Evoked Potentials as an Electrophysiological Marker of Postural Instability in Parkinsonism Patients - An Exploratory Study
Received Date: May 16, 2025 Accepted Date: June 16, 2025 Published Date: June 19, 2025
doi: 10.17303/jnnd.2025.13.102
Citation: Hemant Luniwal, Samhita Panda, Sucharita Anand (2025) Evoked Potentials as an Electrophysiological Marker of Postural Instability in Parkinsonism Patients - An Exploratory Study. J Neurophysiol Neurol Disord 13: 1-14
Abstract
Background: The term parkinsonism includes Parkinson’s disease (PD) and other neurodegenerative diseases presenting with bradykinesia, rest tremor, rigidity and loss of postural reflexes. Such patients may have clinically subtle sensory disturbances demonstrable only by electrophysiological studies. This study aimed to correlate visual, auditory and somatosensory evoked potentials in patients with parkinsonism with presence and severity of postural instability (PI).
Methods: Thirty patients with parkinsonism and 28 healthy controls were enrolled. Disease severity scores- H and Y staging and MDS-UPDRS and scales for PI- PIGD score, FOGQ score and BBS score were assessed. Evoked potentials (EP) [(visual evoked potentials (VEP), brainstem auditory evoked potentials (BAEP), somatosensory evoked potentials (SSEP)] were done.
Results: Mean age was 59.17 ± 9.43 and 57.54 ± 5.81 years for cases and controls, respectively. Mean age of disease onset was 55.93±9.81 years. Majority showed moderate disease severity and moderate PI. A definite negative association was established between SSEP and freezing, VEP and PI, and BAEP latencies with falls and positive pull test. Our study demonstrated lower BAEP latencies with higher PIGD score or a greater risk of PI, shorter VEP and BAEP latencies with higher FOGQ scores and longer freezing episodes and lower VEP and BAEP latencies with lower BBS scores or higher degree of imbalance.
Conclusion: Freezing and PI is a significant cause of disability and morbidity in parkinsonism warranting early recognition and proper management. Significant association exists between PI and EP latencies suggested by our novel observations and may be used to assess disease progression.
Keywords: Postural Instability; PIGD Score; FOGQ Score; BBS Score
Introduction
Clinical features of parkinsonism including Parkinson’s disease (PD) constitute both motor (bradykinesia, rest tremor, rigidity and loss of postural reflexes) and non-motor features [1,2]. Non motor symptoms are increasingly recognized as significant cause of morbidity, predating motor symptoms by several years [3]. Postural instability (PI) due to loss of postural reflexes, is highly prevalent in PD and an important cause of morbidity. Previously thought to be due to involvement of dopaminergic pathways, the current hypothesis is the cortical cholinergic degeneration [4]. Cholinergic neurons in the pedunculopontine nucleus have a powerful influence on motor control of gait and posture. Thalamic acetylcholinesterase activity, derived mainly from neuronal terminals in the pedunculopontine nucleus, reflects cholinergic activity and is reduced in early PD and more severely reduced in PD fallers compared with non-fallers [5].
Brain cholinergic activity can be estimated with short-latency sensory afferent inhibition, that non-invasively assesses the inhibitory circuit in sensorimotor cortex [6]. Patients with parkinsonism may also have sensory disturbances which may not be clinically apparent but demonstrable by electrophysiological studies [7]. Existing literature on evoked potentials (EP) has elucidated that significant delays in EP latencies were observed in parkinsonism, suggesting central conduction abnormalities. Based on above hypothesis, the current study aimed to identify any association between EPs and PI in parkinsonian disorders.
Methods
The study was an observational cross-sectional study conducted over 18 months. Purposive sampling was done from patients of PD attending out-patient and in-patient departments. Recruitment of patients was started after seeking approval from Institutional Ethics Committee (AIIMS/IEC/2022/4121,23/09/2022) and written informed consent from subjects. Patients satisfying the following criteria were enrolled-
Inclusion Criteria
1. Patients with age of 18 or more
2. Patients presenting with hypokinetic extrapyramidal disorder with rigidity, bradykinesia with or without tremor were recruited into the study and divided into typical and atypical parkinsonism.
3. Patients willing to provide written informed consent
Exclusion Criteria
1. Patients with CNS demyelinating disease
2. Patients who are uncooperative for tests
3. Patients with associated dyskinesias
4. Patients with pre-existing peripheral neuropathy, visual or hearing problems
5. Patients who are bedbound and unable to perform tests
Demographic details, clinical history and examination findings, Hoehn & Yahr stage (H and Y stage) and Movement Disorder Society Unified Parkinson’s Disease rating scale (MDS-UPDRS) was recorded. Ophthalmological evaluation, audiometric assessment and nerve conduction studies were done to rule out subclinical involvement. EPs namely visual evoked potential (VEP), brainstem auditory evoked potential (BAEP), and somatosensory evoked potential (SSEP) were done. PI was assessed using PIGD, FOGQ, and BBS score. Those patients fulfilling the UK Parkinson’s disease society brain bank clinical diagnostic criteria were included in the typical parkinsonism group (PD) and 2 patients having onset less than 40 years were considered as early onset parkinsonism but had asymmetric onset and were included along with PD group. Atypical parkinsonism patients were recruited if fulfilling validated clinical diagnostic criteria [8,9]. Two patients with hypokinetic rigid syndrome with preceding history of vaccination and improvement with steroids were considered post vaccineal and included in atypical parkinsonism group. Another two patients with symmetric akinesia and rigidity without significant tremors were considered as drug induced parkinsonism and included in the atypical parkinsonism group [10].
Statistical Analysis
IBM SPSS software version 21.0 and jamovi version 2.5.5 was used for statistical analysis. Qualitative and quantitative variables were represented using frequency and mean and standard deviation respectively. Data was represented using tables, graphs and charts. Unpaired (students) t-test was employed for comparing mean between two groups. Spearmans correlation test was used to find association between EPs and PI severity scores. A p value of less than 0.05 was taken as statistically significant.
Results
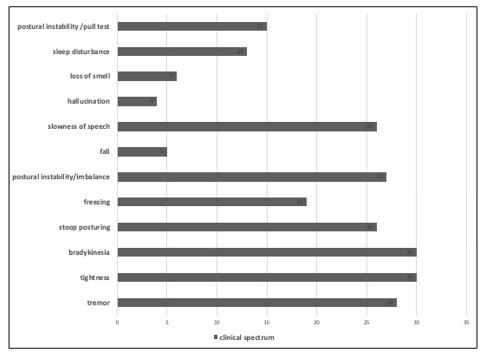
A total of 30 cases with clinical diagnosis of parkinsonism were included. Mean age group was 59.17 ± 9.43 years. The mean duration of illness was 3.23 ± 2.31 years and mean age of onset was 55.93 ± 9.81 years. Assessment of clinical features at presentation revealed that tremor, tightness, bradykinesia, and PI/imbalance affected majority patients (Figure 1). While freezing was also common, falls were the least common symptom. Of the 30 cases, 20 were diagnosed to have PD and 10 had atypical parkinsonism.
Different severity scores were used to assess severity of disease and PI. Mean value of MDS-UPDRS sum score was 76.00 ± 26.04 and mean H & Y stage was 2.80 ± 0.51. Mean values of PIGD, FOGQ and BBS scores were 7.60±4.95, 11.26 ±5.91 and 37.13 ± 12.03 respectively. This indicates that majority of patients in our study had moderate to severe PI.
The mean values of disease severity scores- H and Y stage and MDS-UPDRS, and PI severity scores- PIGD, FOGQ and BBS scores were compared by correlation analysis between PD and atypical parkinsonism cases (Table 1). The mean values of all disease severity scores were more in patients with atypical parkinsonism indicating a higher disease severity though not statistically significant. However, a statistically significant difference was observed for FOGQ (higher score) and a trend towards significance for BBS (lower score) in patients with atypical parkinsonism. Patients having freezing, PI and falls were noted to have higher disease severity in terms of H and Y stage and MDS-UPDRS scores (Table 2).
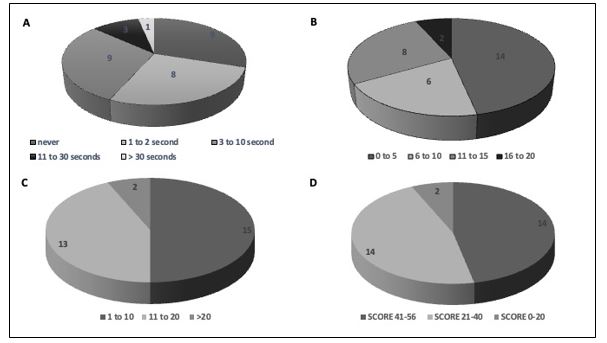
Frequency distribution of subjects based on presence and duration of freezing episodes, PIGD score, FOGQ score, and BBS scores are depicted in Figure 2A-D. Only a minority of patients had moderate (10%) and severe freezing (3%). Patients were grouped into four categories based on PIGD scores: 0-5, 6-10, 11-15 and 16-20 (Figure 2B). The majority had only mild to moderate PI as per PIGD score <10 (66%), while a very small proportion of patients (7%) had very severe PI. Severity of freezing was categorized into 3 groups based on FOGQ score (Figure 2C). Mild gait impairment was observed in 50% (FOGQ scores 1-10), and only 7% had severe gait impairment. Balance was scored based on BBS score and patients divided into three categories- the proportion of patients with low risk of falls was 46%, while 7% had high risk of falls. (Figure 2D).
The mean values of VEP, BAEP and SSEP latencies were compared among patients with and without clinical PI based on falls, freezing of gait, PI, and pull test positivity. No significant difference was noted for VEP among patients with (N=19) and without freezing. However, BAEP I-III IPL left and SSEP P37, N45 left were noted to be shorter in patients with freezing (p values 0.041, 0.033, and 0.042 respectively). Thus, a negative correlation was observed between BAEP/ SSEP latencies and freezing of gait. Amongst the 5 patients having falls, comparison of VEP latencies with those who did not have falls showed prolonged mean N75 and P100 latencies in patients without falls but the difference was not statistically significant. On comparing SSEP latencies, slightly shorter latencies were noted in patients with falls without significant difference. However, BAEP latencies were prolonged in patients without falls with statistical significance for I and I-III IPL right (p values 0.022 and 0.042, respectively).
Further on comparison between patients with and without historical PI, most VEP latencies were shorter in patients with PI. Mean N75 and P100 left latencies were lesser in patients with PI (p values 0.015 and 0.036). On comparing BAEP, all individual wave latencies on left I, II, III, IV, V and I-III IPL, III-V IPL on both sides were more in patients with PI, though not statistically significant. No statistically significant changes were demonstrable in SSEP latencies. Hence, we demonstrated definite negative correlation between VEP and PI.
Similarly, EP latencies were compared between those with positive pull test (N=15) and without. VEP (N75, P100, and N145) and SSEP latencies showed no statistically significant differences between these groups. On the other hand, latencies of BAEP waves I, II III right, both wave V and I-III IPL left showed statistically significant differences (p values 0.033, 0.015, 0.013, 0.011, 0.039 and 0.027, respectively), with lower latencies in patients with positive pull test. Thus, an association of lower BAEP latencies with presence of positive pull test was observed.
The duration of freezing was also compared with EP (Table 3). Mean P100 right was significantly associated with duration, decreasing as freezing duration increased. Likewise, I-III IPL and III-V IPL left showed statistically significant correlation with freezing duration. Overall, a significant negative correlation was observed between VEP and BAEP latencies and duration of freezing of gait.
The various scales for PI and balance were subsequently analysed with respect to EP latencies. No significant association was noted in VEP parameters. Waves I, II, III, V latencies on right and I-III IPL on left showed a significant negative correlation with PIGD scores (Table 4). On comparing SSEP latencies, N20 right showed positive correlation with PIGD score. On subgroup analysis of groups with PIGD scores more than and less than 5, VEP and SSEP parameters showed no statistically significant difference. However, BAEP latencies were more in patients with lesser PIGD scores, the differences in wave I, II, III, IV, V latency on right and V left was statistically significant (p values 0.036, 0.051, 0.018, 0.050, 0.011, 0.027, respectively). Thus, lower BAEP latencies correlated with PIGD score or a greater risk of PI.
On comparison with FOGQ score, significant negative correlation was noted between FOGQ scores and P100 left and N145 right latencies (Table 5). For BAEP, wave I, III right, I-III and III-V IPL left was found to decrease as FOGQ score increased. Further subgroup analysis was done to study the relationship between severity of freezing (FOGQ scores more than and less than 10) and EP. N145 latency right was more in patients with less FOGQ (p = 0.035). BAEP latencies were more in patients with lesser FOGQ, the differences in wave I, II, III, IV latencies on right being statistically significant (p values 0.006, 0.039, 0.018, 0.010, respectively). However, no significant difference was observed in SSEP. Thus, VEP and BAEP latencies were more prolonged with lesser FOGQ scores.
The association between BBS and EP latencies was studied (Table 6). P100 left and BAEP waves I, II, III, IV, V latencies right and I-III IPL left showed a significant negative correlation with BBS scores. However, only N20 right SSEP latency showed a positive correlation with BBS (p value 0.020). Thus, based on these results association between EP latencies and BBS score demonstrated that a lower VEP and BAEP latencies correlated with a lower BBS scores or higher degree of imbalance.
On multivariate analysis of EP latencies between PD and atypical parkinsonism, most of the SSEP latencies were prolonged in atypical parkinsonism group whereas in the case of VEP and BAEP latencies we could not establish a correlation with the type of parkinsonism as shown in Table 7. Though we observed differences between the two groups, they were not statistically significant.
Discussion
This observational, cross-sectional study in patients with clinically diagnosed parkinsonism brought important insights into EP variations that occur in parkinsonism. Distinct differences were observed with respect to type of parkinsonism and severity of PI. Existing literature revealed limited studies on individual EPs in parkinsonism. Our research has focused on all three EPs- VEP, BAEP and SSEP and tried to explore their association with imbalance and PI in parkinsonism.
Demographically, the mean age of onset of parkinsonism in our study was similar to most studies. This aligns with the observations made by Ozek et al, Shalash et al and Roy et al where majority of the study population had onset of disease beyond 50 years of age [11,13].
Apart from clinical features of imbalance and freezing, PI was studied among cases in terms of PIGD, BBS, and FOGQ scores. Our subjects had higher mean PIGD score (7.60±4.95) compared to other studies. Shalash et al reported an average PIGD score of 5.20±4.06 [12]. This indicates that our patients had more severe disease and greater PI. The mean FOGQ score in our study was 11.26 ±5.91 and average BBS score 37.13 ± 12.03, both indicating a moderate to severe disease and greater risk of falls. Klunk et al found average BBS scores to be 54.6±2.0 and 34.7±22.7 among PD and atypical parkinsonism, respectively [14]. We have similarly observed lower BBS scores in patients with atypical parkinsonism compared to IPD. While higher FOGQ were additionally noted in atypical parkinsonism in our study, we did not find literature comparing FOGQ scores between different types of PD. However, Lieberman et al studied freezing of gait in IPD and atypical parkinsonism and observed earlier onset and greater freezing of gait in atypical parkinsonism [15].
A significant negative correlation was established between EP latencies, and historical freezing, PI and falls in our study. A definite negative association was established between SSEP and freezing, VEP and PI and BAEP latencies with falls and positive pull test. This exemplified the fact that a significant correlation exists between increased imbalance and gait issues seen in moderate to severe parkinsonism with a progressive decrease in EP latencies within the group. A detailed literature review did not reveal any contemporary studies on associations between all three EP modalities with PI and freezing.
Using objective scoring of PI, freezing and imbalance, a significant negative correlation was noted with VEP and BAEP parameters and FOGQ and BBS scores. Similar observations were made for PIGD scores, where BAEP parameters showed significant negative correlation. Thus, we have observed an increased objective severity of PI, freezing and imbalance associated with significant reduction in EP latencies mainly for VEP and BAEP parameters. When analyzing between PD and atypical parkinsonism patients, SSEP latencies were more prolonged in atypical parkinsonism patients who also had higher mean disease severity and PI severity scores. This aligns with observations made by Roy et al. On comparing VEP and BAEP latencies with type of parkinsonism, we could not establish a definite correlation. So, this requires further studies.
Our findings are novel as no other literature supports our observations comparing PIGD, FOGQ and BBS scores with all three modalities of EP. However, a few similar studies that were related to our observations are reported. Roy et al compared EP latencies between tremor dominant PD and PIGD variety of PD and observed that BAEP latencies III, V, III-V were more prolonged in PIGD variant [13]. This observation was also statistically significant and it indicated that PI is positively associated with prolongation of BAEP latencies. On the other hand, negative correlation was found by Klunk et al between VEMP amplitude and BBS scores [14].
The major highlight of our study is the consistent lowering of EP latencies in patients with longer duration of parkinsonism and more severe PI and freezing. The pathophysiology behind this observation is unclear at present. While motor impairments in PI were earlier thought to be predominantly caused by dopaminergic neuronal deficits, lack of response of PI and freezing to dopamine therapy may indicate that other neurotransmitters may also be involved. It is currently postulated that gait disturbances in PI maybe linked to cholinergic system mediated cortical and subcortical connections and their degeneration [5]. Bohnen et al showed lower levels of cholinergic activity and increased acetylcholine hydrolysis rates in PD fallers compared to non-fallers [16]. It maybe speculative that while severity of imbalance increases with duration and type of parkinsonism due to the above mechanisms, relative shortening of EP latencies may be influenced by alternate pathways and higher doses of dopaminergic medications that this subgroup is usually subjected to. Higher disease severity would have lead to more severe motor impairment and hence a higher dose of dopamine being used for treatment, the effect of which was not considered in our study. This is a relatively novel concept which needs further exploration as prior studies have contrary observations regarding disease severity and EP latencies [17].
Major limitation of our study was the sample size. The study was primarily designed to analyse relation between EP latencies and parkinsonism with postural instability and the recruitment of study subjects between different disease severity stages was not equal. The number of PD and atypical parkinsonism cases were also not similar (20 vs 10) and hence the observations made cannot be generalised to all PD and atypical parkinsonism patients and limits applicability of our findings. Similarly, most of the patients recruited had moderate disease and moderate PI. Hence the EP latencies in patients with early PD and early PI cannot be commented upon here. Though none of the patients were drug naïve, the effect of dopamine agonists and other drugs on EP latencies were not considered in this study. Some patients were observed to have prolongation of latencies on one side only. Whether this correlates with the side of symptoms has to be further studied in detail and currently we did not analyze the EP latencies based on laterality of clinical feature.
But this study highlights the existence of such associations between disease severity and degree of PI and EP latency and paves the way for future research and breakthroughs. Further studies are required comparing all three EP modalities with different disease stages of PD and atypical parkinsonism with patients recruited based of type and stage of parkinsonism and compared with drug effect and across time and if same association is proven, then EP latencies can be used as a marker for disease progression. This novel study has paved the way for future research which might lead to non-invasive investigations such as EPs being used for assessment of disease progression in PD.
Conclusions
EP abnormalities are common in PD and significant associations exist between PI and its severity and all three modalities of VEP, BAEP and SSEP. Our study revealed shorter EP latencies in patients with more severe PI. Thus, EPs maybe a useful marker of severity of disease and possibly predict PI. However, the exact pathophysiological mechanisms behind this observation cannot be explained with current knowledge, mandating more research.
Acknowledgements
The authors would like to ackowledge the support of the technical staff of the electrophysiology laboratory in the Department of Neurology of All India Institute of Medical Sciences Jodhpur, India.
Disclaimer
NIL
Disclosure of Conflict of Interest
None of the authors has any conflict of interest to disclose.
Source(s) of Support/Funding
None of the authors has received funding from any source for the study.
Patient Consent
Informed consent has been taken from the patients’ relative for publication
Ethical Publication Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
- Marsden CD (1994) Parkinson's disease. J Neurol Neurosurg Psych. 57: 672.
- Váradi C (2020) Clinical features of Parkinson’s disease: the evolution of critical symptoms. Biology. 9: 103.
- Kumaresan M, Khan S (2021) Spectrum of non-motor symptoms in Parkinson's disease. Cureus. 13.
- Allen NE, Schwarzel AK, Canning CG (2013) Recurrent falls in Parkinson's disease: a systematic review. ParkinsonsDis. 2013: 906274
- Bohnen NI, Muller ML, Koeppe RA, Studenski SA, Kilbourn MA, Frey KA, et al. (2009) History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 73: 1670-6.
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, et al. (2008) The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 119: 504-32.
- Menšíková K, Matěj R, Colosimo C, Rosales R, Tuckova L, Ehrmann J, et al. (2022) Lewy body disease or diseases with Lewy bodies? NPJ Parkinsons Dis. 8: 3.
- Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, et al. (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 71: 670-6.
- Williams DR, Lees AJ (2009) Progressive supranuclear palsy: clinicopathological concepts and diagnostic challenges. The Lancet Neurology. 8: 270-9.
- Shin HW, Chung SJ (2012) Drug-induced parkinsonism. Journal of clinical neurology (Seoul, Korea). 8: 15.
- Ozek SU, Jamoussi E, Orken C (2019) Evaluation of visual evoked potential and optical coherence tomography results in idiopathic Parkinson’s disease patients. Euras J Med Investig. 3: 7-13.
- Shalash AS, Hassan DM, Elrassas HH, Salama MM, Méndez-Hernández E, Salas-Pacheco JM, et al. (2017) Auditory-and vestibular-evoked potentials correlate with motor and non-motor features of Parkinson’s disease. Front Neurol. 8: 243178.
- Roy M, Misra AK, Mukherjee J, Mandal M, Chaudhuri J, Ghosh KC, et al. (2023) Evoked potential response in patients with idiopathic Parkinson’s disease and atypical parkinsonian syndromes: A comparative study. Adv Neurol. 2: 1907.
- Klunk D, Woost TB, Fricke C, Classen J, Weise D (2021) Differentiating neurodegenerative parkinsonian syndromes using vestibular evoked myogenic potentials and balance assessment. Clin Neurophysiol. 132: 2808-19.
- Lieberman A, Deep A, Dhall R, Tran A, Liu MJ (2015) Early freezing of gait: atypical versus typical parkinson disorders. Parkinson’s Dis. 2015: 951645.
- Bohnen NI, Albin RL (2011) The cholinergic system and Parkinson disease. Beh Brain Res. 221: 564-73.
- Onofrj M, Fulgente T, Malatesta G, Ferracci F, Thomas A, Curatola L, et al. (1995) The abnormality of N30 somatosensory evoked potential in idiopathic Parkinson's disease is unrelated to disease stage or clinical scores and insensitive to dopamine manipulations. Mov Disord. 10: 71-80.

Tables at a glance
Figures at a glance