date: October 11, 2024
doi: 10.17303/jnnd.2024.12.104
Citation: Muaaz Ather, Gerard Mullane, Eoghan Donlon, Manisha Coran, Hassan Bhatti, et al. (2024) Hemiplegic Migraine with Hemibody Dysautonomia: First Presentation of Hemiplegic Migraine with CT Perfusion and Electrographic Abnormalities Triggered by Influenza B Infection. J Neurophysiol Neurol Disord 12: 1-7
Abstract
This report details a unique case of an 18-year-old female presenting with left-sided hemiplegia, hemibody dysautonomia, and headache triggered by influenza B infection. The patient exhibited profound hemispheric dysfunction, confirmed by CT perfusion showing subtle abnormalities and EEG demonstrating right hemisphere attenuation and slowing. Genetic testing revealed a CACNA1A mutation, suggesting a diagnosis of hemiplegic migraine (HM). The case highlights the importance of considering viral infections as potential triggers for HM and introduces the novel observation of lateralized autonomic dysfunction. Early recognition of imaging and electrographic features was essential in differentiating this from other conditions such as stroke, emphasizing the need for accurate diagnostic approaches in similar presentations.
Keywords: Hemiplegic Migraine; Neurological Disorder; Headache; Influenza B Infection; Electroencephalography; Hemibody Dysautonomia
Introduction
Hemiplegic Migraine (HM) is a rare disorder characterized by attacks of headache associated with motor weakness and can be associated with encephalopathy and other cortical signs. HM can be sporadic or hereditary with causative mutations identified in CACNA1A, ATP1A2 and SCN1A genes [1]. The pathophysiology of HM is hypothesized to be similar to that of migraine with aura, but more severe with prominent electrophysiological abnormalities and occasionally CT perfusion abnormalities. HM is an established stroke mimic that demonstrates normal angiographic imaging, but can be associated with subtle regional hypoperfusion on CT perfusion imaging.
Case Report
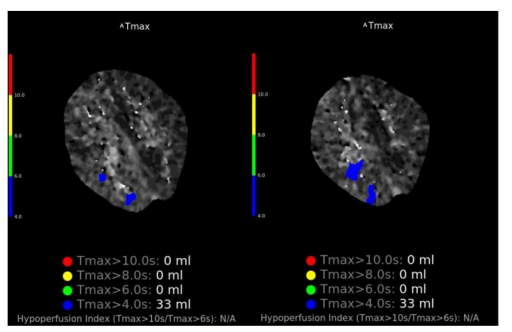
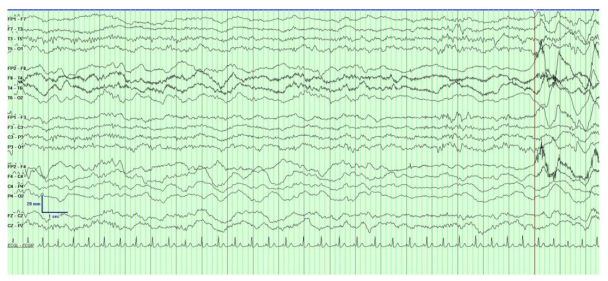
An 18 year old lady presented to the emergency department with left sided weakness and headache. Background history was significant for migraine with visual aura. At the time of presentation she reported gradual onset of left upper limb reduced sensation over the course of minutes, followed by left upper limb weakness, which spread to her left lower limb and face over the course of 45 minutes. One hour later she developed a throbbing right frontal headache associated with photophobia. She had reported mild coryzal symptoms 2 days prior to presentation, resolved by time of presentation. On arrival to the emergency department, given the rapid onset and focality of her symptoms, a stroke call was activated. On examination the patient was alert, but mildly encephalopathic, responding slowly to questioning. Clinical examination was notable for a left homonymous hemianopia, subtle dysarthria, left sided upper motor neuron facial droop with forehead sparing, left sided hemiparesis (MRC grade 1/5 upper limb, 2/5 lower limb), left sided sensory neglect and extinction, brisk reflexes on left compared to right with sustained clonus at the left knee and ankle and upgoing left plantar. The left upper limb was noticeably cold and pale compared to the right side with slowed capillary refill, suggestive of lateralized autonomic dysfunction. Computed Tomography (CT) and angiography of the brain was normal. CT perfusion did not detect a large perfusion mismatch to explain severity of symptoms but did demonstrate patchy subtle perfusion abnormalities exclusively in the right hemisphere with a “time to maximum greater than 4seconds” (Tmax >4s) volume of 33ml (figure 1). A bedside electroencephalogram (EEG) demonstrated significant asymmetry of the background with significant attenuation and slowing over the entire right hemisphere. Anterior posterior organization was preserved over the left which was generating alpha range frequencies with some intermittent mild theta slowing. There were no epileptiform abnormalities, periodic patterns or electrographic seizures (figure 2).
Given the negative CT perfusion and CTA and EEG showing hemispheric dysfunction without epileptiform acivity in conjunction with the patient’s history of migraine a diagnosis of hemiplegic migraine was considered and the patient was treated with IV paracetamol, naproxen, metoclopramide and IV fluids. She improved over the next 24 hours with subsequent normal neurological examination. The patient tested positive for Influenza B infection but was asymptomatic. The patient self-discharged before an MRI of the brain could be performed.
There was a strong family history of migraine with aura but no known family history of hemiplegic migraine. Based on the patient’s clinical presentation and family history, genetic testing was requested which identified a variant of unknown significance in CACNA1A (c.632G>A p. (Ser211Asn)) in close proximity to a highly conserved acceptor splice site.
Discussion
This case highlights a unique first presentation of CACNA1A associated HM and important acute electrophysiological and radiological features that can aid in earlier diagnosis of this condition to be made.
Hemiplegic migraine (HM) is defined by the ICHD-3 as both fulfilling diagnostic criteria for migraine with aura, as well as having necessary features of motor weakness and visual, sensory or speech disturbance, with all features showing full reversibility. The term “Familial hemiplegic migraine (FHM)” necessitates at least one first or second degree family member also fulfilling diagnostic criteria for HM. Causative mutations have been identified in CACNA1A, ATP1A2 and SCN1A [2]. The prevalence of hemiplegic migraine is estimated to be 0.01%, based on a large scale Danish epidemiological study conducted in 1999 [3]. Our patient had a history of migraine with visual aura, there were no prior episodes of hemibody sensorimotor dysfunction. There was a history of migraine with aura but not hemiplegia in a first degree relative, therefore they met criteria for sporadic hemiplegic migraine rather than FHM. Over 60% of patients presenting with HM report a clear trigger leading to the development of symptoms [4]. Emotional stress, bright lights and head trauma are among the most commonly reported triggers. There have been documented cases of HM triggered by fever and viral infection but these seem to be rare [5]. In our case, influenza B infection with mild coryzal symptoms in the days leading up to presentation was likely the trigger of this first clinical HM presentation.
Along with seizures and metabolic disturbances such as hypoglycemia, migraine is a common stroke mimic. In our case, a clear history of hemiplegia was pre-alerted by paramedics leading to the activation of a stroke call and immediate review by a neurologist. In addition to hemiplegia other findings on examination suggested widespread dysfunction of the right hemisphere including homonymous hemianopia, hemineglect and hemiparesis with marked corticospinal tract dysfunction. Emergent imaging included non-contrast CT brain, CT perfusion and CT Angiogram arch to vertex with a venous phase, which effectively out ruled a vascular etiology such a haemorrhage, ischaemic stroke or cerebral venous sinus thrombosis. A bedside EEG was performed within 30 minutes of presentation which allowed acute electroencephalographic assessment of the right hemisphere at peak symptom severity and demonstrated marked asymmetry of the cerebral hemisphere with diffuse background slowing in the delta range without epileptiform discharges (Figure 2). The development of a typical unilateral headache along with photophobia and photopsia then made a diagnosis of hemiplegic migraine most likely.
Mild lateralized sensorimotor symptoms are not uncommon in migraineurs and sometimes lead to patient being given a somewhat casual label of hemiplegic migraine. This case clearly demonstrates the difference in symptom severity in a true HM secondary to CACNA1A channelopathy causing severe dysfunction of an entire hemisphere for a prolonged period of time rather than a more focal disturbance in typical migraine with aura.
Radiological features in HM are typically exclusively unilateral and demonstrate complete reversibility. CT may demonstrate unilateral sulcal effacement, but often is unremarkable [6,7]. Magnetic resonance imaging (MRI) may demonstrate unilateral high T2 Fluid attenuated inversion recovery (FLAIR) signal with occasional cortical enhancement on T1 post gadolinium sequences [7,8]. Perfusion studies (both CT and MRI) have shown focal areas of hypo perfusion in the affected hemisphere [8]. Radiological features consistently show full resolution over time, but permanent sequelae have also been rarely reported [9]. In our case the perfusion abnormalities were subtle, but were exclusively right hemispheric, which we hypothesis are secondary to the widespread metabolic disturbance in this hemisphere.
The role of genetic sequencing has had significant impacts on both diagnostic and prognostic factors in HM. While there are a number of genes which have been linked to HM, the most closely associated and commonly described are CACNA1A, ATP1A2 and SCN1A. Other implicated genes include PRRT2 and SLC4A4, with less robust associations. CACNA1A encodes the α1 subunit of neuronal voltage-gated Cav2.1 (P/Q type) channels which predominantly localizes to presynaptic terminals of cortical and cerebellar neurons. More than 25 pathogenic CACNA1A variants associated with autosomal dominant FHM1 have been described. They often have gain of function in vivo effects increasing Ca2+ cellular influx leading to increased glutamatergic activity and neuronal hyperexcitability. In our case a heterozygous mutation in the CACNA1A (NM_001127222.2 (CACNA1A):c.632G>A (p.Ser211Asn)) gene was found on testing. This mutation has been reported on ClinVar database twice previously and is currently classified as a variant of uncertain significance. It has a relatively low frequency in the gnomAD database (0.006%) and in silico analysis supports that this missense variant has a deleterious effect on protein structure/function, but has not previously been published as pathogenic or benign.
Migraine was accurately described existing in the borderlands of epilepsy by Williams Gowers in 1906 [10]. The electrographic changes and genetics of FHM provide a fascinating insight into the similarities between migraine and epilepsy, two paraxoysmal neurological disorders that can, at times, be difficult to differentiate [11]. A recent study looked at the coexistence of epilepsy in patient with FHM and confirmed mutations in CACNA1A, ATP1A2, SCN1A or PRRT2. Of the 195 individuals, 78 had a diagnosis of focal or generalized epilepsy with the highest rate of association in ATP1A2 (57.7%), followed by PRRT2 (17%), SCN1A (16.7%) and CACNA1A (7.7%) [12]. EEG findings in FHM reported in the literature vary from slow sharp waves, diffusely slow with polymorphic theta with some reports of epileptiform discharges in the contralateral hemisphere to hemiplegia [13-15]. The EEG in our case demonstrated diffuse slowing of the right hemisphere fitting with some of the EEG patterns described in HM.
This case reports a previously undescribed phenomenon of hemibody autonomic dysfunction as evidence by marked pallor and reduced temperature of the left upper limb and delayed capillary refill compared to the right. Dysautonomia is well described in migraine often in the premonitory phase with features including nausea, vomiting, polyuria, eyelid oedema, lacrimation, nasal congestion and ptosis. Dysautonomia manifesting as pallor of the limbs on one side of the body and temperature differential has not been described in the literature in migraine. Unilateral autonomic dysfunction is described in epilepsy in the form of ictal piloerection (“goose bumps”) principally caused by temporal lobe seizures, but also reported in frontal and hypothalamic seizure focuses. The most well described lateralized dysautonomic syndrome is Harlequin syndrome which is characterized by unliateral facial flushing and hyperhidrosis caused by unilateral blockade of sympathetic vascodilator and sudomotor fibres to the face [16]. There are two reports of a crossed sympathetic deficit of the face and arm with flushing and hyperhidrosis on right side of the face with relative pallor of the left face induced by exercise with reduced temperature and pallor of the right arm [17,18]. Furthermore, a study looking at the connection between autonomic dysfunction and stroke showed significant statistical association of specific central lesions in stroke with contralateral autonomic dysfunction [19]. We speculate that the diffuse right-sided hemispheric dysfunction evident based on EEG find may have led to dysfunction of subcortical structures including the hypothalamus affecting first order sympathetic neurons.
This case also suggests the likely effect of a virus as a trigger for an acute neurological episode. Although case reports linking influenza and hemiplegic migraine are rare, the literature does mention case reports of other viruses as a trigger for hemiplegic syndromes, including that of a girl with serologic evidence of active Epstein-Barr virus infection who presented with acute hemiplegic migraine syndrome [20], and a 5-year-old Japanese girl with hemiconvulsion-hemiplegia-epilepsy syndrome after infection with parvovirus B19 infection [21]. While there is ample literature describing the connection between certain viruses and central nervous system infections, our understanding of the connection between other neurological disorders and viral infections is more limited. It is hypothesized that multiple factors could contribute to the indirect damage of viruses which can trigger certain neurological disorders, and these include increased oxidative stress, destruction of neurons, protein aggregation and alteration of autophage function [22].
Our case highlights novel clinical observation of hemibody dysautonomia along with EEG and imaging findings that can aid in making the diagnosis early and avoiding inappropriate treatment for stroke. We also propose a novel mutation in CACNA1A (c.632G>A (p.Ser211Asn)) as likely causative for this patient’s condition.
- Di Stefano V, Rispoli MG, Pellegrino N, Graziosi A, Rotondo E, Napoli C, et al. (2020) Diagnostic and therapeutic aspects of hemiplegic migraine. J Neurol Neurosurg Psychiatry. 91: 764-71.
- Headache Classification Committee of the International Headache Society (IHS) (2018) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 38: 1-211.
- Lykke Thomsen L, Kirchmann Eriksen M, Faerch Romer S, Andersen I, Ostergaard E, Keiding N, et al. (2002) An epidemiological survey of hemiplegic migraine. Cephalalgia. 22: 361-75.
- Hansen JM, Hauge AW, Ashina M, Olesen J (2011) Trigger factors for familial hemiplegic migraine. Cephalalgia. 31: 1274-81.
- Toldo I, Brunello F, Morao V, Perissinotto E, Valeriani M, Pruna D, et al. (2019) First Attack and Clinical Presentation of Hemiplegic Migraine in Pediatric Age: A Multicenter Retrospective Study and Literature Review. Front Neurol. 10: 1079.
- Sugrue G, Bolster F, Crosbie I, Kavanagh E (2014) Hemiplegic migraine: neuroimaging findings during a hemiplegic migraine attack. Headache. 54: 716-8.
- Tee TY, Khoo CS, Raymond AA, Syazarina SO (2019) Teaching NeuroImages: Neuroimaging in hemiplegic migraine. Neurology. 93: e626-e7.
- Fear D, Patel M, Zand R (2021) Serial magnetic resonance imaging findings during severe attacks of familial hemiplegic migraine type 2: a case report. BMC Neurol. 21: 173.
- Schwedt TJ, Zhou J, Dodick DW (2014) Sporadic hemiplegic migraine with permanent neurological deficits. Headache. 54: 163-6.
- Gowers WR (1906) Clinical Lectures ON THE BORDERLAND OF EPILEPSY. III.-MIGRAINE. Br Med J. 2: 1617-22.
- Paungarttner J, Quartana M, Patti L, Sklenarova B,Farham F, Jimenez IH, et al. (2024) Migraine - a borderland disease to epilepsy: near it but not of it. J Headache Pain. 25: 11.
- Hasirci Bayir BR, Tutkavul K, Eser M, Baykan B (2021) Epilepsy in patients with familial hemiplegic migraine. Seizure. 88: 87-94.
- Gastaut JL, Yermenos E, Bonnefoy M, Cros D (1981) Familial hemiplegic migraine: EEG and CT scan study of two cases. Ann Neurol. 10: 392-5.
- Chastan N, Lebas A, Legoff F, Parain D, Guyant-- Marechal L (2016) Clinical and electroencephalographic abnormalities during the full duration of a sporadic hemiplegic migraine attack. Neurophysiol Clin. 46: 307-11.
- Lee CH, Seo MW, Shin BS, Yang TH, Shin HJ, Ryu HU (2016) Intermittent Theta Slowings in Contralateral Side of Weakness after Sleep Deprivation on Spot EEG in Sporadic Hemiplegic Migraine. J Epilepsy Res. 6: 100-3.
- Valerio E, Barlotta A, Lorenzon E, Antonazzo L, Cutrone M (2015) Harlequin Color Change: Neonatal Case Series and Brief Literature Review. AJP Rep. 5: e73-6.
- Corbett M, Abernethy DA (1999) Harlequin syndrome. J Neurol Neurosurg Psychiatry. 66: 544.
- Moon SY, Shin DI, Park SH, Kim JS (2005) Harlequin syndrome with crossed sympathetic deficit of the face and arm. J Korean Med Sci. 20: 329-30.
- Diserens K, Vuadens P, Michel P, Reichhart M, Herrmann FR, Arnold P, et al. (2006) Acute autonomic dysfunction contralateral to acute strokes: a prospective study of 100 consecutive cases. European Journal of Neurology. 13: 1245-50.
- Leavell R, Ray CG, Ferry PC, Minnich LL (1986) Unusual Acute Neurologic Presentations With Epstein-Barr Virus Infection. Archives of Neurology. 43: 186-8.
- Yamazaki S, Ikeno K, Abe T, Tohyama J, Adachi Y (2011) Hemiconvulsion-Hemiplegia Epilepsy Syndrome Associated With CACNA1A S218L Mutation. Pediatric Neurology. 45: 193-6.
- Wouk J, Rechenchoski DZ, Rodrigues BCD, Ribelato EV, Faccin-Galhardi LC (2021) Viral infections and their relationship to neurological disorders. Archives of Virology. 166: 733-53.

Figures at a glance