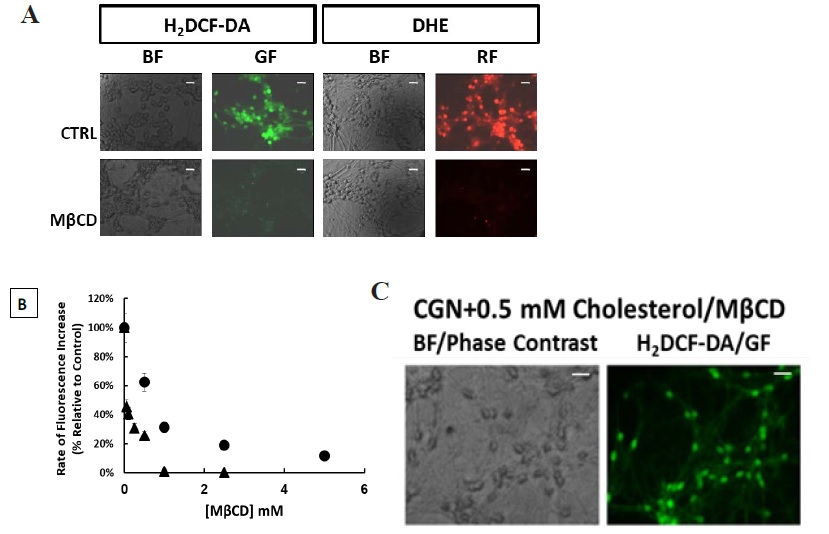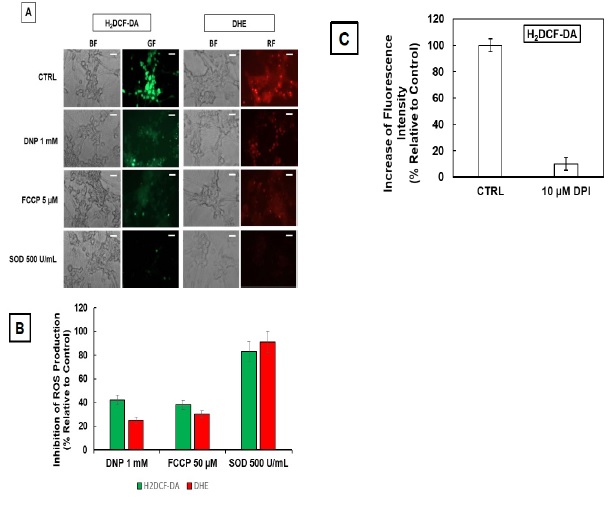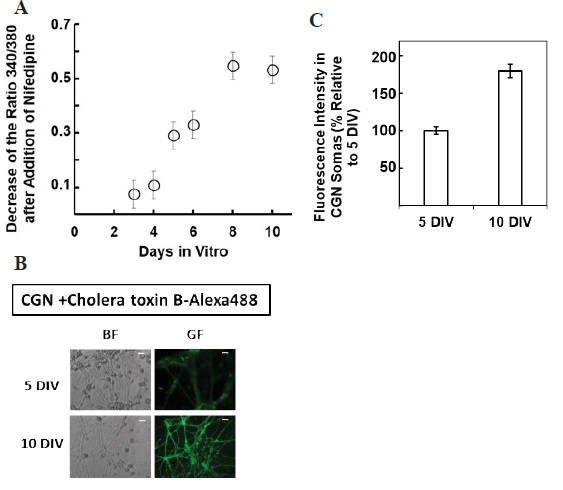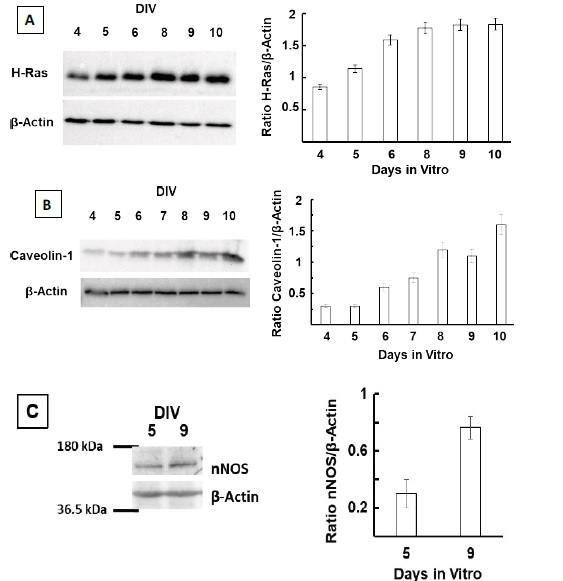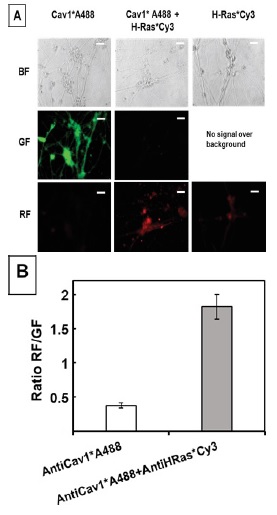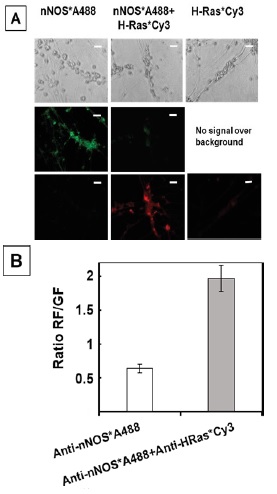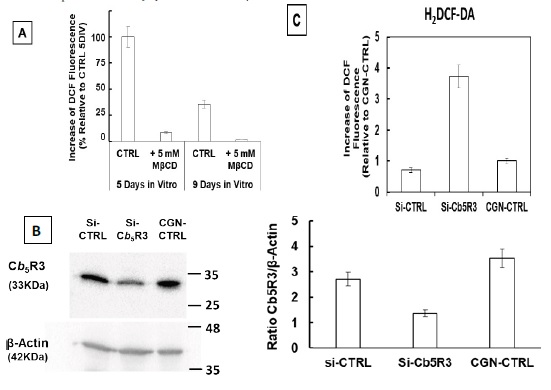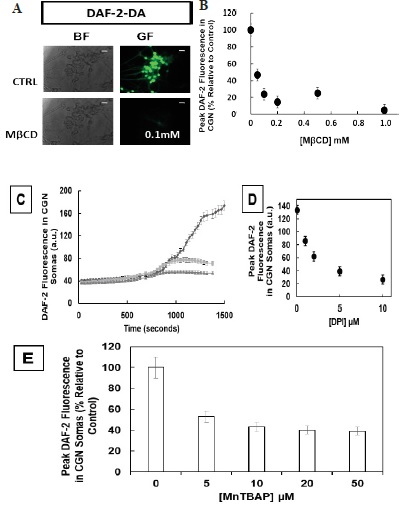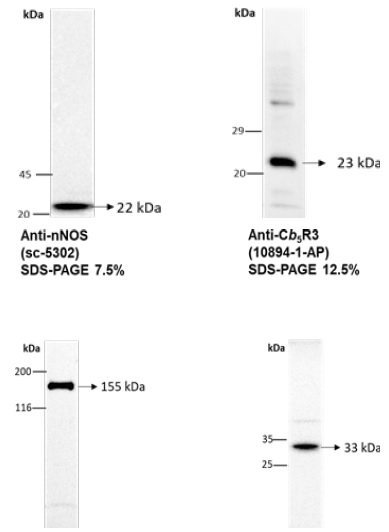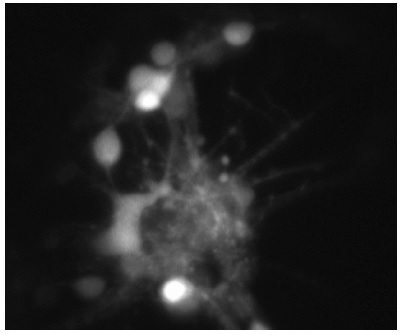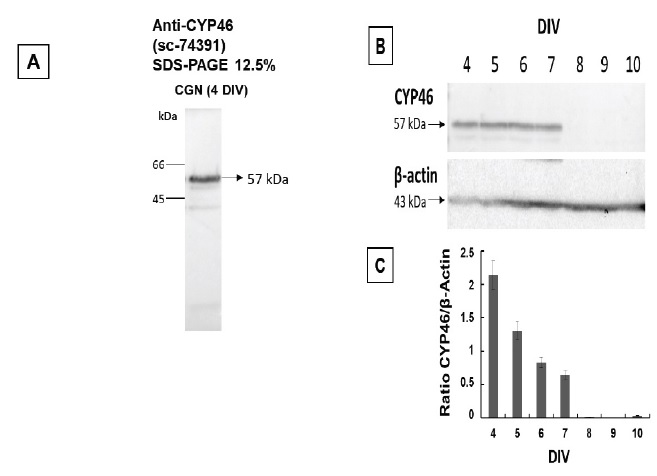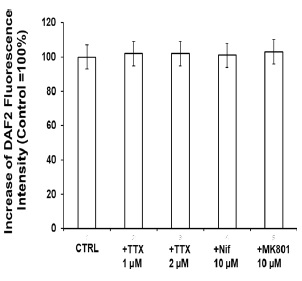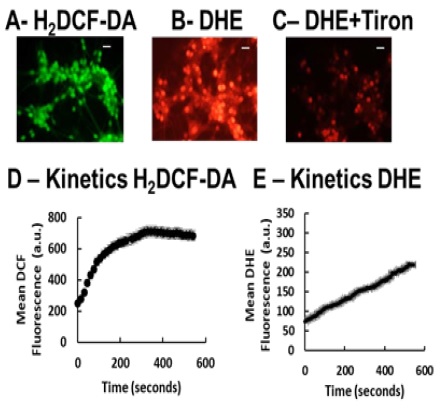
Figure 1 H2DCF-DA and DHE monitors ROS production upon incorporation to CGN. Representative fluorescence microscopy images of mature CGN showed that they accumulated a significant amount of DCF (Panel A) and oxidized DHE (PanelB) only after 10 min of addition of 10 µM H2DCF-DA and DHE to MLocke's K25. Addition of 20 mM Tiron to the extracellular medium 1 hour before the addition of DHE largely attenuated the increase of DHE fluorescence (Panel C). The kinetics of an increase of the average fluorescence intensity per pixel in the soma of mature CGN after the addition of H2DCF-DA and DHE to MLocke's K25 medium is shown in Panels D and E, respectively. Scale bar inserted in fluorescence microscopy images = 10 µm.